Hot-Dip Aluminizing on AISI F55–UNS S32760 Super Duplex Stainless Steel Properties: Effect of Thermal Treatments
Abstract
:1. Introduction
2. Experimental Procedure
3. Theory and Calculation
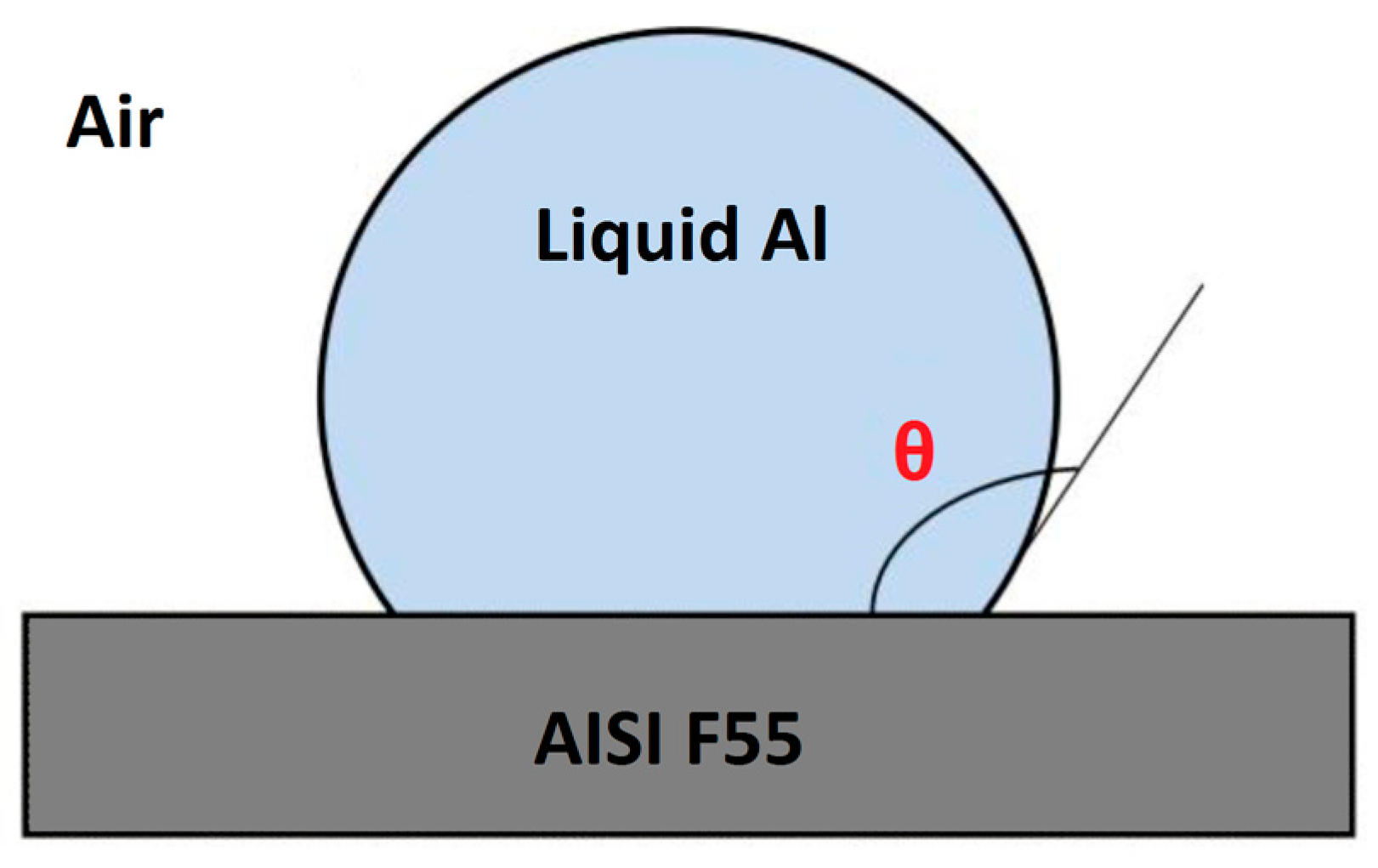
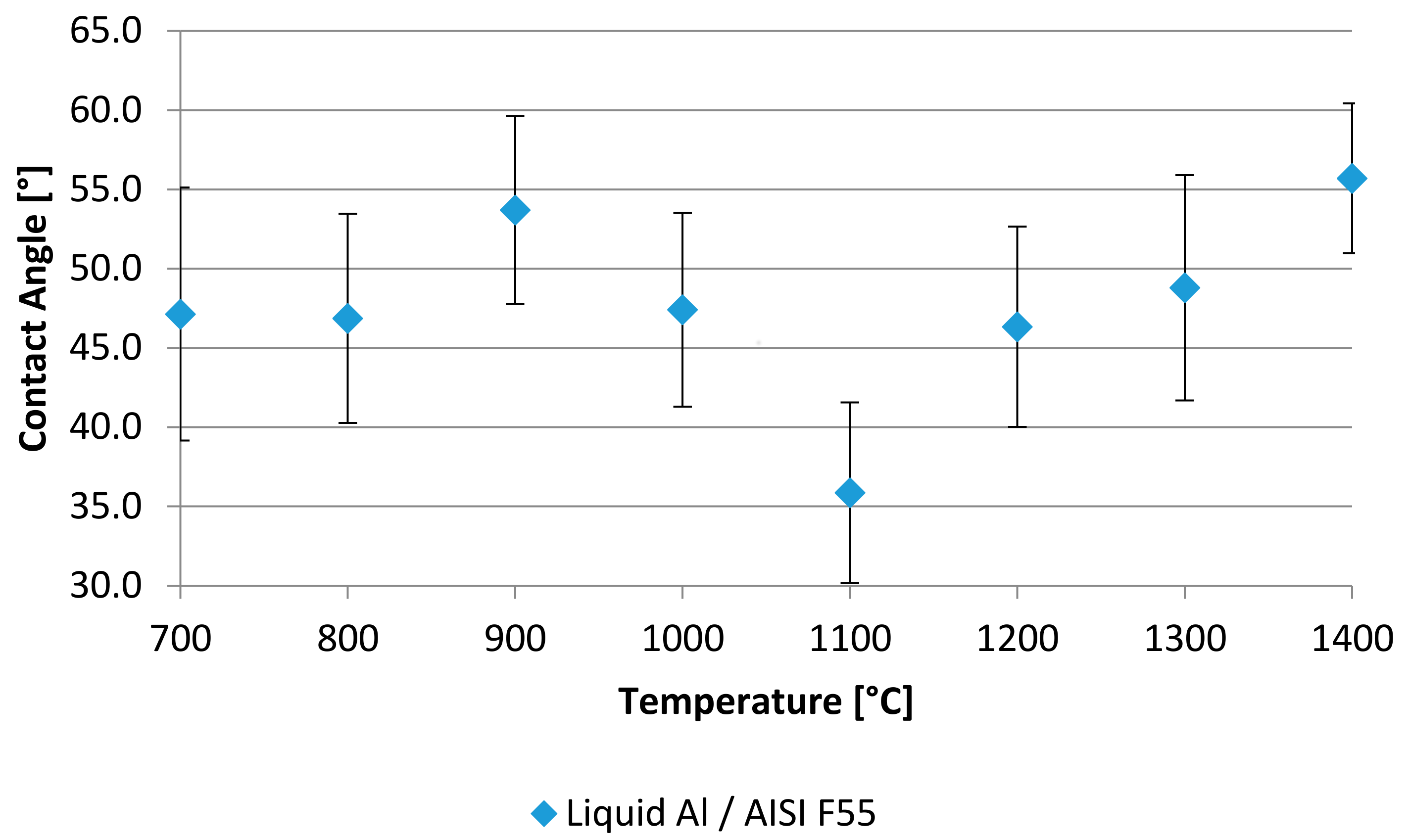
3.1. Wetting Contact Angle
3.2. Diffusion
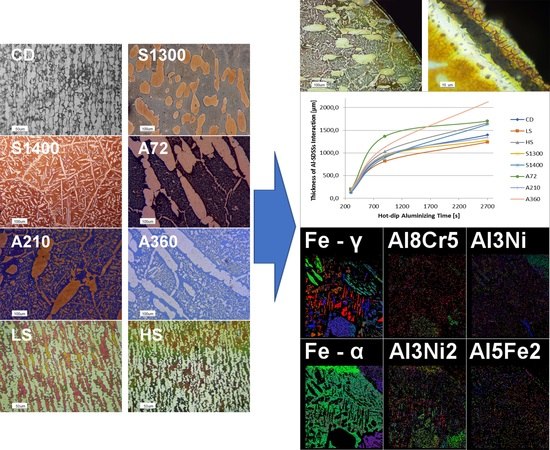
4. Results
5. Discussion
6. Conclusions
Author Contributions
Conflicts of Interest
References
- Żaba, K. Wear Resistance of Aluminized Steel Plates. Arch. Metall. Mater. 2011, 56. [Google Scholar] [CrossRef]
- Heo, N.H.; Kim, M.T.; Shin, J.H.; Kim, C.Y. Simultaneous chromizing and aluminizing using chromium oxide and aluminum: (II) on austenitic stainless steel. Surf. Coat. Technol. 2000, 124, 39–43. [Google Scholar] [CrossRef]
- Sharafi, S.; Farhang, M.R. Effect of aluminizing on surface microstructure of an HH309 stainless steel. Surf. Coat. Technol. 2006, 200, 5048–5051. [Google Scholar] [CrossRef]
- Messaoudi, K.; Huntz, A.M.; Lesage, B. Diffusion and growth mechanism of Al2O3 scales on ferritic Fe-Cr-Al alloys. Mater. Sci. Eng. A 1998, 247, 248–262. [Google Scholar] [CrossRef]
- Sadique, S.E.; Mollah, M.A.H.; Ali, M.M.; Haque, M.M.; Basri, S.; Ahmad, M.M.H.M.; Sapuan, S.M. Influence of Aluminium Additions on the Rate of Oxidation of Iron-Chromium Alloys. J. Corros. Sci. Eng. 2000, 1, 18. [Google Scholar]
- Badini, C.; Laurella, F. Oxidation of FeCrAl alloy: Influence of temperature and atmosphere on scale growth rate and mechanism. Surf. Coat. Technol. 2001, 135, 291–298. [Google Scholar] [CrossRef]
- Perujo, A.; Forcey, K.S. Tritium permeation barriers for fusion technology. Fusion Eng. Des. 1995, 28, 252–257. [Google Scholar] [CrossRef]
- Kalin, B.A.; Yakushin, V.L.; Fomina, E.P. Tritium barrier development for austenitic stainless steel by its aluminizing in a lithium melt. Fusion Eng. Des. 1998, 41, 119–127. [Google Scholar] [CrossRef]
