Effect of Dynamic Reheating Induced by Weaving on the Microstructure of GTAW Weld Metal of 25% Cr Super Duplex Stainless Steel Weld Metal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
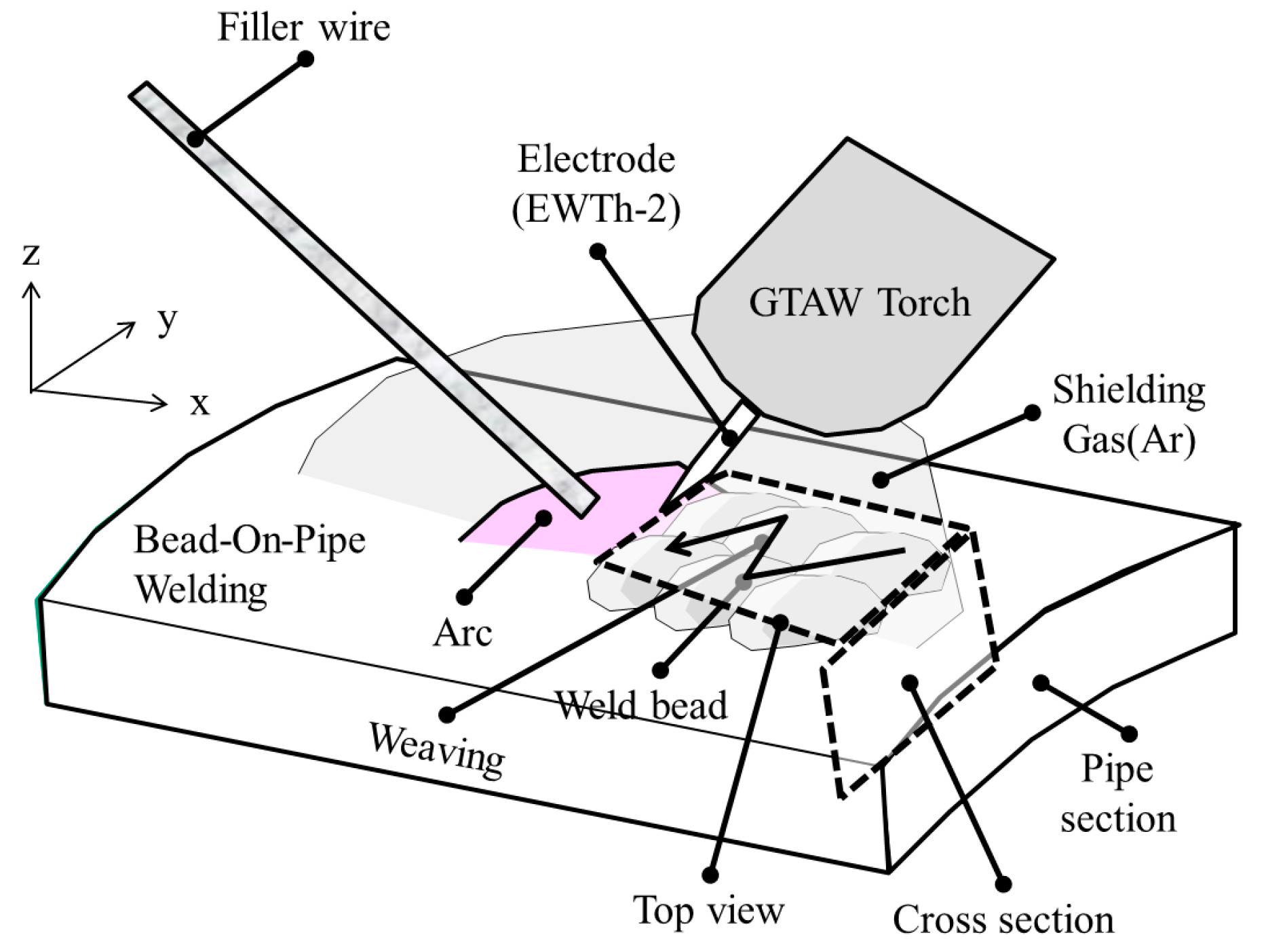
2.2. Welding Conditions
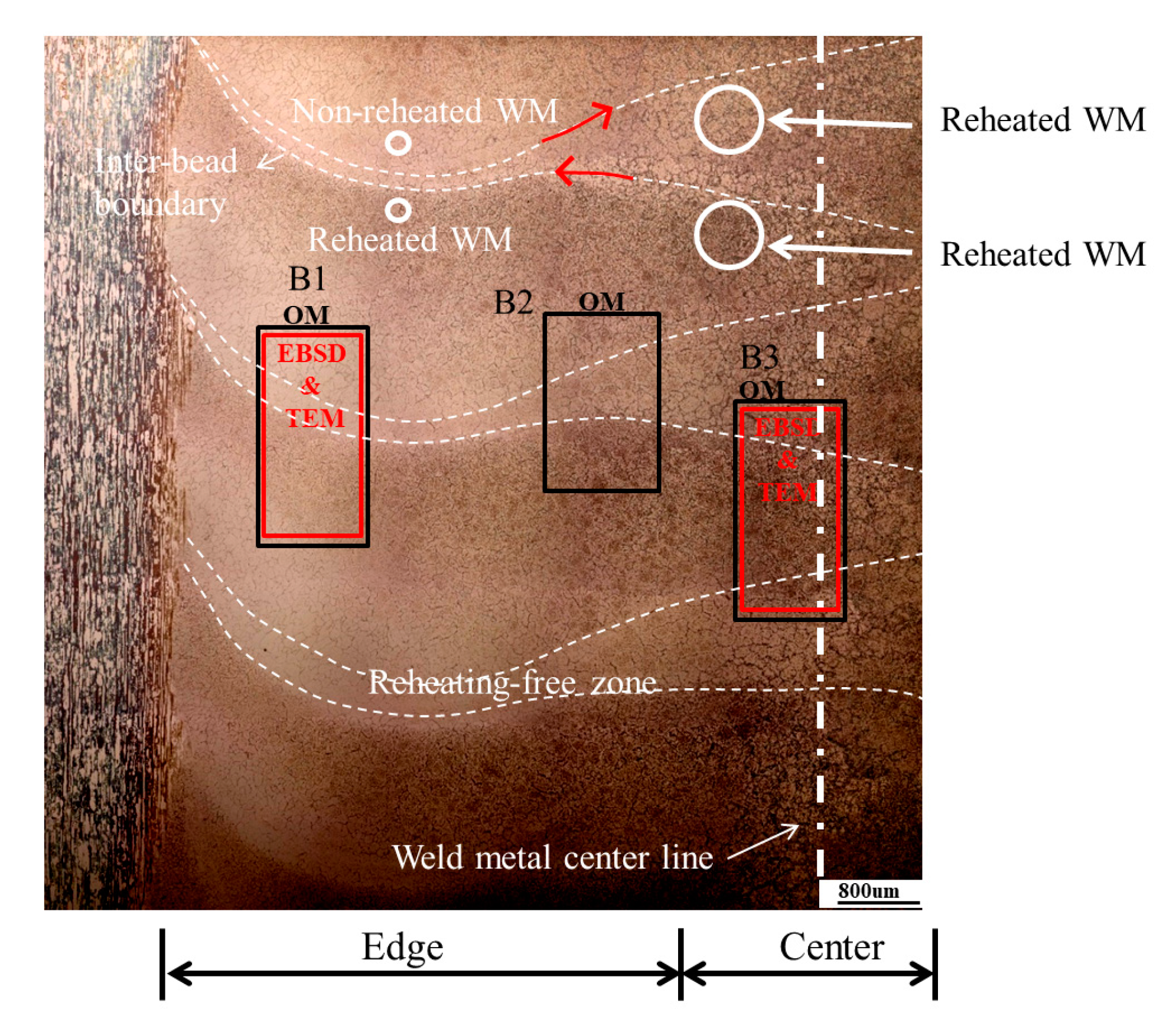
2.3. Macro-Micro Structural Analysis
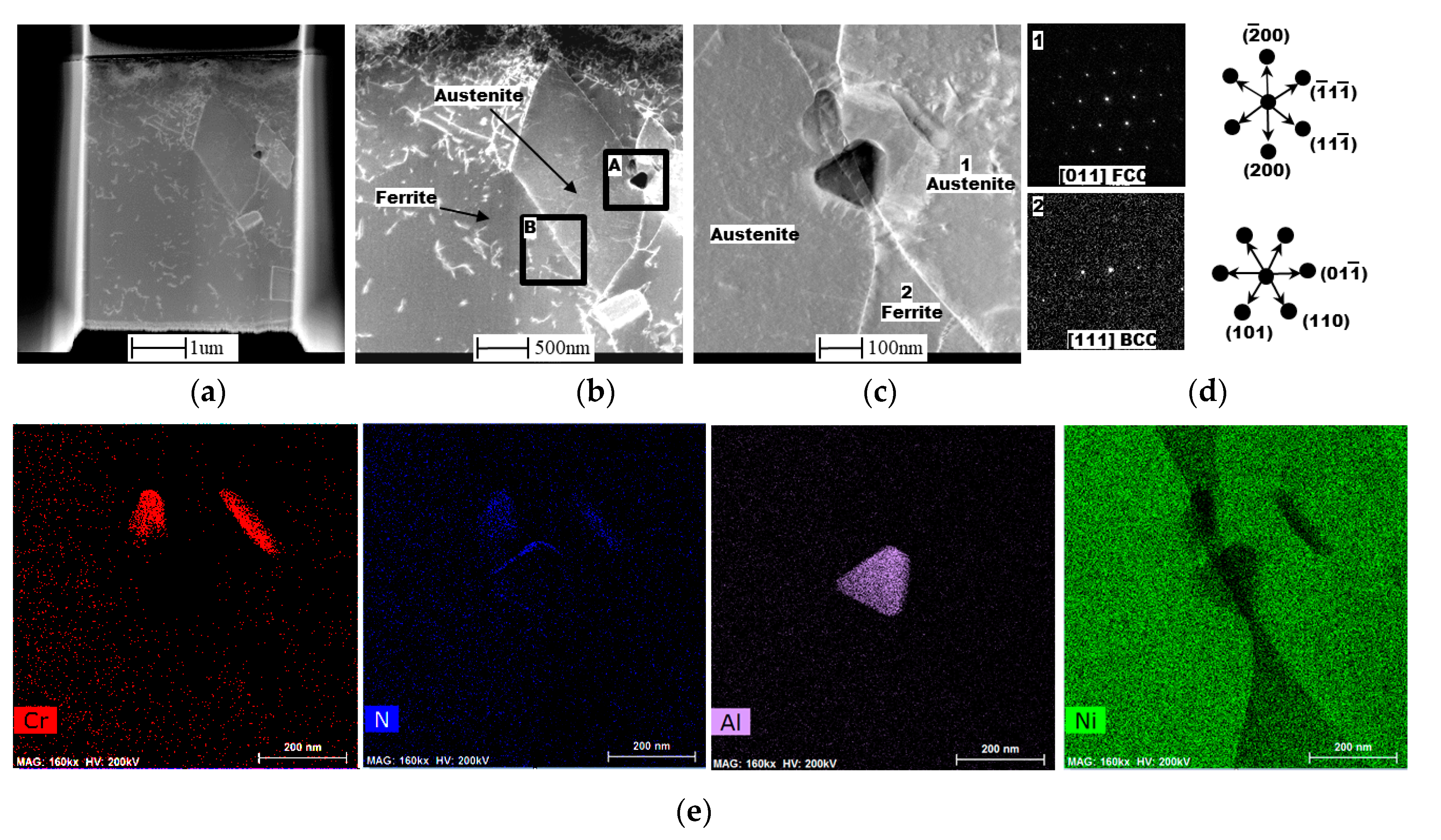
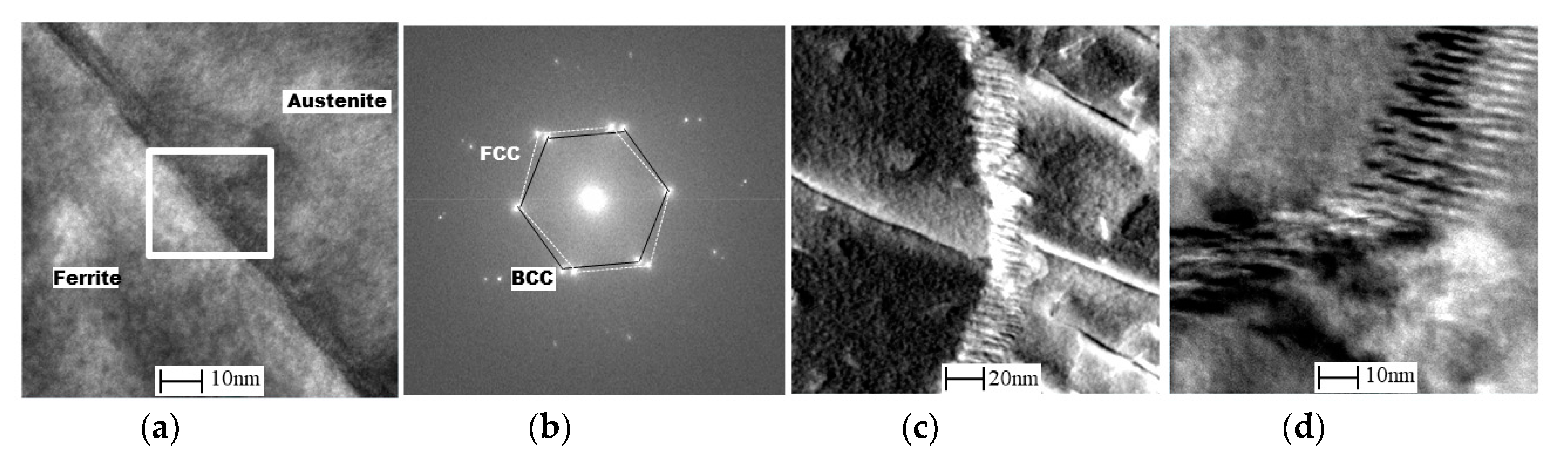
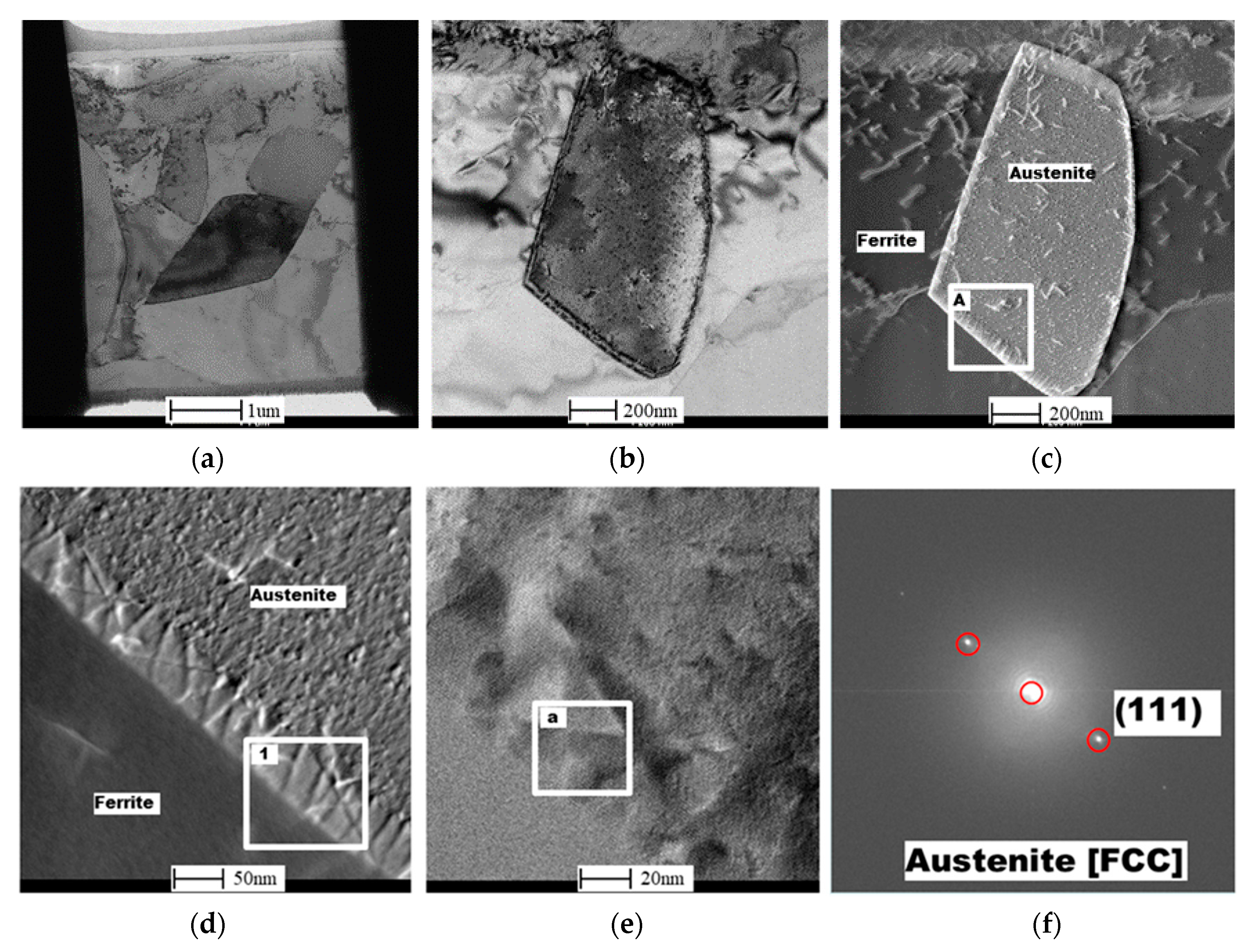
2.4. EBSD and TEM Investigation
3. Results
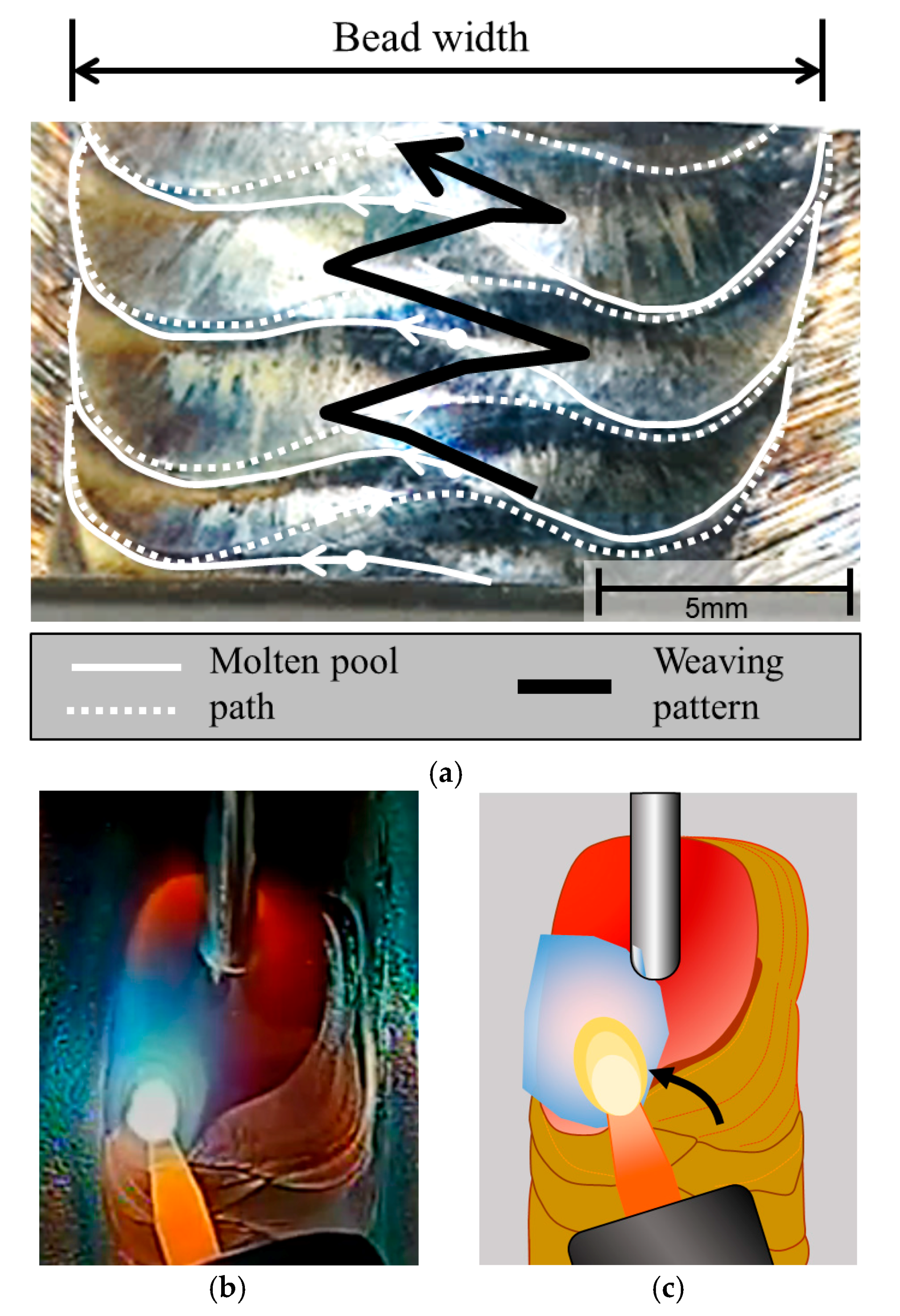
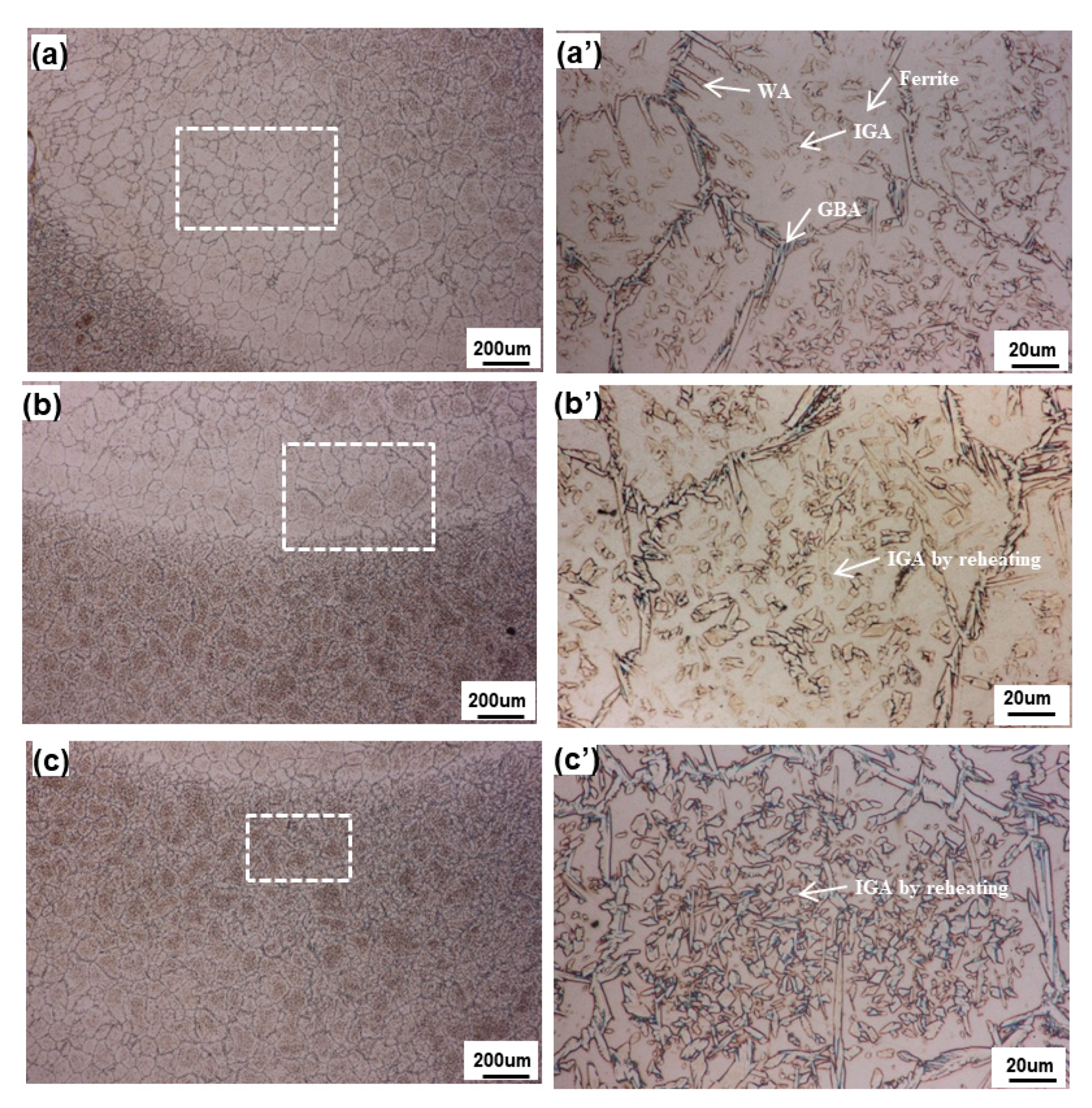
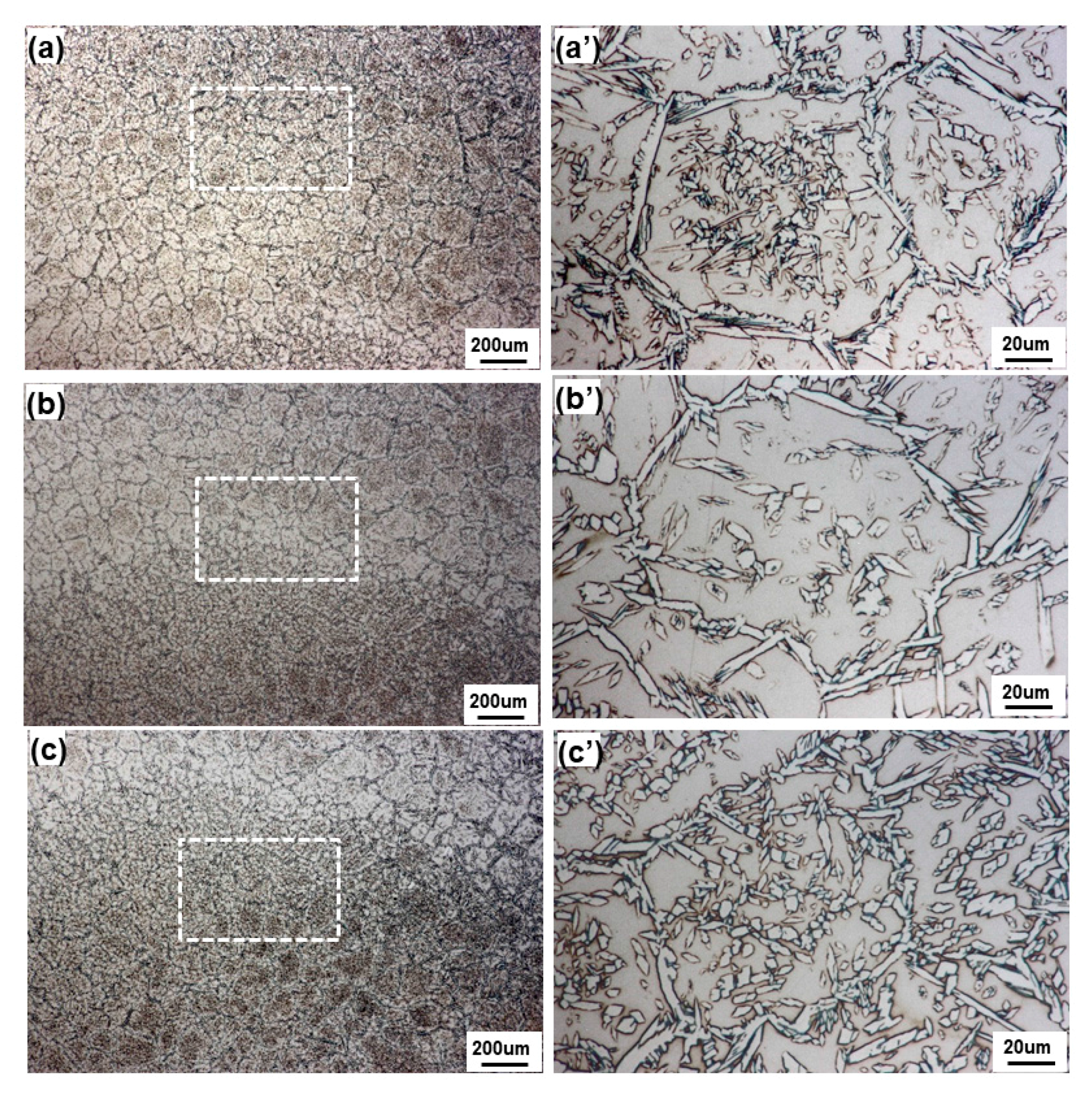
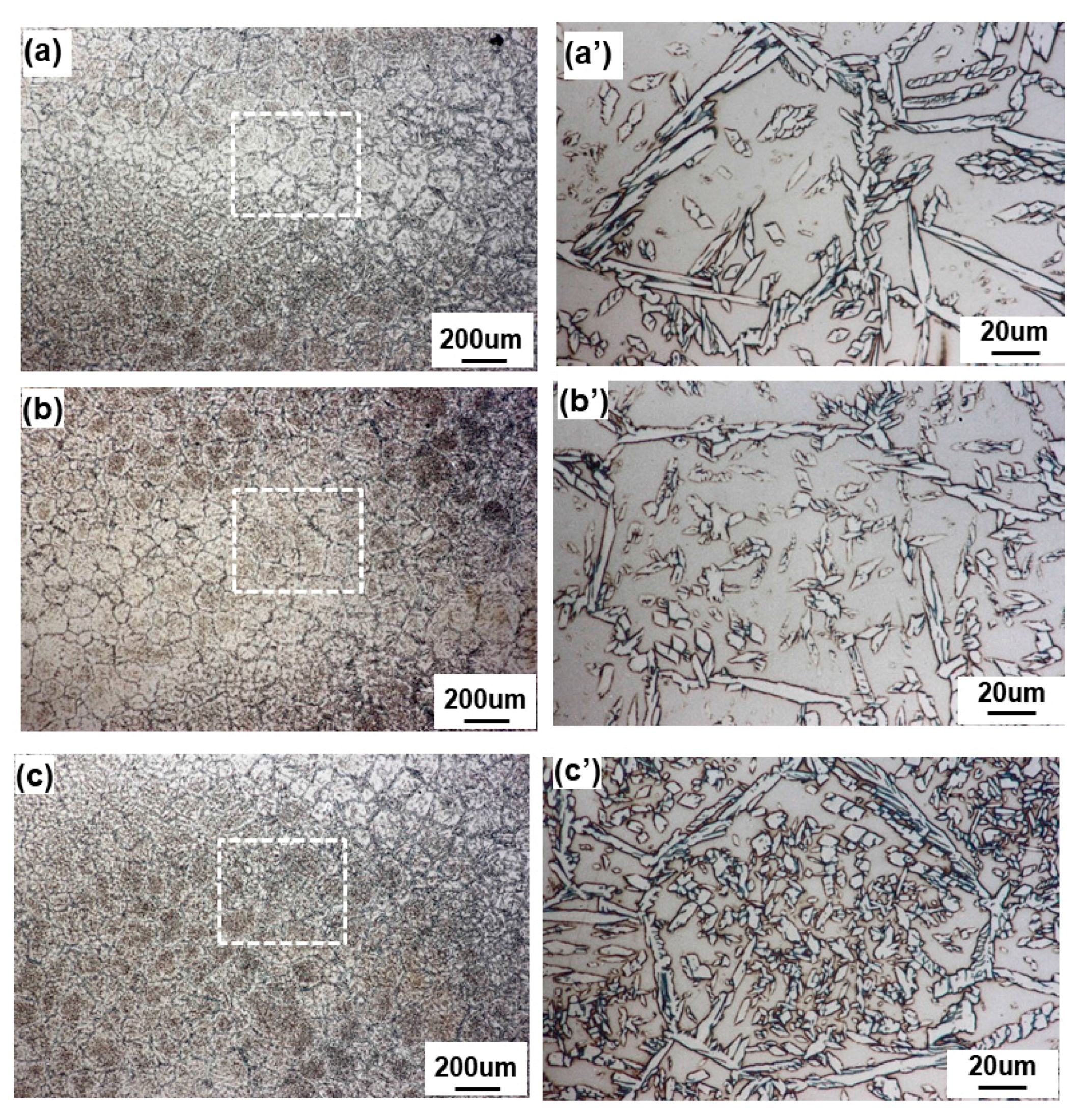
3.1. Macrostructural and Microstructural Analyses
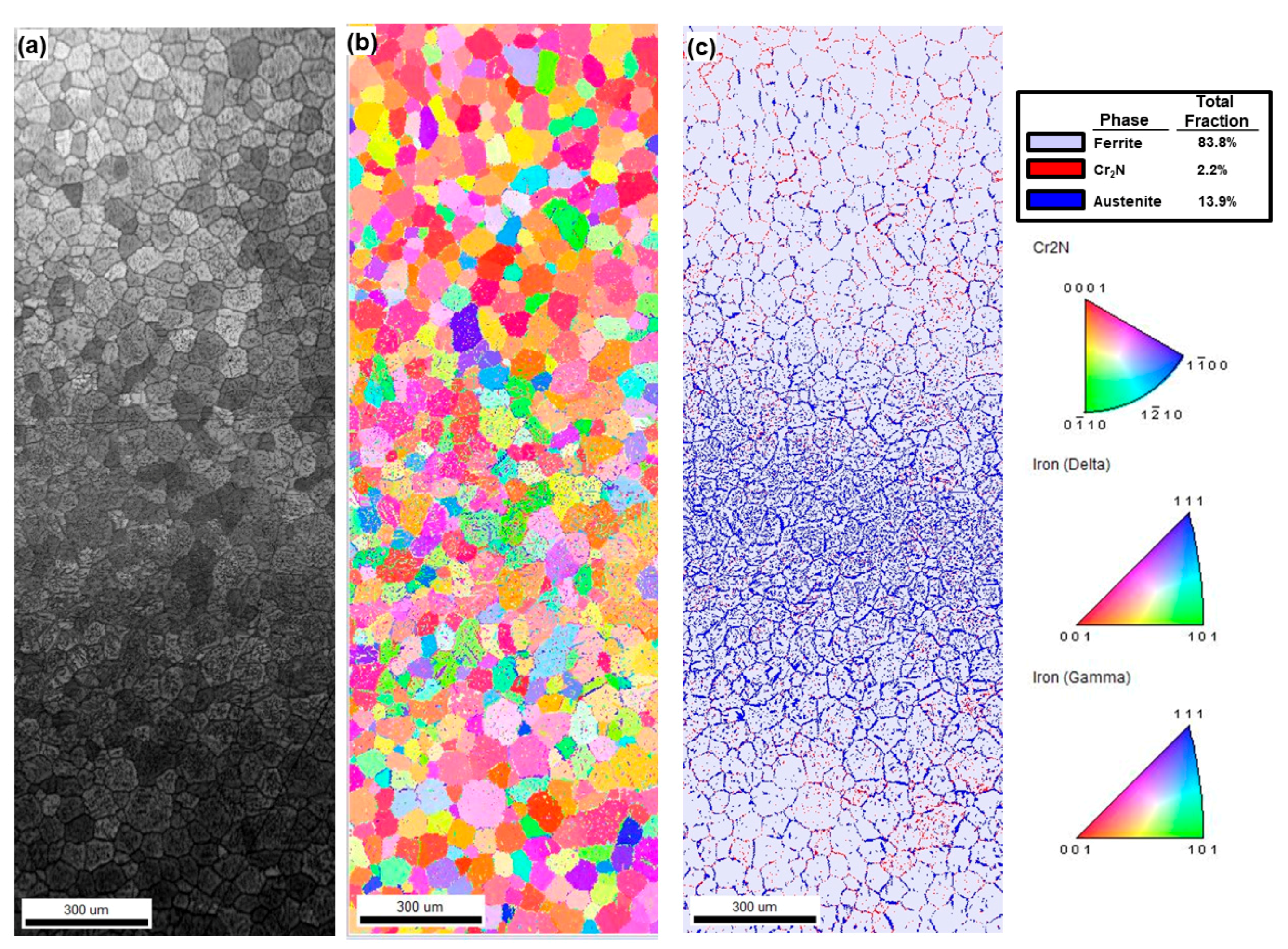
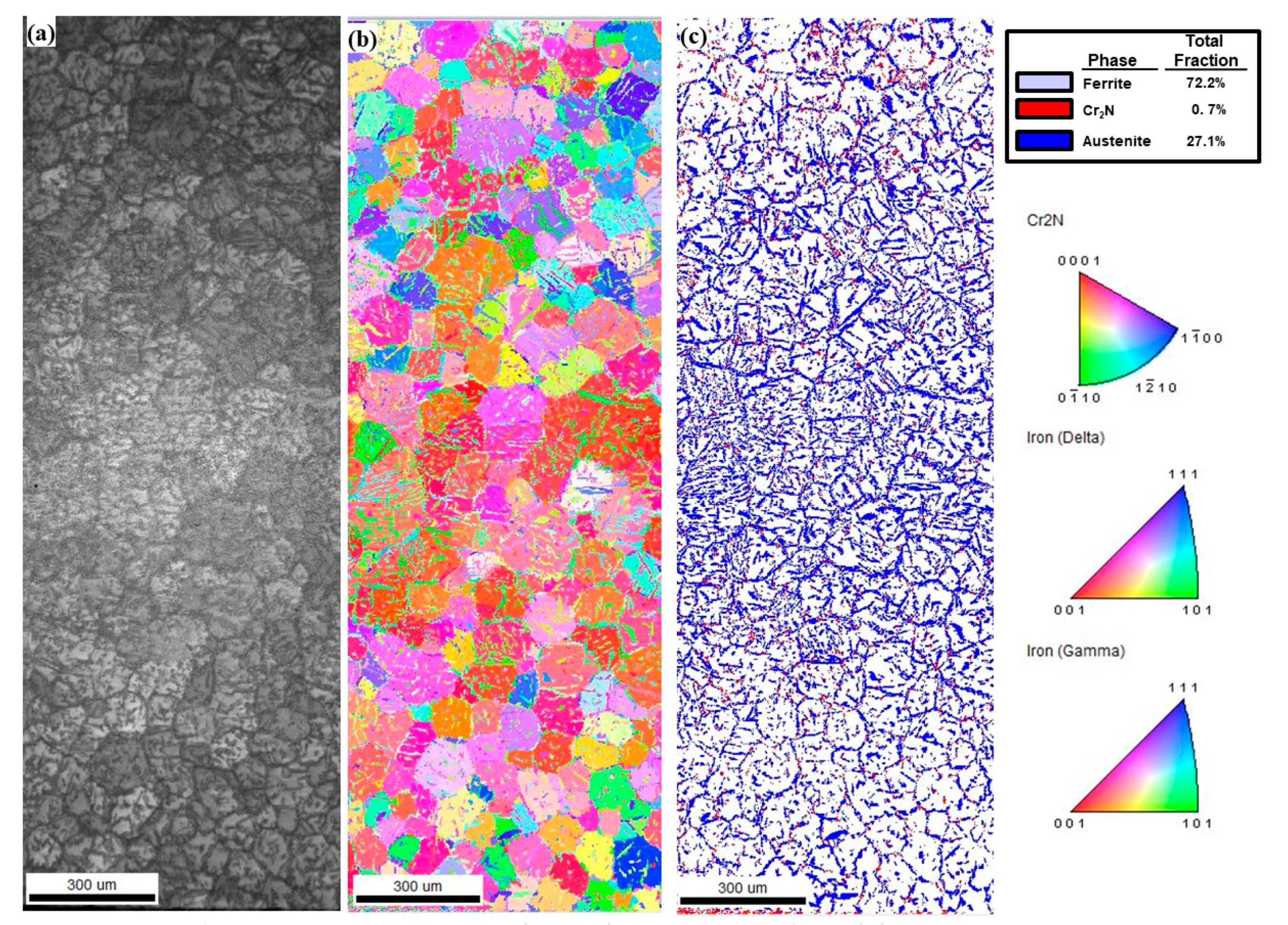
3.2. EBSD Analysis
4. Discussion
4.1. Phase Fraction at the Edge and the Center of Weld Metal
4.2. Phase Fraction between Reheated Weld Metal and Non-Reheated Weld Metal
4.3. Reheating-Free Zone
4.4. Chromium Nitride (Cr2N) Fraction between the Edge and the Center Weld Metal
5. Conclusions
- Even though single-pass bead-on-pipe welding was carried out, there was a reheating phenomenon, called “dynamic reheating”. It was found that top-view investigation was a better method to recognize the entire dynamic reheating phenomena, compared with cross-section investigation.
