Revisiting Mg–Mg2Ni System from Electronic Perspective
Abstract
:1. Introduction
2. Materials and Methods
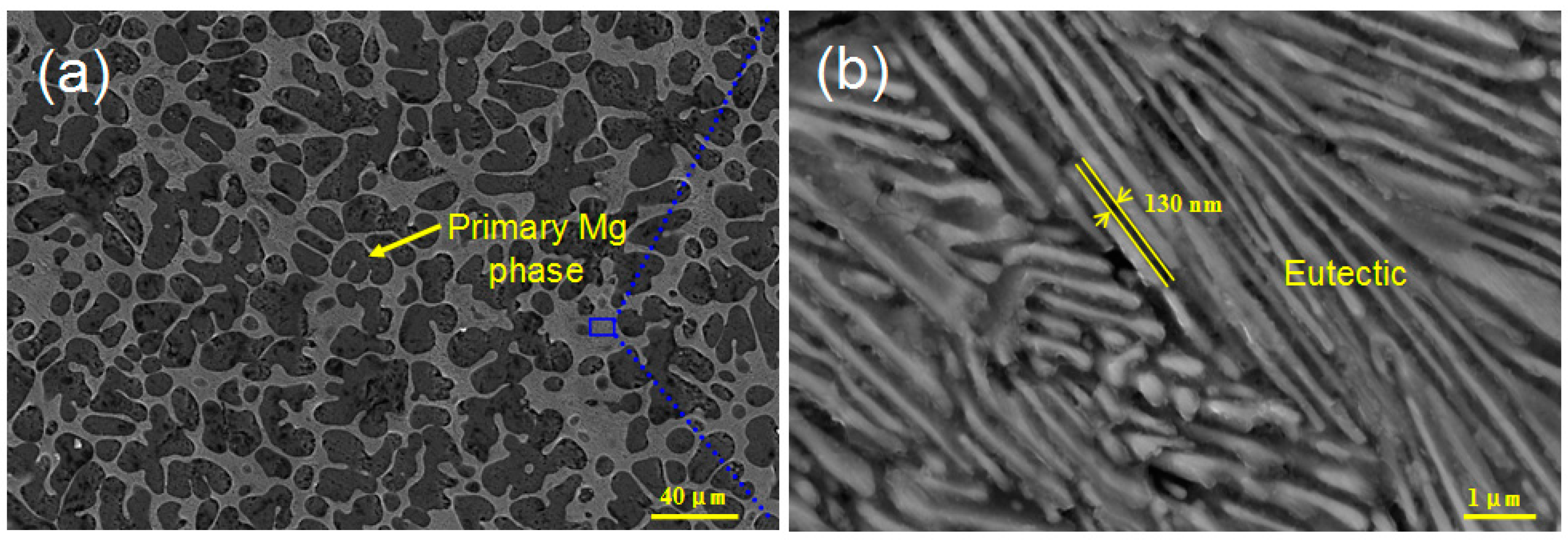
