1. Introduction
Since the long-term integrity of a thermal neutron poison is essential for effective criticality control of used nuclear fuel during storage, boron containing alloys or composites have been used in both used fuel pool storage racks and dual-purpose (storage/transportation) canisters and casks. Boral is currently used in fuel pools and storage/transportation casks. However, its NRC (Nuclear Regulatory Commission) license is only 20 years [
1]. Al/B
4C metal matrix composites (MMCs), as innovative new materials, are considered to be excellent candidates that used as neutron absorber materials in both dry storage casks and wet storage pools of spent nuclear fuel for preventing criticality due to the high neutron absorption cross-section of elemental
10B, excellent corrosion properties and light weight [
2,
3,
4,
5]. Meanwhile, because Al/B
4C MMCs have high thermal conductivity and thermal stability, they are tremendously beneficial for dry storage casks that will be loaded with mixed oxide (MOX) fuel and highly enriched fuel.
Al/B
4C MMCs could be manufactured using 1000, 5000 and 6000 series aluminum alloys [
6]. 5000 series of Al/B
4C MMCs have a better aging response to the higher thermal environment from enriched/MOX fuel and 6090 series of Al/B
4C MMCs have previously been qualified for wet and dry used fuel storage. As a candidate of neutron absorber, the microstructure and properties of Al/B
4C MMCs should be fully understood. In-service environment, Al/B
4C MMCs will be subjected to irradiate from gamma rays with approximately 0.1–10 kGy/h dose rate and neutrons with the fluxes in a range of (0.25–26) × 10
4/cm
2·s [
2]. Therefore, the distribution features and microstructure characteristics of B
4C particles are important to the service behavior because of the nuclear features of elemental B. The main investigations conducted so far include the followings: (1) The defect structure in gamma or neutron irradiated B
4C [
2,
3,
7,
8,
9]; (2) The microstructure evolution in ion irradiated Al/B
4C MMCs, such as helium bubbles in Al matrix [
4], amorphization occurred in severe conditions [
10], and interfacial microstructure in Al/B
4C [
11]; (3) The degradation mechanism including irradiation damage [
5], boron leaching, corrosion and mechanical damage [
12]; (4) The discussion of preparation process, mechanical properties [
13,
14,
15,
16], microstructural characteristics [
17]. Despite these extensive investigations, there are still some questions associated with the microstructure characteristics in the Al/B
4C MMCs that need to be answered. For example, because the hardness value between boron carbide and aluminum matrix is of great different, the smooth surface with low roughness is difficultly obtained by conventional metallographic methods. Therefore, few researchers could obtain the distribution characteristics and size factor of B
4C particles in aluminum matrix, which is actually very important to assess the properties of Al/B
4C MMCs.
In the present work, in order to investigate the distribution features and microstructure characteristics of B4C particles in the Al/B4C MMCs, a series of experiments were designed and then carried out. In addition, as neutron absorbers used in fuel storage pool, Al/B4C MMCs were subjected chemical corrosion. Therefore, the corrosion properties and corresponding mechanism were also investigated in an aqueous solution with 5000 ppm boric acid at 100 °C and atmospheric pressure.
2. Experiment
Three kinds of Al/B
4C MMCs prepared by powder metallurgy were used as original materials in the present work. The chemical composition of Al/B
4C MMCs were listed in
Table 1. Except for the main chemical compositions of Al and B
4C, these Al/B
4C MMCs also had some trace chemical elements such as Cr, Cu, Fe, Mg, and so on. Regarding the easy usage in the following paragraphs, these three kinds of Al/B
4C MMCs were simplified as Al-15%B
4C, Al-25%B
4C and Al-30%B
4C MMCs.