- The center area had a comparatively low cooling rate, owing to the fact that the molten pool produced during welding remained for longer than in the edge area. It was found that the austenite phase fraction at the center was higher than that of the edge area, while the Cr2N content was lower at the center than in the edge area.
- A “reheating-free zone” was detected, even though this area was definitely reheated. Reheating phenomena had disappeared in this area, because it was at a very high temperature and/or was liquid, even though it was located in the reheated zone.
- The austenite phase volume fraction of reheated weld metal was higher than that of non-reheated weld metal, while the Cr2N volume fraction showed the opposite tendency. However, the Cr2N content of the reheated area decreased, because it had dissolved during the austenite phase transformation and/or growth as a result of reheating.
- From the TEM result, Cr2N was found to exist at the ferrite/austenite phase boundary of the non-reheated area, with 10 nm width. However, it was not detected at the phase boundary of the reheated area; instead, a 50-nm-wide band, consisting of secondary austenite, was found, as expected.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sedriks, A.J. Effects of alloy composition and microstructure on the passivity of stainless steels. Corrosion 1986, 42, 376. [Google Scholar] [CrossRef]
- Bernhardsson, S. Duplex Stainless Steels’91; Charles, J., Bernhardsson, S., Eds.; Les Editions de Physique: Beaune, France, 1991; Volume 1, p. 185. [Google Scholar]
- Nilsson, J.-O. Super duplex stainless steels. Mater. Sci. Technol. 1992, 8, 685–700. [Google Scholar] [CrossRef]
- Charles, J. Why and where duplex stainless steels. In Proceedings of the Duplex Stainless Steel ’97 International Conference & Expo, Maastricht, The Netherlands, 21–23 October 1997; pp. 29–42. [Google Scholar]
- Alvarez-Armas, I. Duplex stainless steels: Brief history and some recent alloys. Recent Pat. Mech. Eng. 2008, 1, 51–57. [Google Scholar] [CrossRef]
- Messer, B.; Wright, A.; Oprea, V. Duplex Stainless Steel Welding. Best Practices; Fluor Canada Ltd.: Calgary, AB, Canada, 2007; Volume 11, pp. 45–63. [Google Scholar]
- Calliari, I.; Bassani, P.; Brunelli, K.; Breda, M.; Ramous, E. Effect of continuous cooling on secondary phase precipitation in the super duplex stainless steel ZERON-100. J. Mater. Eng. Perform. 2013, 22, 3860–3866. [Google Scholar] [CrossRef]
- Tan, H.; Jiang, Y.; Deng, B.; Sun, T.; Xu, J.; Li, J. Effect of annealing temperature on the pitting corrosion resistance of SDSS UNS S32750. Mater. Charact. 2009, 60, 1049–1054. [Google Scholar] [CrossRef]