- Hollenberg, G.W.; Simonen, E.P.; Kalinin, G.; Terlain, A. Tritium/hydrogen barrier development. Fusion Eng. Des. 1995, 28, 190–208. [Google Scholar] [CrossRef]
- Benamati, G.; Chabrol, C.; Perujo, A.; Rigal, E.; Glasbrenner, H. Development of tritium permeation barriers on Al base in Europe. J. Nucl. Mater. 1999, 271–272, 391–395. [Google Scholar] [CrossRef]
- Wang, D.Q. Phase evolution of an aluminized steel by oxidation treatment. Appl. Surf. Sci. 2008, 254, 3026–3032. [Google Scholar] [CrossRef]
- Josh, D.L.; Easton, D.S.; Liu, C.T.; Babu, S.S.; David, S.A. Processing of Fe3Al and FeAl alloys by reaction synthesis. Intermetallics 1995, 3, 467–481. [Google Scholar]
- Lauwerens, W.; De Boeck, A.; Thijs, M.; Claessens, S.; Van Stappen, M.; Steenackers, P. PVD Al-Ti and Al-Mn coatings for high temperature corrosion protection of sheet steel. Surf. Coat. Technol. 2001, 146–147, 27–32. [Google Scholar] [CrossRef]
- Lee, P.-Y.; Chiu, L.-H.; Yang, C.-F.; Chen, W.-D.; Kai, W.; Chu, J.P. Improvement of environmental degradation resistance of Fe–Al-based alloys by surface modification treatments. Mater. Sci. Eng. A 1997, 239–240, 736–740. [Google Scholar] [CrossRef]
- Su, C.W.; Lee, J.W.; Wang, C.S.; Chao, C.G.; Liu, T.F. The effect of hot-dipped aluminum coatings on Fe-8Al-30Mn-0.8C alloy. Surf. Coat. Technol. 2008, 202, 1847–1852. [Google Scholar] [CrossRef]
- Li, Y.J.; Zhang, Y.L.; Liou, Y.X. Characteristics of phase constitution in the Fe-Al alloy layer of calorized steel pipe. J. Mater. Sci. 1995, 30, 2635–2639. [Google Scholar] [CrossRef]
- Wang, D.; Shi, Z.; Zou, L. A liquid aluminum corrosion resistance surface on steel substrate. Appl. Surf. Sci. 2003, 214, 304–311. [Google Scholar] [CrossRef]