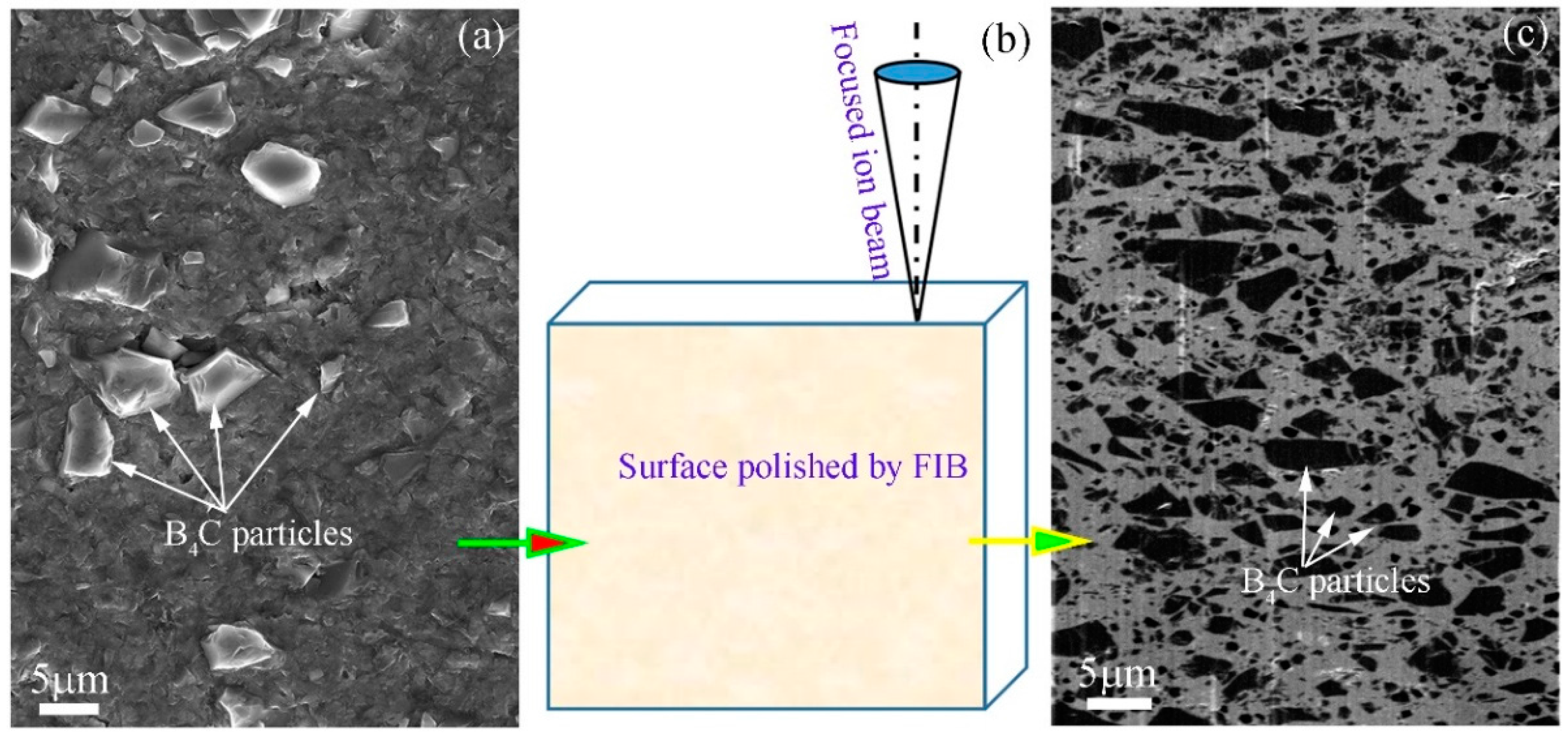
Because of the great difference of hardness value between boron carbide and the aluminum matrix, in general, SEM image of Al/B
4C MMCs looks like that which is shown in
Figure 1a, as an example in Luo’s research report [
11] and Shorowordi’s literature [
13]. Due to the fact that the sample surface was polished using conventional metallographic methods, it is very difficult to characterize B
4C particles in the aluminum matrix. In here, we provide a method to prepare an ideal sample surface of Al/B
4C MMCs with very low roughness, which can be used to characterize B
4C particles and can be carried out for ion-irradiation research with high-energy charge particles.
The Al/B
4C MMCs slices with approximately 10 mm × 10 mm × 1.5 mm size were first cut from as-prepared plates, using a precision diamond knife cutting machine and then grinded by SiC sandpaper from 320 to 2500 grid, and mechanically polished using 6–1 μm series diamond pastes. After that, the morphology of sample surface is as shown in
Figure 1a. Although some B
4C particles can be observed in the sample surface, not only does the aluminum matrix show severe plastic deformation, but also B
4C particles protrude on the sample surface. Therefore, the non-ideal surface was further polished using focused ion beam (FIB) under a dual beam workstation (FEI Nova Workstation) equipped with a focused Ga
+ ion beam column and a high-resolution field emission scanning electron microscope (FEG SEM, Nova 200, FEI, Hillsboro, OH, USA). The sketch of sample surface polished by FIB is shown in
Figure 1b. The incidence direction of focused Ga
+ ions was almost parallel to the sample surface. The morphology of the finally polished sample surface is shown in
Figure 1c, which was taken at the FEI Nova Workstation. The polished area is over 50 μm × 300 μm. Compared
Figure 1c with
Figure 1a, it can be seen that after being polished by FIB, the distribution characteristics and boundary dimension of B
4C particles within the aluminum matrix are very clear.
The samples for transmission electron microscopy (TEM) observation were prepared using the FIB “lift-out” technique [
18] and then performed using a JEOL 3011 high resolution transmission electron microscope (JEOL, Tokyo, Japan) (300 kV) and a JEOL 2010F field emission gun analytical electron microscope (JEOL, Tokyo, Japan) (200 kV).