- Deng, B.; Jiang, Y.M.; Gao, J.; Li, J. Effect of annealing treatment on microstructure evolution and the associated corrosion behavior of a super-duplex stainless steel. J. Alloys Compd. 2010, 493, 461–464. [Google Scholar] [CrossRef]
- Cervo, R.; Ferro, P.; Tiziani, A. Annealing temperature effects on super duplex stainless steel UNS s32750 welded joints. I: Microstructure and partitioning of elements. J. Mater. Sci. 2010, 45, 4369–4377. [Google Scholar] [CrossRef]
- Chan, K.; Tjong, S. Effect of secondary phase precipitation on the corrosion behavior of duplex stainless steels. Materials 2014, 7, 5268–5304. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, A.J.; Lippold, J.C.; Brandi, S.D. The relationship between chromium nitride and secondary austenite precipitation in duplex stainless steels. Met. Mater. Trans. Coruña 2003, 34, 1575–1597. [Google Scholar] [CrossRef]
- Zhang, Z.; Jing, H.; Xu, L.; Han, Y.; Gao, Z.; Zhao, L.; Zhang, J. Microstructural characterization and electron backscatter diffraction analysis across the welded interface of duplex stainless steel. Appl. Surf. Sci. 2017, 413, 327–343. [Google Scholar] [CrossRef]
- Pettersson, N.; Pettersson, R.F.A.; Wessman, S. Precipitation of chromium nitrides in the super duplex stainless steel 2507. Met. Mater. Trans. Coruña 2015, 46, 1062–1072. [Google Scholar] [CrossRef]