- Young, G.A.; Tucker, J.D.; Lewis, N.; Plesko, E.; Sander, P. Assessment of lean grade duplex stainless steels for nuclear power applications. In Proceedings of the 15th International Conference on Environmental Degradation of Materials in Nuclear Power Systems -Water Reactors-2011, Colorado Springs, CO, USA, 7–11 August 2011; The Minerals, Metals & Materials Society: Pittsburgh, PA, USA. [Google Scholar]
- Wang, Y.Q.; Yang, B.; Han, J.; Dong, F.; Wang, Y.L. Localized Corrosion of Thermally Aged Cast Duplex Stainless Steel for Primary Coolant Pipes of Nuclear Power Plant. Procedia Eng. 2012, 36, 88–95. [Google Scholar] [CrossRef]
- Gunn, R.N. Duplex Stainless Steels, 1st ed.; Woodhead Publishing: Sawston, Cambridge, UK, 1997; pp. 1–12. [Google Scholar]
- Mateo, A.; Girone, A.; Keichel, J.; Llanes, L.; Akdut, N.; Anglada, M. Cyclic deformation behaviour of superduplex stainless steels. Mater. Sci. Eng. A 2001, 314, 176–185. [Google Scholar] [CrossRef]
- Ciuffini, A.; Barella, S.; Di Cecca, C.; Gruttadauria, A.; Crugnola, S.; Mapelli, C. Local pitting corrosion resistance evaluation in large forged UNS-S32760 super duplex stainless steel parts of a sphere valve for the oil & gas industry. Metall. Ital. 2017, 10, 33–45. [Google Scholar]
- Charles, J. 10 years later, Obviously duplex grades in industrial applications look like a success story. In Proceedings of the 6th World Duplex Conference, Venice, Italy, 2000; AIM: Milan, Italy. [Google Scholar]
- Johansson, K. Duplex stainless steels: Past, present and future. In Proceedings of the 6th World Duplex Conference, Venice, Italy, 2000; AIM: Milan, Italy. [Google Scholar]
- Nilsson, J.O. Super duplex stainless steels. Mater. Sci. Technol. 1992, 8, 685–700. [Google Scholar] [CrossRef]
- Ciuffini, A.; Barella, S.; Di Cecca, C.; Gruttadauria, A.; Mapelli, C.; Mombelli, D. Isothermal Austenite–Ferrite Phase Transformations and Microstructural Evolution during Annealing in Super Duplex Stainless Steels. Metals 2017, 7, 368. [Google Scholar] [CrossRef]