The Al/B4C MMCs samples of approximately 10 mm × 10 mm × 1.5 mm size were corroded with different time in an aqueous solution with 5000 ppm boric acid at 100 °C and atmospheric pressure using a high temperature high pressure autoclave (Dalian runchang petrochemical equipment Co. Ltd., Dalian, China). The sample mass was measured after a certain corrosion time. The mass increment rate was calculated according to the equation of αi = (mi − m0)/m0, where, αi is the mass increment rate at i time measurement; mi and m0 are the sample mass before the corrosion and after i time corrosion, respectively. The morphology of the sample surface after a certain corrosion time was observed by scanning electron microscopy (SEM) (FEG SEM, ZEISSEVO18, ZEISS, Heidenheim, Germany).
3. Results and Discussion
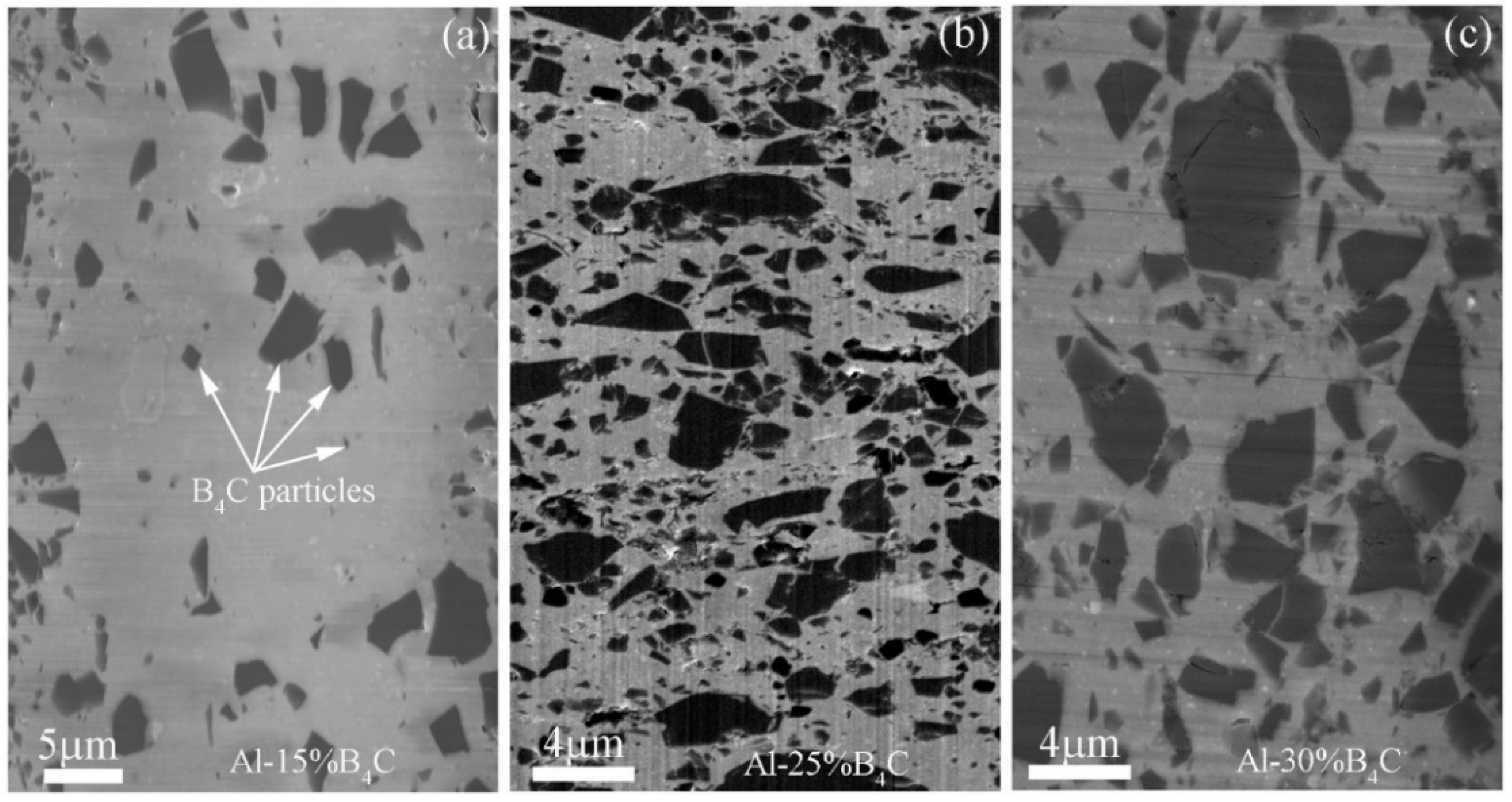
The surface morphology of the polished Al-15%B
4C MMCs, Al-25%B
4C MMCs and Al-30%B
4C MMCs is shown in
Figure 2a‒c, respectively. It can be seen that B
4C particles indicated by white arrows homogeneously distribute in the Al matrix. The size of B
4C particles, ranging from several hundred nanometers to several microns, is not uniform. The shape of the B
4C particles is as a polygon. In addition, some pores and cracks can be found in the particles. A high concentration of damaged particles may have a critical impact on nucleation and propagation of cracks in the composites. However, Alizadeh [
19] reported B
4C particles uniformly dispersed in Al matrix and the pores in the composites could be negligible.