- Smiderle, J.; Pardal, J.M.; Tavares, S.S.M.; Vidal, A.C.N. Premature failure of superduplex stainless steel pipe by pitting in sea water environment. Fail. Anal. 2014, 46, 134–139. [Google Scholar] [CrossRef]
- Linton, V.M.; Laycock, N.J.; Thomsen, S.J.; Klumpers, A. Failure of a SDSS reaction vessel. Eng. Fail. Anal. 2004, 11, 243–256. [Google Scholar] [CrossRef]
- Kim, S.-T.; Lee, I.-S.; Kim, J.-S.; Jang, S.-H.; Park, Y.-S.; Kim, K.-T.; Kim, Y.-S. Investigation of the localized corrosion associated with phase transformation of tube-to-tube sheet welds of hyper duplex stainless steel in acidified chloride environments. Corros. Sci. 2012, 64, 164–173. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, Z.; Li, H.; Feng, H.; Zhang, B. Detection of susceptibility to intergranular corrosion of aged super austenitic stainless steel S32654 by a modified electrochemical potentiokinetic reactivation method. J. Alloys Compd. 2017, 695, 3083–3093. [Google Scholar] [CrossRef]
- Ha, H.; Kwon, H. Effects of Cr2N on the pitting corrosion of high nitrogen stainless steels. Electrochim. Acta 2007, 52, 2175–2180. [Google Scholar] [CrossRef]
- Eleonora Bettini, U.K.; Leygraf, C.; Pan, J. Study of corrosion behavior of a 2507 SDSS: Influence of quenched-in and isothermal nitrides. Int. J. Electrochem. Sci. 2014, 9, 61–80. [Google Scholar]