- Southwick, P.D.; Honeycombe, R.W.K. Decomposition of ferrite to austenite in 26%Cr-5%Ni stainless steel. Met. Sci. 1980, 7, 253–261. [Google Scholar] [CrossRef]
- Hsieh, C.-C.; Wu, W. Precipitation of Phase Using General Diffusion Equation with Comparison to Vitek Diffusion Model in Dissimilar Stainless Steels. J. Metall. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Jackson, E.M.L.E.M.; Visser, P.E.; Cdornish, L.A. Distinguishing between Chi and Sigma phases in duplex stainless steels using potentiostatic etching. Mater. Charact. 1993, 31, 185–190. [Google Scholar] [CrossRef]
- Miranda, M.A.R.; Sasaki, J.M.; Tavares, S.S.M.; De Abreu, H.F.G.; Neto, J.M. The use of X-ray diffraction, microscopy, and magnetic measurements for analysing microstructural features of a duplex stainless steel. Mater. Charact. 2005, 54, 387–393. [Google Scholar] [CrossRef]
- Champion, J.A.; Keene, B.J.; Sillwood, J.M. Wetting of aluminium oxide by molten aluminium and other metals. J. Mater. Sci. 1969, 4, 39–49. [Google Scholar] [CrossRef]
- Hashim, J.; Looney, L.; Hashmi, M.S.J. The wettability of SiC particles by molten aluminium alloy. J. Mater. Process. Technol. 2001, 119, 324–328. [Google Scholar] [CrossRef]
- Bracco, G.; Holst, B. Surface Science Techniques, 1st ed.; Springer-Verlag Berlin Heidelberg: Berlin, Germany, 2013; pp. 3–34. [Google Scholar]
- Lam, H.S. Multicomponent diffusion revisited. Phys. Fluids 2006, 18, 1–8. [Google Scholar] [CrossRef]
- Baluffi, R.W.; Allen, S.M.; Carter, W.C. Kinetics of materials, 1st ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; pp. 77–98. [Google Scholar]
- Alvarez-Armas, I.; Degallaix-Moreuil, S. Duplex Stainless Steels, 1st ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 115–140. [Google Scholar]
- Murray, J.L. The Al-Cr (aluminum-chromium) system. J. Phase Equilib. 1998, 19, 367–375. [Google Scholar] [CrossRef]