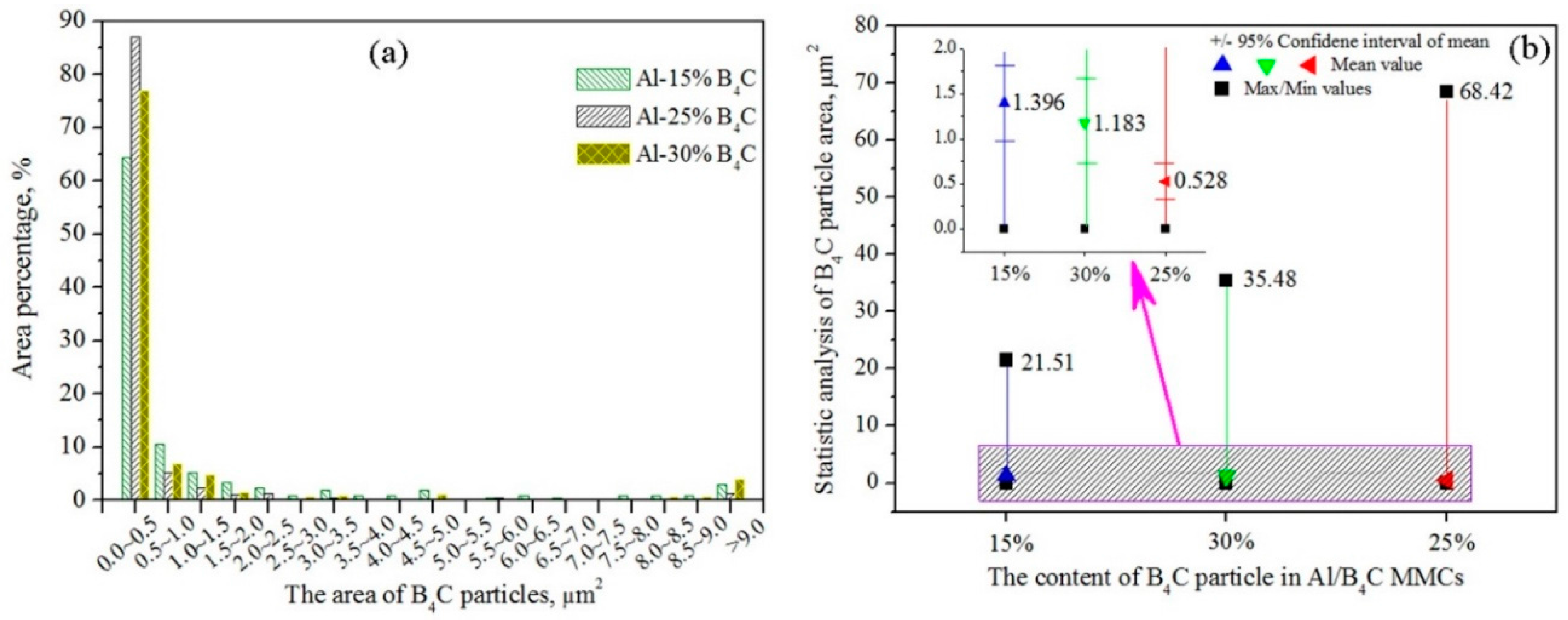
Appropriate dimension of dispersion B
4C particles is one of the significant methods to optimize the neutron irradiation and corrosion properties of Al/B
4C MMCs. Homogeneous distribution of particles is a prerequisite to enhance the related properties of the matrix alloy [
20]. Therefore, the characteristics of B
4C particles become very important. The statistical analysis results of B
4C particles in Al/B
4C MMCs are shown in
Figure 3. The counting numbers is exceeded 150. The error bar is less than 1%. Area percentage of B
4C particles is shown in
Figure 3a, which indicates that the area of B
4C particles mainly concentrates on the range from 0 to 0.5 μm
2. The proportion of B
4C particle size ranged from 0 to 0.5 μm
2 in the Al-15%B
4C, Al-25%B
4C and Al-30%B
4C MMCs are 64.29%, 86.99% and 76.86% of total statistical results, respectively. In addition, according to the statistical results and SEM observation, there are also several large-sized B
4C particles distributing in the Al matrix. The proportion of B
4C particle size that is larger than 9.0 μm
2 in the Al-15%B
4C, Al-25%B
4C and Al-30%B
4C MMCs are about 2.86%, 1.33% and 4.0%, respectively.