- Nilsson, J.O.; Wilson, A. Influence of isothermal phase transformations on toughness and pitting corrosion of super duplex stainless steel SAF 2507. Mater. Sci. Technol. 1993, 9, 545–554. [Google Scholar] [CrossRef]
- Liao, J. Nitride Precipitation in weld HAZs of a duplex stainless steel. ISIJ Int. 2001, 41, 460–467. [Google Scholar] [CrossRef]
- Nilsson, J.-O.; Huhtala, T.; Jonsson, P.; Karlsson, L.; Wilson, A. Structural stability of super duplex stainless weld metals and its dependence on tungsten and copper. Met. Mater. Trans. Coruña 1996, 27, 2196–2208. [Google Scholar] [CrossRef]
- Devendranath Ramkumar, K.; Mishra, D.; Ganesh Raj, B.; Vignesh, M.K.; Thiruvengatam, G.; Sudharshan, S.P.; Arivazhagan, N.; Sivashanmugam, N.; Rabel, A.M. Effect of optimal weld parameters in the microstructure and mechanical properties of autogeneous gas tungsten arc weldments of super-duplex stainless steel UNS S32750. Mater. Des. (1980–2015) 2015, 66, 356–365. [Google Scholar] [CrossRef]
- Eghlimi, A.; Shamanian, M.; Raeissi, K. Effect of current type on microstructure and corrosion resistance of super duplex stainless steel claddings produced by the gas tungsten arc welding process. Surf. Coat. Technol. 2014, 244, 45–51. [Google Scholar] [CrossRef]