- Li, X.; Scherf, A.; Heilmaier, M.; Stein, F. The Al-Rich Part of the Fe-Al Phase Diagram. J. Phase Equilibria Diffus. 2016, 37, 162–173. [Google Scholar] [CrossRef]
- Kuentzler, R. Ordering effects in the Fe-Al system. J. Phys. 1983, 44, 1167–1178. [Google Scholar] [CrossRef]
- Ghatee, M.H.; Zare, M.; Zolghadr, A.R.; Moosavi, F. Temperature dependence of viscosity and relation with the surface tension of ionic liquids. Fluid Phase Equilib. 2010, 291, 188–194. [Google Scholar] [CrossRef]
- Huang, W.; Chang, Y.A. Thermodynamic properties of the Ni-Al-Cr system. Intermetallics 1999, 7, 863–874. [Google Scholar] [CrossRef]
- Palm, M. Concepts derived from phase diagram studies for the strengthening of Fe-Al-based alloys. Intermetallics 2005, 13, 1286–1295. [Google Scholar] [CrossRef]
- Palm, M. The Al-C-Fe syste-Phases and phase equilibria in the Al-rich corner. J. Alloys Compd. 1997, 252, 192–200. [Google Scholar] [CrossRef]
- Eustathopoulos, N. Wetting by Liquid Metals—Application in Materials Processing: The Contribution of the Grenoble Group. Metals 2015, 5, 350–370. [Google Scholar] [CrossRef]
- Oikawa, H. Review on lattice diffusion of substitutional impurities in iron. A summary report. Technol. Rep. Tohoku Univ. 1982, 47, 215–224. [Google Scholar]
- Ennis, B.L.; Jimenez-Melero, E.; Mostert, R.; Santillana, B.; Lee, P.D. The role of aluminium in chemical and phase segregation in a TRIP-assisted dual phase steel. Acta Mater. 2016, 115, 132–142. [Google Scholar] [CrossRef]
- Baczmański, A.; Braham, C. Elastoplastic properties of duplex steel determined using neutron diffraction and self-consistent model. Acta Mater. 2004, 52, 1133–1142. [Google Scholar] [CrossRef]
- Bugat, S.; Besson, J.; Pineau, A. Micromechanical modeling of the behavior of duplex stainless steels. Comput. Mater. Sci. 1999, 16, 158–166. [Google Scholar] [CrossRef]