The average area of B4C particles in the Al-15%B4C, Al-25%B4C and Al-30%B4C MMCs are about 1.396, 0.528 and 1.183 μm2, respectively. It clear that the average area of B4C particles in the Al-15%B4C MMCs is about three times that of the Al-25%B4C MMCs. In addition, the maximum area of B4C particles in the current test is 21.51 μm2, 68.42 μm2 and 35.48 μm2 in the Al-15%B4C, Al-25%B4C and Al-30%B4C MMCs, respectively.
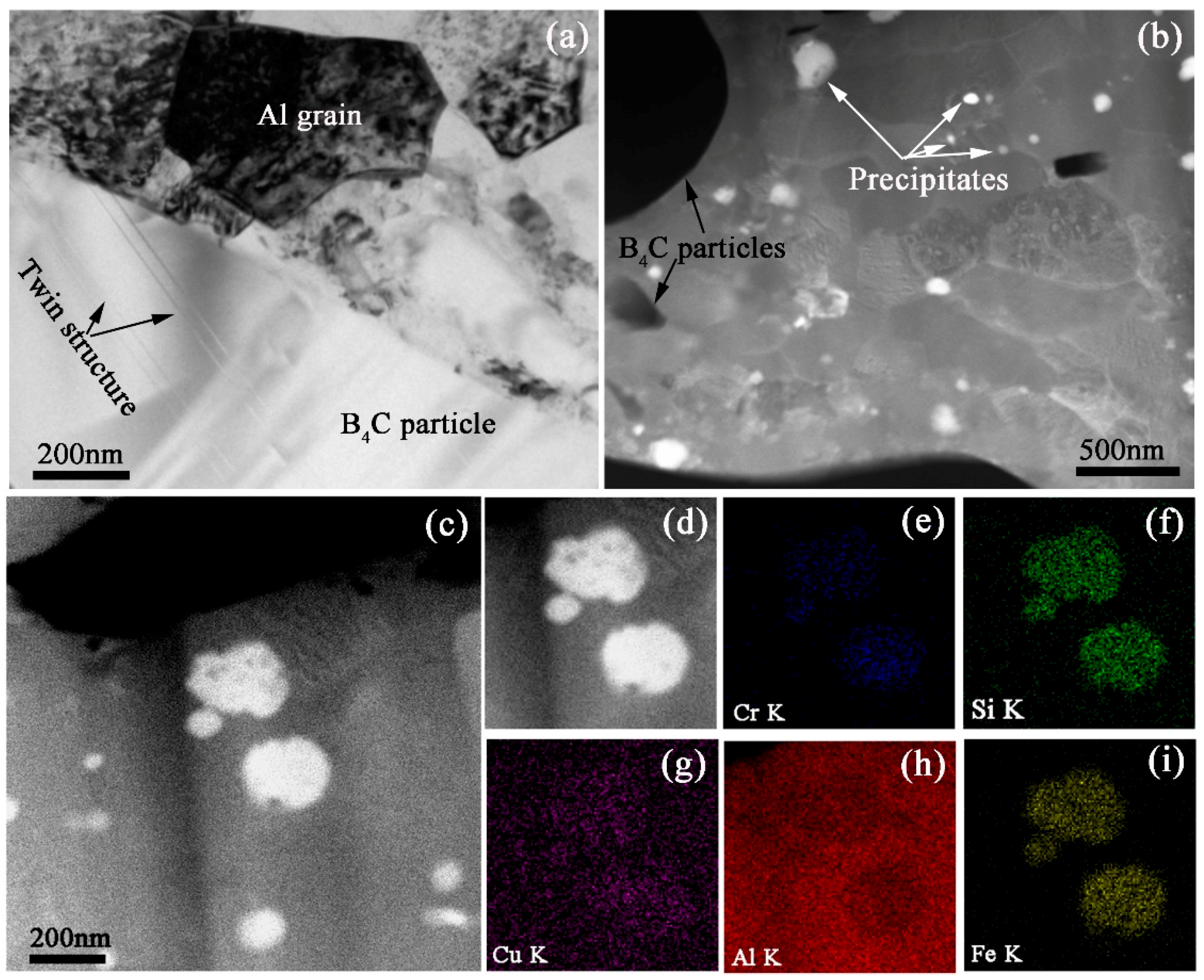
The bright field TEM image of the Al-30% B
4C MMCs is shown in
Figure 4a. The Al grains and B
4C particles can be observed. Large amount of dislocations distribute in Al matrix. Meanwhile, twin structures can be observed in B
4C particles as denoted by dark arrows. Li [
21] also reported that the deformation twins were found in boron carbide particles within a nanostructured Al 5083/B
4C MMCs. Heian [
22] researched both of the milled and unmilled B
4C samples and observed numerous nanometric twins in the micro-size grains. High angle annual dark field scanning transmission electron microscopy (HAADF-STEM) image in
Figure 4b shows not only B
4C particles as denoted by dark arrows but also alloy precipitates with bright color as denoted by white arrows. The grain size of the Al matrix is relatively homogeneous. However, from a more microscopic point of view, some grains show that they are round shape, while others show ellipse or lath-shaped. Similar phenomena could be found in the literature [
23].