- Hosseini, V.A.; Wessman, S.; Hurtig, K.; Karlsson, L. Nitrogen loss and effects on microstructure in multipass TIG welding of a SDSS. Mater. Des. 2016, 98, 88–97. [Google Scholar] [CrossRef]
- Kiesche, M.; Donath, V. TIG welding of root runs in the vertical position. Weld. Int. 1991, 5, 828–830. [Google Scholar] [CrossRef]
- Chul, S.J. Weld Camera YouTube. 27 June 2016. Available online: https://www.youtube.com/watch?v=3mpyWP9OKGo (accessed on 30 September 2017).
- Garzon, C.; Ramirez, A. Growth kinetics of secondary austenite in the welding microstructure of a UNS S32304 duplex stainless steel. Acta Mater. 2006, 54, 3321–3331. [Google Scholar] [CrossRef]
- Sieurin, H.; Sandström, R. Austenite reformation in the heat-affected zone of duplex stainless steel 2205. Mater. Sci. Eng. Coruña 2006, 418, 250–256. [Google Scholar] [CrossRef]


| Material | Chemical Composition (wt %) | PREN 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C | Si | Mn | Cr | Ni | Mo | N | Other | ||
| Base metal 1 (UNS S32750) | 0.022 | 0.23 | 0.64 | 25.5 | 6.7 | 3.52 | 0.26 | Cu: 0.16 | 42.8 |
| Welding wire 2 (AWS ER2594) | 0.01 | 0.41 | 0.38 | 25.0 | 9.5 | 3.93 | 0.24 | - | 42.1 |
| Material | Strength (MPa) | El. (%) | Absorbed Energy (J) | Ferrite Content (%) 1 | |
|---|---|---|---|---|---|
| Y.S. | T.S. | ||||
| Base metal (UNS S32750) | 561–625 | 812–856 | 34–42 | 120–231 @ −51 °C | 47.61–50.46 |