- Jia, N.; Wang, Y.D.; Peng, L.R. Micromechanical behaviors of duplex steel: in situ neutron diffraction measurements and simulations. J. Phys. Condens. Matter 2008, 20, 104259. [Google Scholar] [CrossRef]
- Song, J.L.; Bate, P.S. Plastic anisotropy in a superplastic duplex stainless steel. Acta Mater. 1997, 45, 2747–2757. [Google Scholar] [CrossRef]
- Fargas, G.; Akdut, N.; Anglada, M.; Mateo, A. Microstructural evolution during industrial rolling of a duplex stainless steel. ISIJ Int. 2008, 48, 1596–1602. [Google Scholar] [CrossRef]
- Howard, R.E.; Lidiard, A.B. Matter transport in solids. Rep. Prog. Phys. 1964, 27, 161–240. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Chen, W. Interfacial reactions of duplex stainless steels with molten aluminum. Surf. Interface Anal. 2015, 47, 648–656. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, W.; Luo, H.; Li, S.; Zhou, T.; Shi, L. Corrosion resistance and interfacial morphologies of novel Fe-Cr-Mo-B cast steels in molten aluminum. Corros. Sci. 2017, 125, 20–28. [Google Scholar] [CrossRef]
- Yun, J.-G.; Lee, J.-H.; Kwak, S.-Y.; Kang, C.-Y. Microstructural Evolution of Intermetallic Compound Formed in Boron Steel Hot-Dipped in Al–7%Ni Alloy. Metals 2017, 7, 393. [Google Scholar] [CrossRef]
- Barmak, K.; Dybkov, V.I. Interaction of iron-chromium alloys containing 10 and 25 mass% chromium with liquid aluminium Part II Formation of intermetallic compounds. J. Mater. Sci. 2004, 9, 4219–4230. [Google Scholar] [CrossRef]

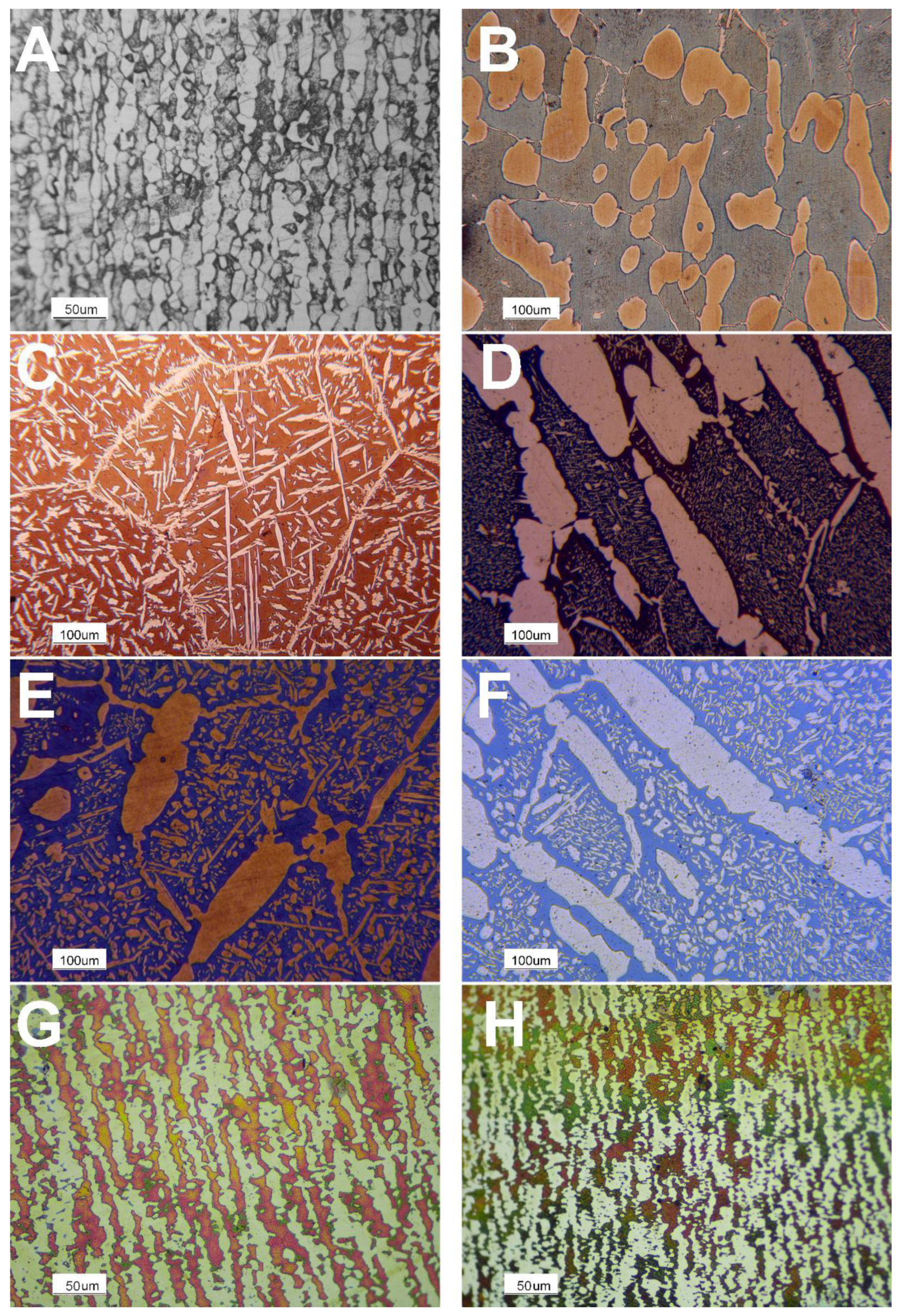
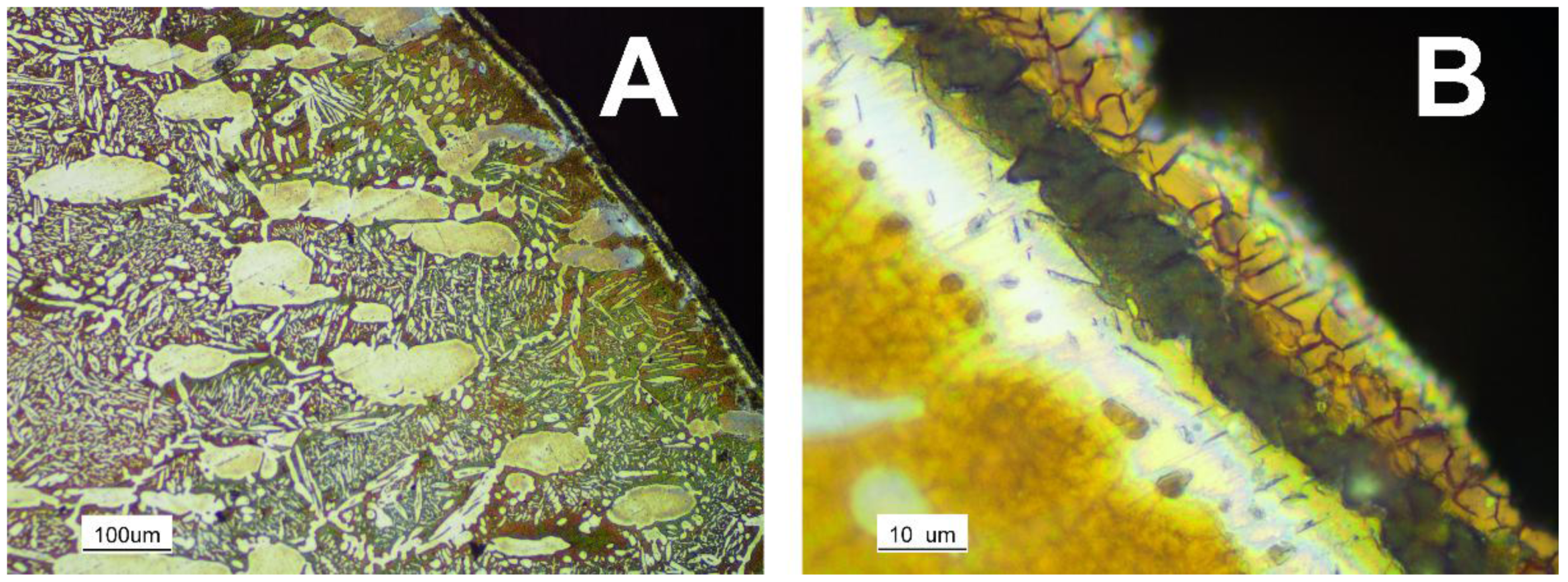
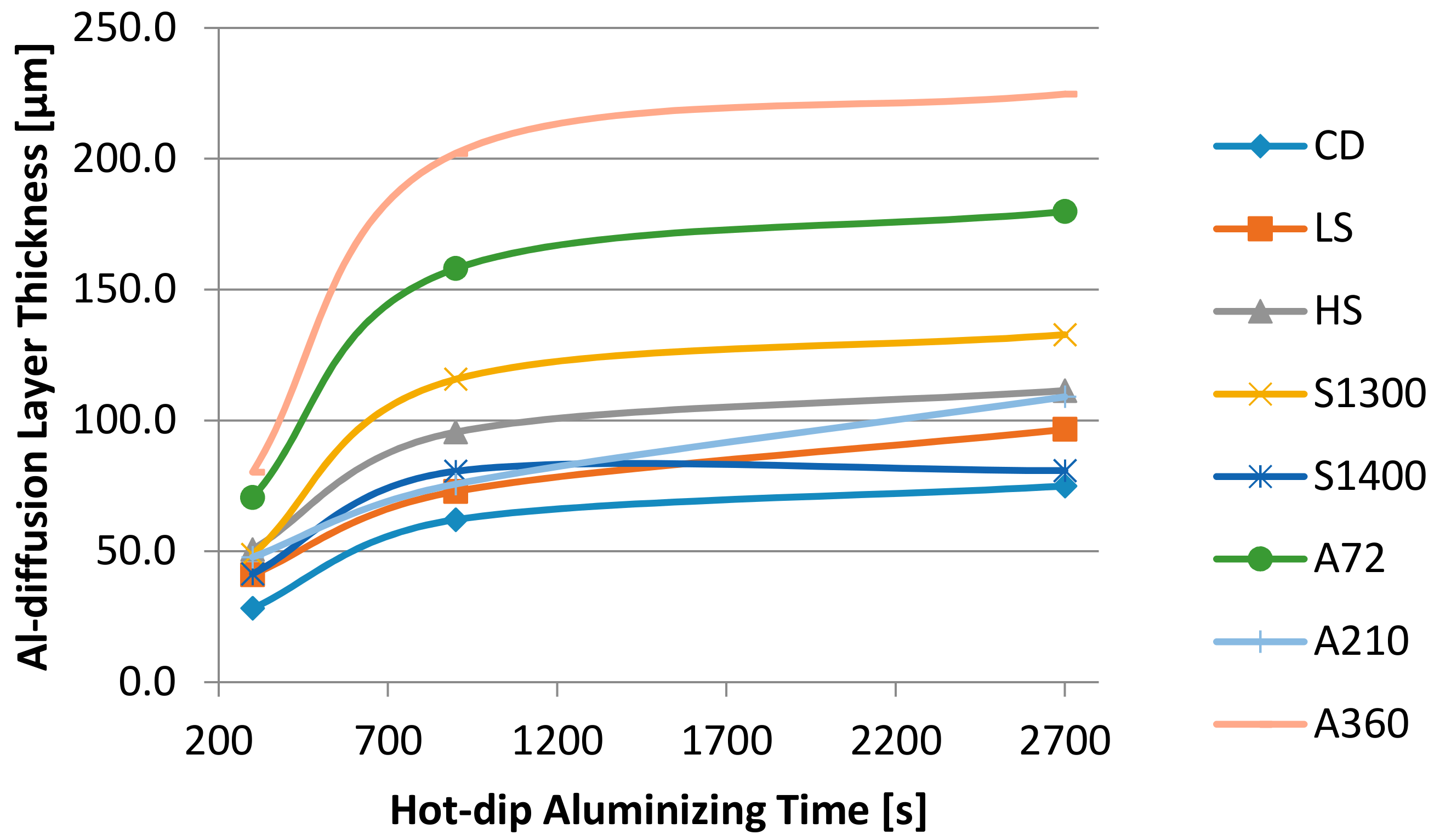
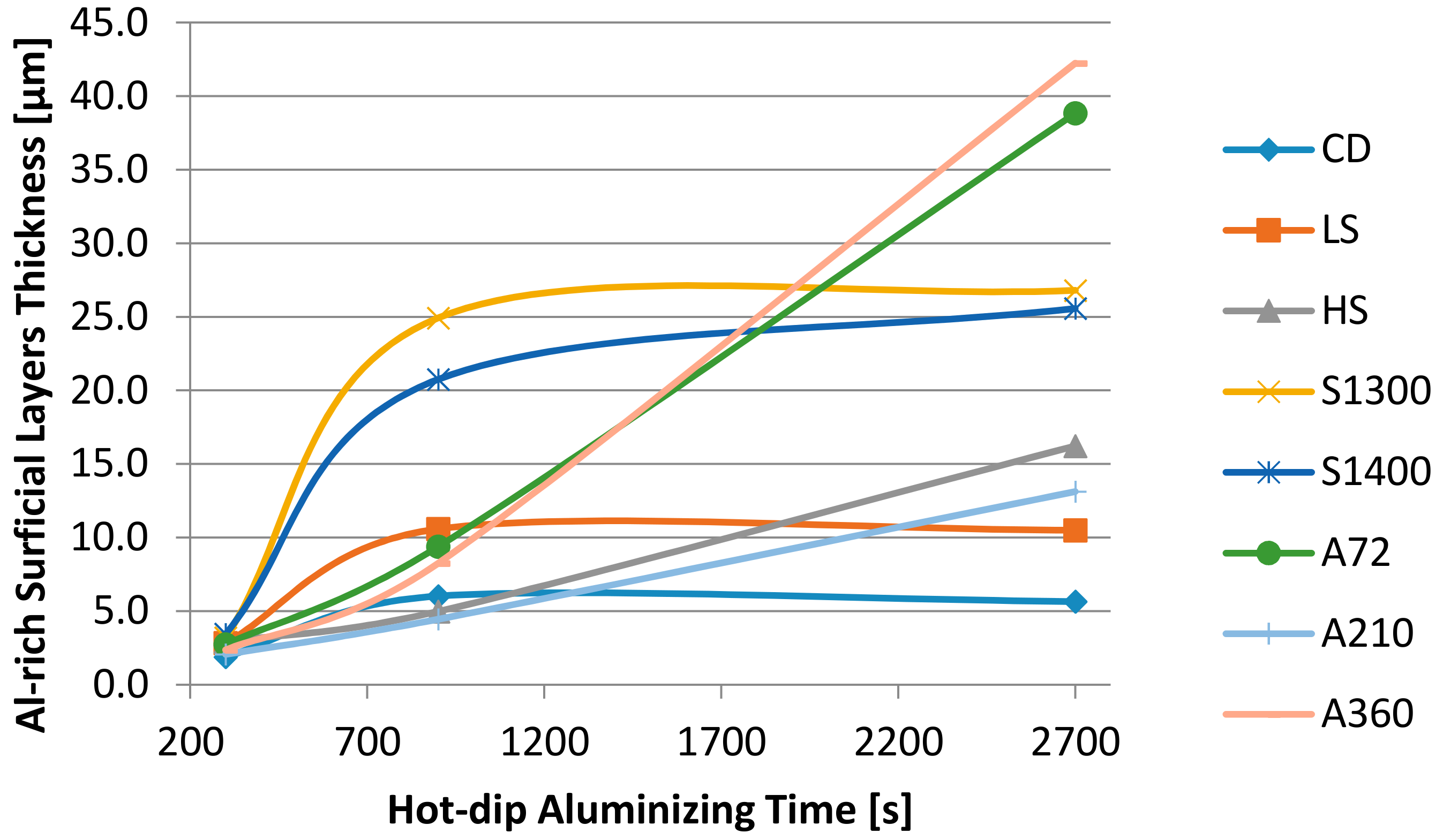
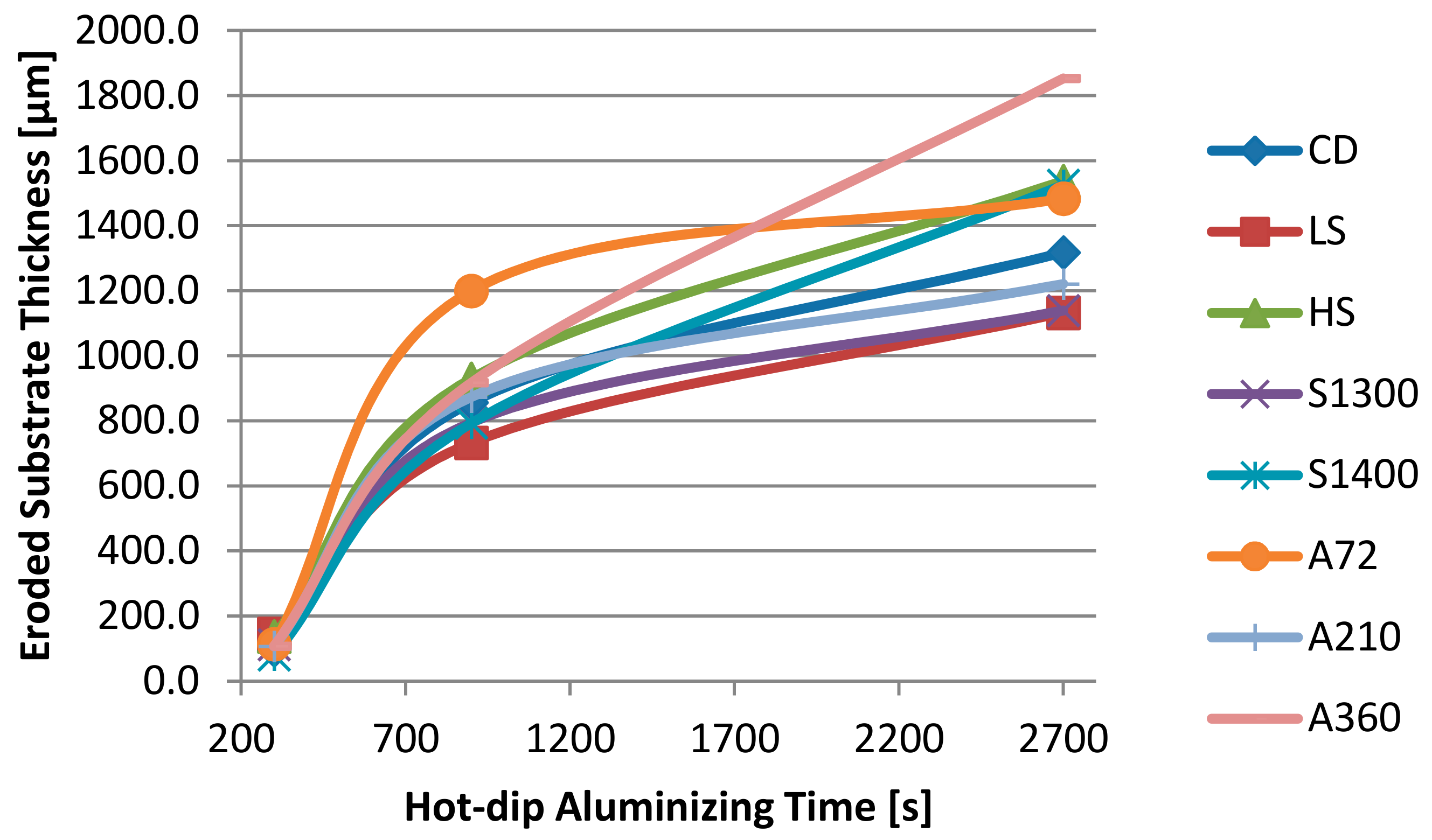
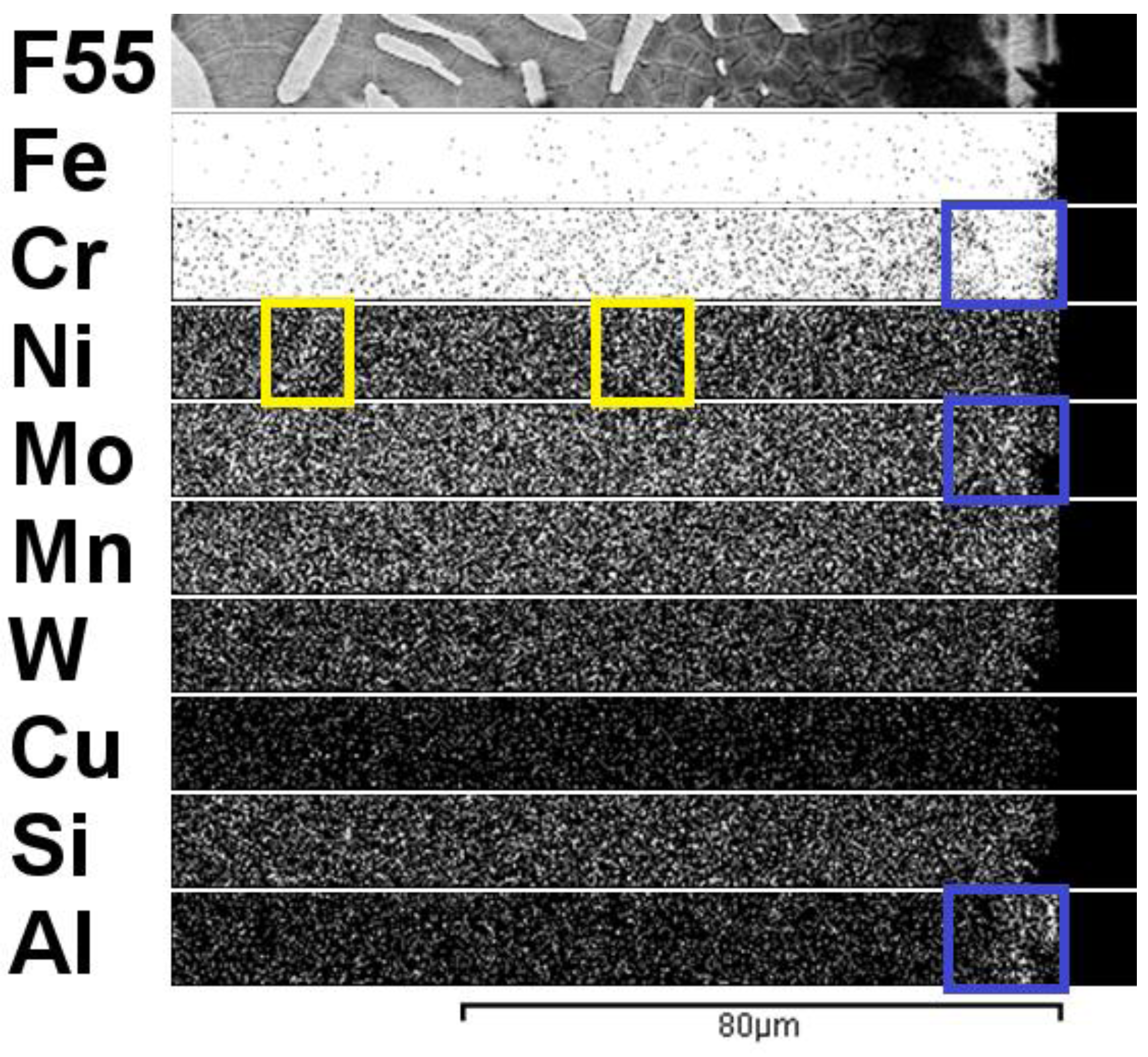
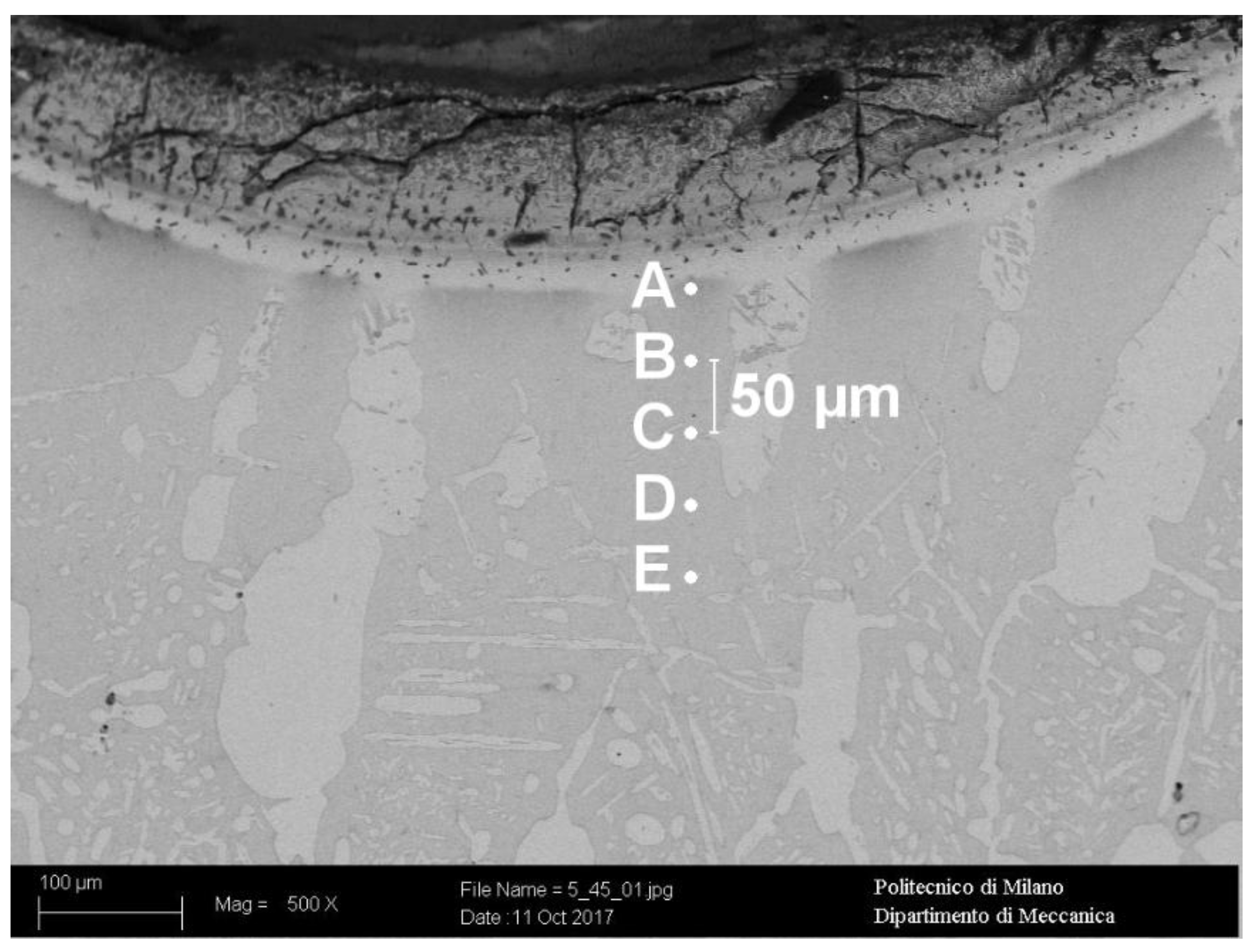
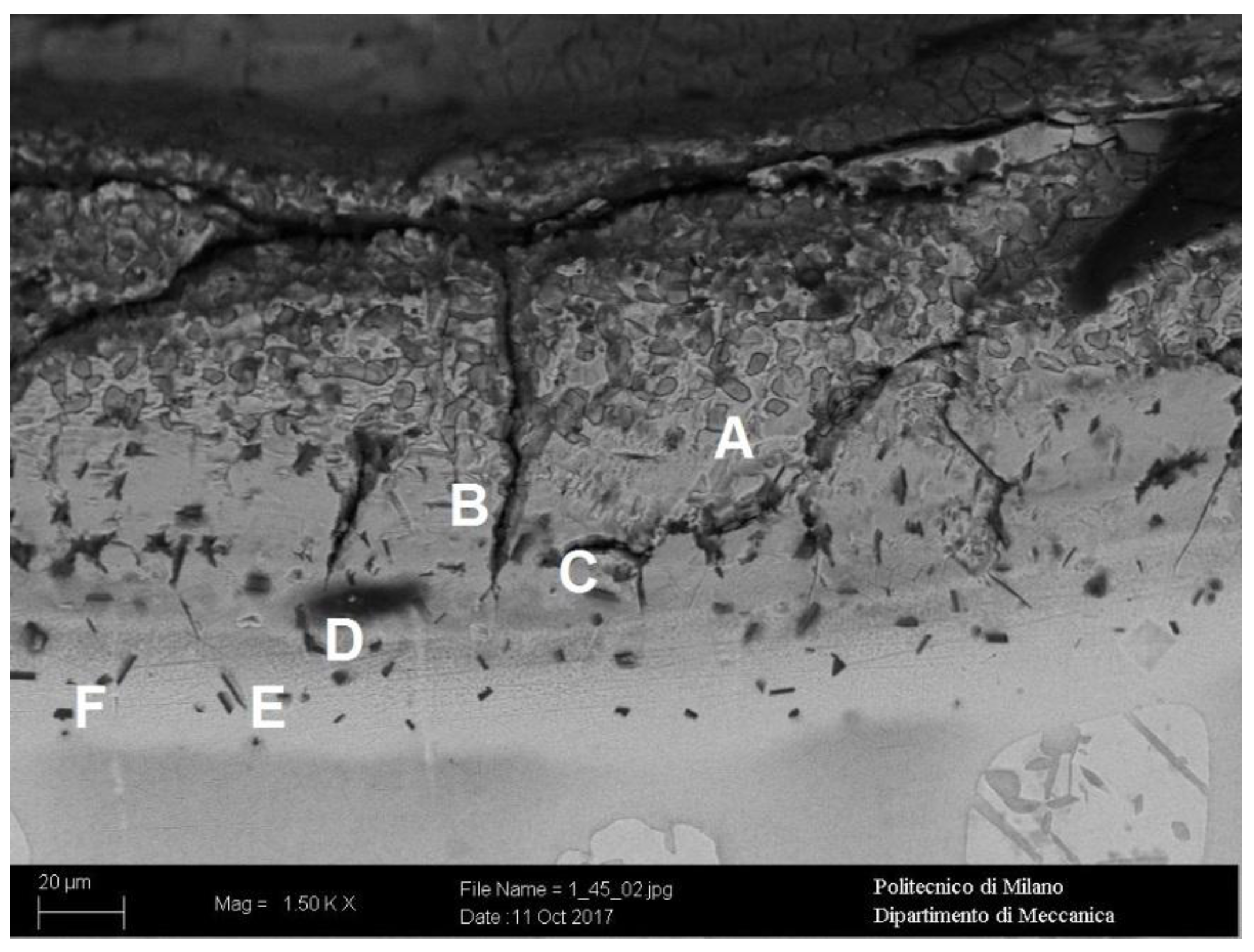