The size of alloy precipitates ranges from few tens of nanometers to several hundred nanometers. The grain boundaries of Al matrix can be obviously observed.
Figure 4c,d are the HAADF-STEM images of the precipitates in the Al-30%B
4C MMCs. The shape of the alloy precipitates shows an elliptic geometry. The element mapping results of Cr K, Si K, Cu K, Al K and Fe K are shown in
Figure 4e–i, respectively. The elemental Cu, Cr, Fe and Si are found to be bright in the region of alloy precipitates. At the same time, elemental Al displays a shadow. The elemental mapping results show that the precipitates are the alloying of elemental Cu, Cr, Fe and Si, not including elemental Al.
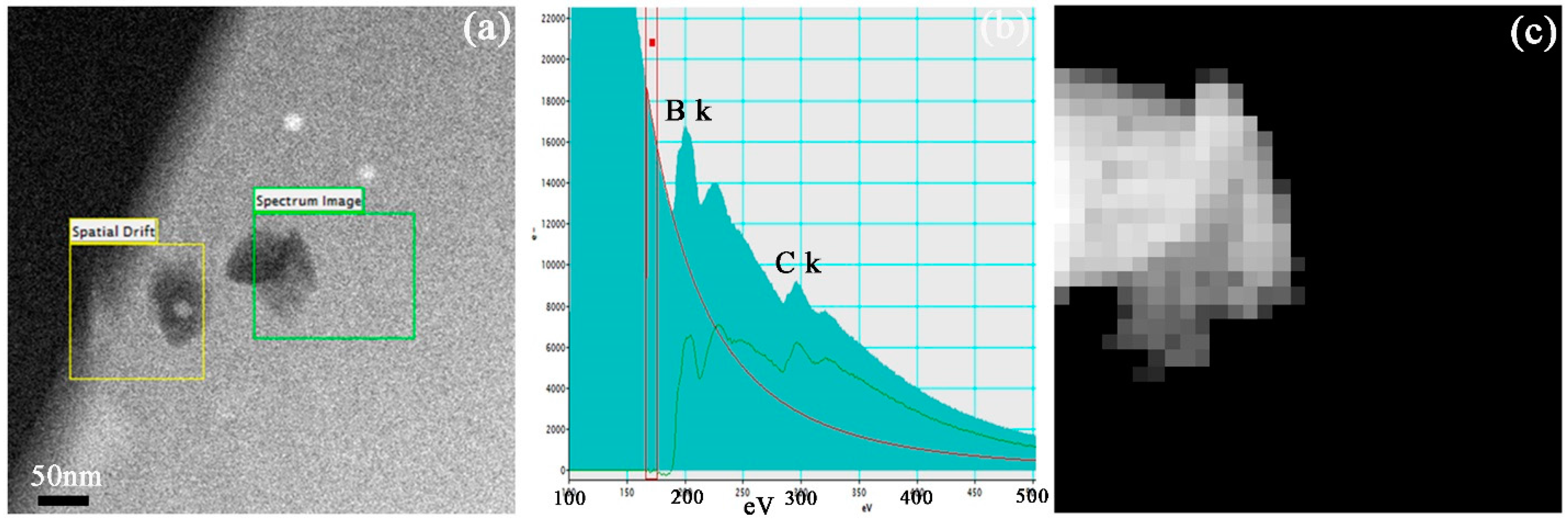
In order to confirm that the nano-sized particles with dark color in HAADF-STEM images are the B
4C particles, the electron energy loss spectroscopy (EELS) is used to analyze the elements. Two particles with approximately 100 nm are distributed in the Al-15% B
4C MMCs matrix as shown in
Figure 5a. The green box named “Spectrum Image” symbolizes the EELS map scanning region of elemental boron, while the yellow box named “Spatial Drift” is set to achieve the range migration correction accurately by choosing a black particle as reference point during the EELS mapping process. The B k and C k peaks are detected in the black particle as shown in
Figure 5b. Therefore, it indicates that the effective detection region is composed by B and C elements. The mapping result of elemental boron in the dark particle is shown in
Figure 5c, which indicates that the distribution of boron element is in good agreement with the morphology contour of the chosen black particle. The same experiment results were also obtained in the Al-25% B
4C and Al-30% B
4C MMCs samples. In addition, it is worth noting that the elemental boron cannot be detected in the Al gains at the precision scope of the EELS technology. However, in the present work, there is still a doubt that whether a few B or C atoms can diffuse into interstitial void of Al grains. The experimental results above suggest a way to calculate the distribution of boron element in Al/B
4C MMCs, by figuring out the distribution of B
4C particles in aluminum matrix.