| Welding wire (AWS A5.9 ER2594) | 685 | 874 | 30 | 181–194 @ −50 °C | 44 |
| Current (A) | Voltage (V) | Welding Speed (cm/min) | Heat Input (kJ/cm) | Bead Width (mm) |
|---|---|---|---|---|
| 97 | 11 | 4.4 | 14.7 | 14.2 |
| Phase Volume Fraction (%) | Edge | Center | ||
|---|---|---|---|---|
| Ferrite | 83.8 | 72.2 | ||
| Austenite | 13.9 | 27.1 | ||
| Cr2N | 2.2 | 0.7 | ||
| Location | Non-Reheated WM | Reheated WM | Non-Reheated WM | Reheated WM |
| Ferrite | 92.9 | 72.1 | 77.2 | 67.2 |
| Austenite | 4.4 | 26.6 | 21.7 | 32.3 |
| Cr2N | 2.7 | 1.4 | 0.11 | 0.04 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, H.-J.; Na, H.-S.; Kang, C.-Y. Effect of Dynamic Reheating Induced by Weaving on the Microstructure of GTAW Weld Metal of 25% Cr Super Duplex Stainless Steel Weld Metal. Metals 2017, 7, 490. https://doi.org/10.3390/met7110490
Sung H-J, Na H-S, Kang C-Y. Effect of Dynamic Reheating Induced by Weaving on the Microstructure of GTAW Weld Metal of 25% Cr Super Duplex Stainless Steel Weld Metal. Metals. 2017; 7(11):490. https://doi.org/10.3390/met7110490
Chicago/Turabian StyleSung, Hee-Joon, Hye-Sung Na, and Chung-Yun Kang. 2017. "Effect of Dynamic Reheating Induced by Weaving on the Microstructure of GTAW Weld Metal of 25% Cr Super Duplex Stainless Steel Weld Metal" Metals 7, no. 11: 490. https://doi.org/10.3390/met7110490