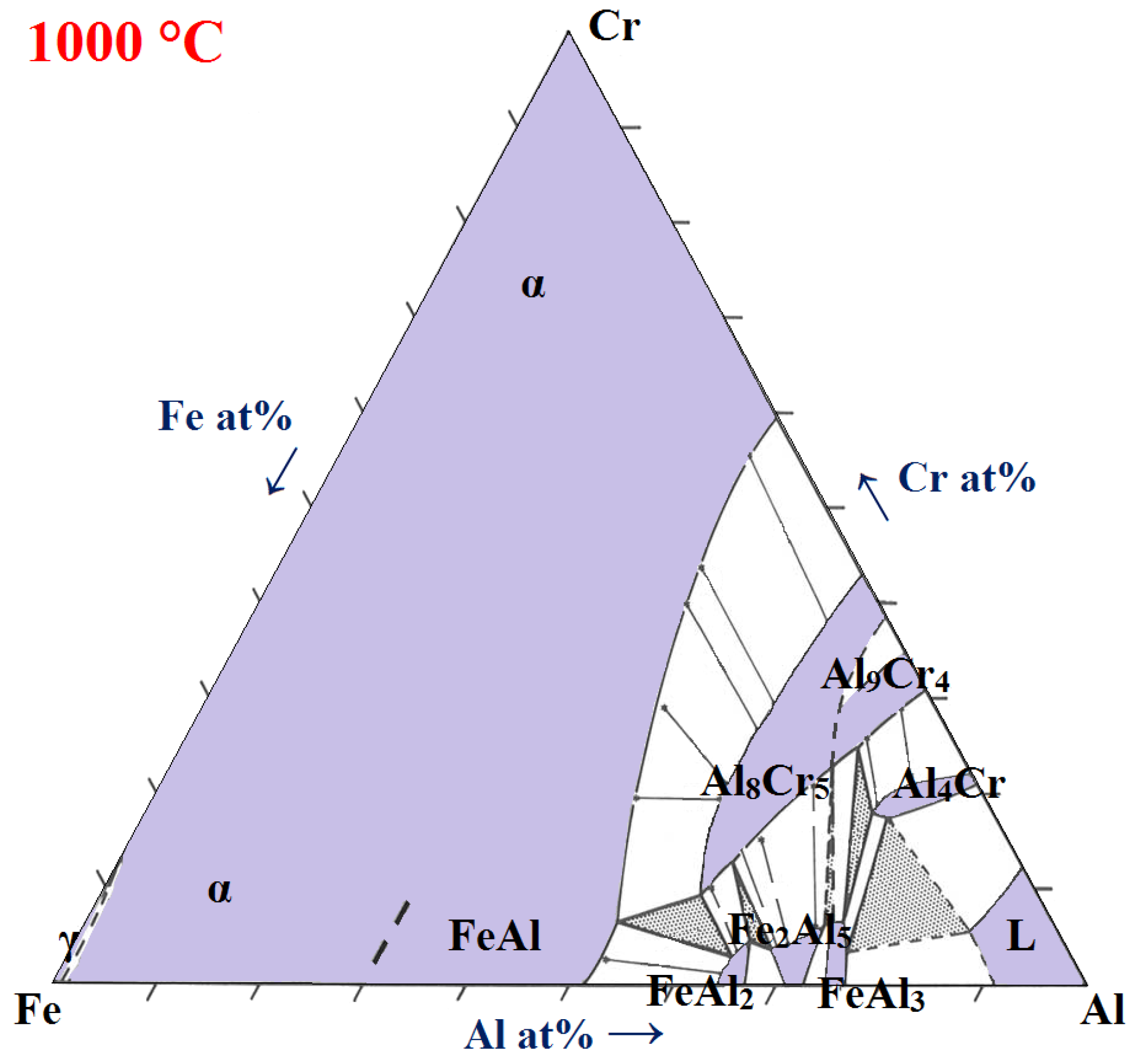

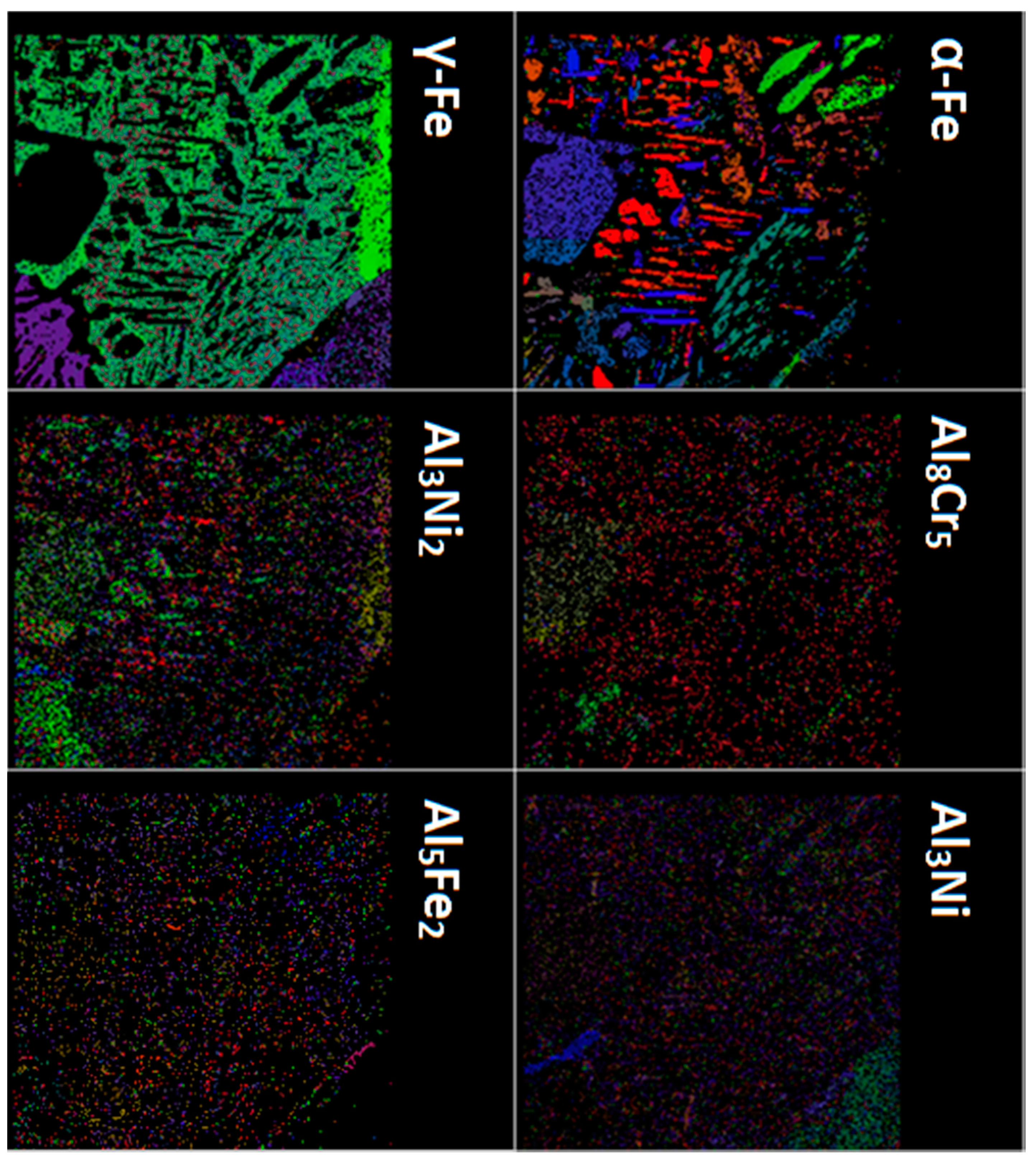
| Sample | CD | LS | HS | A72 | A210 | A360 | S1300 | S1400 |
|---|---|---|---|---|---|---|---|---|
| Austenite (Vol. %) | 55.3 | 49.2 | 54.6 | 47.7 | 45.9 | 53.7 | 31.8 | 37.3 |
| Error (Vol. %) | 2.3 | 0.9 | 1.8 | 3.4 | 2.5 | 2.7 | 2.5 | 1.7 |
| Sample | Phase | Cr (%) | Mn (%) | Fe (%) | Ni (%) | Cu (%) | Mo (%) | W (%) |
|---|---|---|---|---|---|---|---|---|
| D | α | 27.01 | 0.44 | 60.46 | 5.91 | 0.55 | 4.74 | 0.89 |
| γ | 24.13 | 0.63 | 62.14 | 8.52 | 0.78 | 3.19 | 0.61 | |
| LS | α | 26.79 | 0.51 | 60.56 | 5.22 | 0.67 | 5.14 | 1.11 |
| γ | 24.53 | 0.66 | 61.87 | 7.59 | 0.82 | 3.58 | 0.95 | |
| HS | α | 26.21 | 0.58 | 60.45 | 6.03 | 0.72 | 4.97 | 1.04 |
| γ | 24.33 | 0.82 | 62.27 | 7.13 | 0.93 | 3.78 | 0.74 | |
| A72 | α | 24.18 | 0.76 | 58.50 | 5.31 | 1.07 | 8.12 | 2.06 |
| γ | 22.87 | 0.77 | 60.10 | 9.05 | 1.11 | 4.28 | 1.82 | |
| A210 | α | 26.60 | 0.65 | 58.61 | 4.80 | 0.54 | 7.15 | 1.65 |
| γ | 24.50 | 0.57 | 61.15 | 7.95 | 0.71 | 3.65 | 1.48 | |
| A360 | α | 26.13 | 0.48 | 57.37 | 4.48 | 1.36 | 8.49 | 1.69 |
| γ | 24.76 | 0.32 | 61.75 | 7.71 | 0.53 | 3.52 | 1.42 | |
| S1300 | α | 26.04 | 0.59 | 59.17 | 6.61 | 0.47 | 5.52 | 1.60 |
| γ | 24.21 | 0.98 | 61.22 | 7.85 | 1.36 | 3.03 | 1.36 | |
| S1400 | α | 25.64 | 0.70 | 59.56 | 6.99 | 0.82 | 4.77 | 1.53 |
| γ | 24.38 | 0.88 | 61.19 | 7.41 | 0.81 | 3.89 | 1.44 |
| Depth | Al (%) | Si (%) | Cr (%) | Mn (%) | Fe (%) | Ni (%) | Cu (%) | Mo (%) | W (%) |
|---|---|---|---|---|---|---|---|---|---|
| A-0 µm | 0.63 | 0.57 | 23.12 | 0.48 | 61.88 | 7.18 | 0.52 | 4.38 | 1.23 |
| B-50 µm | 0.25 | 0.78 | 25.68 | 0.79 | 59.97 | 6.63 | 0.55 | 5.23 | 0.11 |
| C-100 µm | 0.12 | 0.68 | 24.92 | 0.89 | 60.97 | 6.93 | 0.46 | 4.27 | 0.76 |
| D-150 µm | 0.07 | 0.54 | 24.64 | 0.77 | 61.28 | 6.43 | 0.89 | 4.59 | 0.79 |