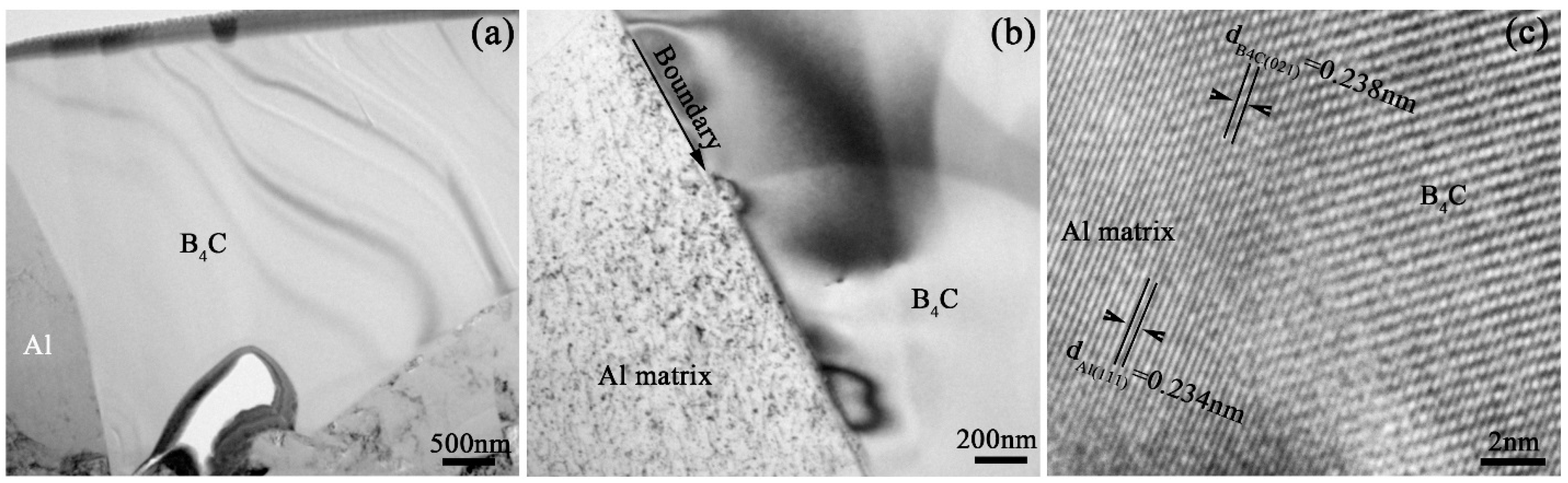
Figure 6a is a bright field TEM image with low magnification showing the microstructure of Al grain and B
4C particle. A pore with approximately 1 μm size can be observed at the boundary of Al grain and B
4C particle.
Figure 6b is high magnification of crystal boundary between Al matrix and B
4C particle and
Figure 6c is a high resolution TEM (HRTEM) image of their crystal boundary. The crystal lattice of Al grain and B
4C particle can be observed. The crystal structure of Al grain and B
4C phase are belong to face-centered cubic (FCC) and hexagonal close packed (HCP) lattice, respectively. The lattice parameter of B
4C phase is
a = 0.56003 nm,
c = 1.2086 nm, and
α = 90°,
β = 90°,
γ = 120°. The crystal plane distance d
B4C(021) of B
4C phase is equal to 0.238 nm. The distance of {111} crystal planes of Al phase (d
Al(111)) is 0.234 nm. When the (021) crystal plane of B
4C phase and (111) crystal plane of Al phase grow together, the lattice mismatch is approximately 1.68% and calculated as the following equation:
where,
δ is the lattice mismatch. The calculation suggests that (021) crystal plane of B
4C phase and (111) crystal plane of Al phase located in the interface are in parallel relationship closely, which is shown in
Figure 6c.