| E-200 µm | - | 0.54 | 25.86 | 0.63 | 59.44 | 6.47 | 0.89 | 5.03 | 1.14 |
| Analyzed Area | Al (at. %) | Si (at. %) | Cr (at. %) | Mn (at. %) | Fe (at. %) | Ni (at. %) | Cu (at. %) | Mo (at. %) | W (at. %) | Intermetallic Phase |
|---|---|---|---|---|---|---|---|---|---|---|
| A | 27.23 | 1.36 | 9.07 | 0.41 | 57.62 | 2.69 | - | 1.38 | 0.24 | Al-Me3 |
| B | 33.90 | 2.70 | 10.36 | 0.06 | 29.74 | 12.92 | 1.81 | 8.41 | 0.10 | Al-Me2 |
| C | 53.30 | 2.70 | 13.19 | 0.39 | 26.05 | 2.56 | 0.15 | 1.39 | 0.28 | Al-Me |
| D | 88.23 | 0.14 | 4.37 | - | 6.44 | 0.46 | - | 0.27 | 0.09 | Al7-Me |
| E | 78.04 | 1.07 | 6.60 | 0.21 | 12.05 | 1.20 | 0.25 | 0.50 | 0.07 | Al3-Me |
| F | 86.48 | 0.15 | 4.37 | 0.03 | 7.70 | 0.51 | 0.23 | 0.44 | 0.09 | Al7-Me |
| Alloying Elements Content | Phase | CD | LS | HS | A72 | A210 | A360 | S1300 | S1400 |
|---|---|---|---|---|---|---|---|---|---|
| Ni + Cr (wt. %) | α | 32.92 | 32.01 | 32.24 | 29.49 | 31.4 | 30.61 | 32.65 | 32.63 |
| γ | 32.65 | 32.12 | 31.46 | 31.92 | 32.45 | 32.47 | 32.06 | 31.79 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciuffini, A.F.; Barella, S.; Di Cecca, C.; Gruttadauria, A.; Mombelli, D.; Mapelli, C. Hot-Dip Aluminizing on AISI F55–UNS S32760 Super Duplex Stainless Steel Properties: Effect of Thermal Treatments. Metals 2017, 7, 525. https://doi.org/10.3390/met7120525
Ciuffini AF, Barella S, Di Cecca C, Gruttadauria A, Mombelli D, Mapelli C. Hot-Dip Aluminizing on AISI F55–UNS S32760 Super Duplex Stainless Steel Properties: Effect of Thermal Treatments. Metals. 2017; 7(12):525. https://doi.org/10.3390/met7120525
Chicago/Turabian StyleCiuffini, Andrea Francesco, Silvia Barella, Cosmo Di Cecca, Andrea Gruttadauria, Davide Mombelli, and Carlo Mapelli. 2017. "Hot-Dip Aluminizing on AISI F55–UNS S32760 Super Duplex Stainless Steel Properties: Effect of Thermal Treatments" Metals 7, no. 12: 525. https://doi.org/10.3390/met7120525