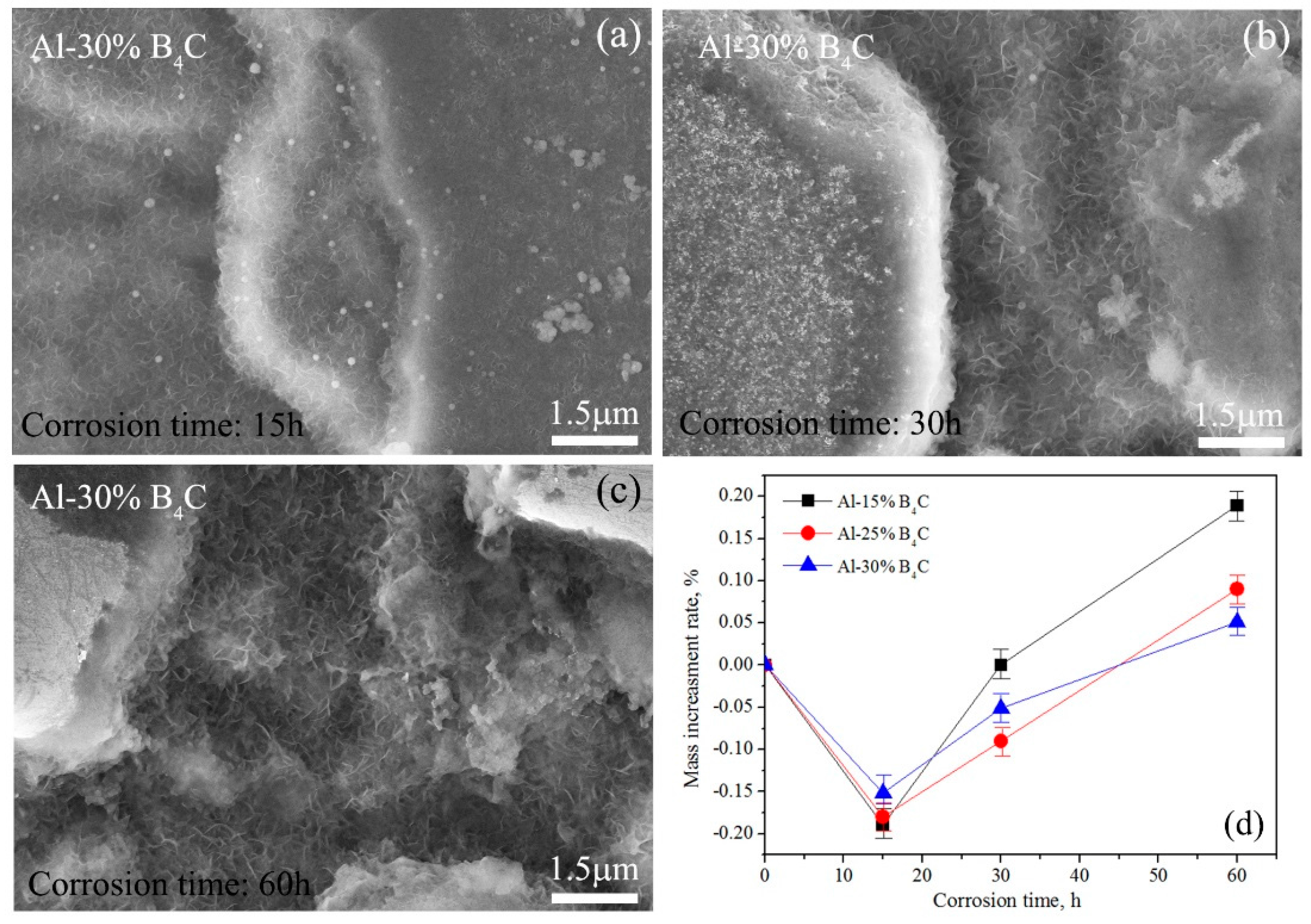
Figure 7 shows the SEM images showing the surface morphology of Al-30%B
4C MMCs corroded for (a) 15 h, (b) 30 h and (c) 60 h in an aqueous solution with 5000 ppm boric acid at 100 °C and atmospheric pressure. Some flocculent oxide and pit etching are observed in the sample surface. The longer the corrosion time is, the more the amount of flocculent oxide and pit etching. The values of the mass increment rate after certain corrosion times are listed in
Table 2. The relationship between the mass increment rate and corrosion time is shown in
Figure 7d. It can be seen that the mass increment rate is first decreased with increasing corrosion time and then increased. The negative mass increment rate at 0–15 h corrosion time means that the mass of Al/B
4C MMCs sample is decreased in that time period. During the procedure of sample corrosion, the oxide such as Al
2O
3 will be generated and also be simultaneously dissolved by boric acid. In the initial stage, the dissolution rate of corrosion products is larger than generation rate. Therefore, the sample mass is decreased and the mass increment rate occurs the negative. When the generation rate is larger than the dissolution rate, the mass increment rate will be changed to positive value and shows an increment trend. The reason should be due to the rapid oxidation of aluminum matrix, which is also used to explain that the mass increment rate of Al-15%B
4C MMCs is largest at 15–60 h corrosion time because of the maximal aluminum content in these three kinds of Al/B
4C MMCs. The mass increment is Δm
(Al-30%B4C) < Δm
(Al-25%B4C) < Δm
(Al-15%B4C). However, more detailed corrosion properties and behaviors will be investigated in future work.