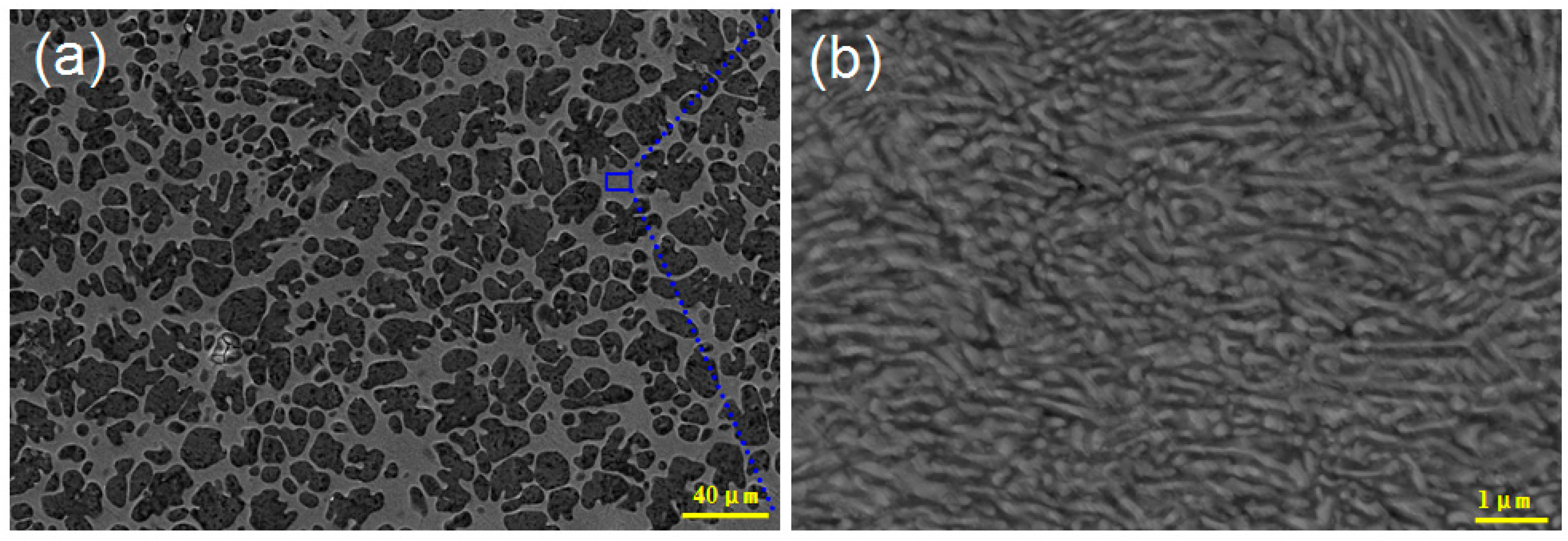
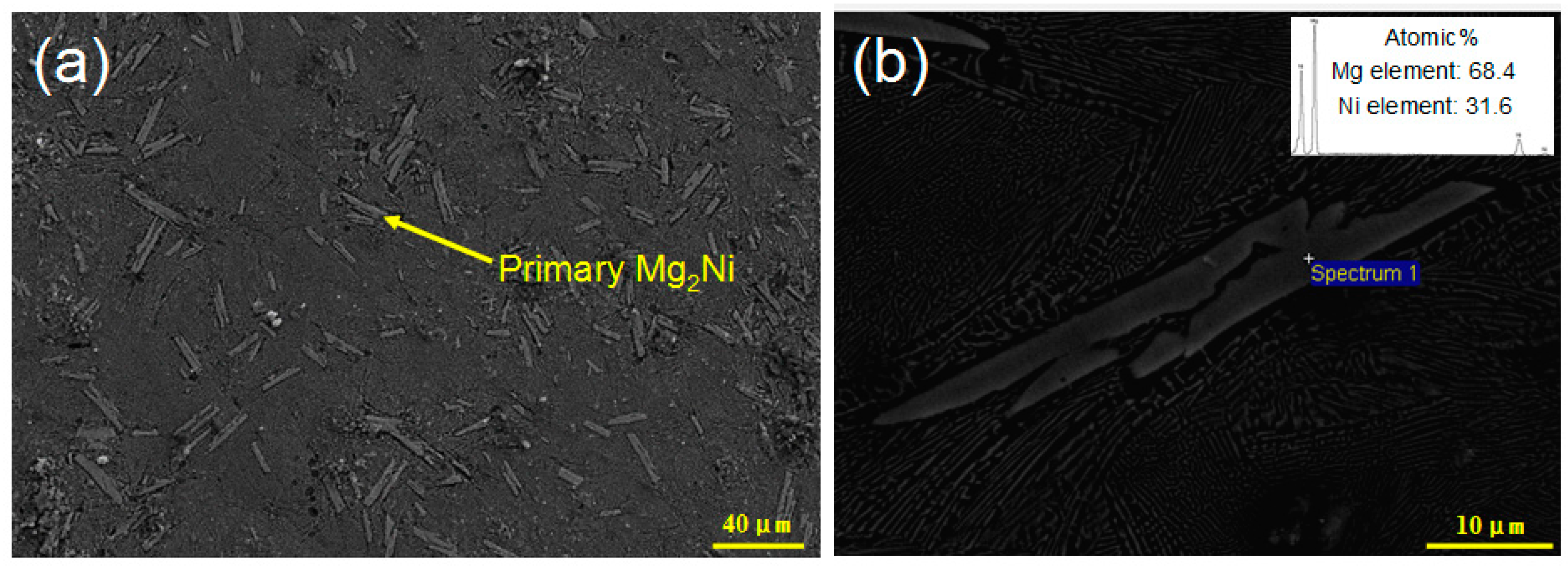
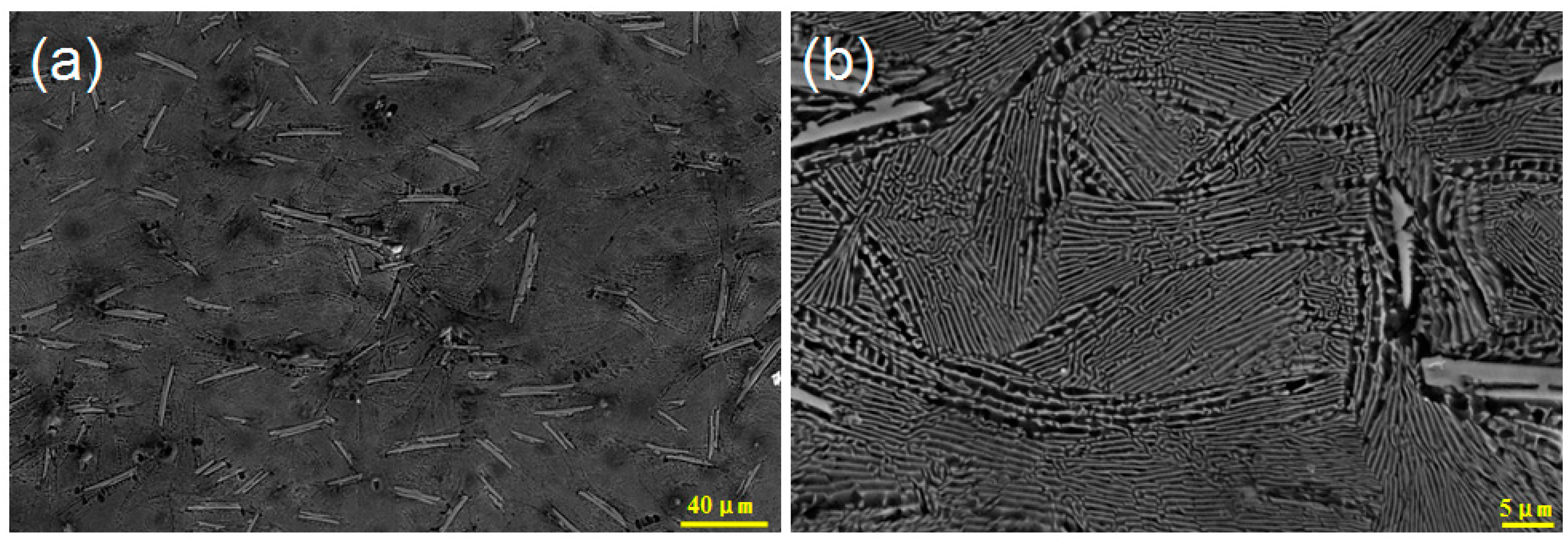
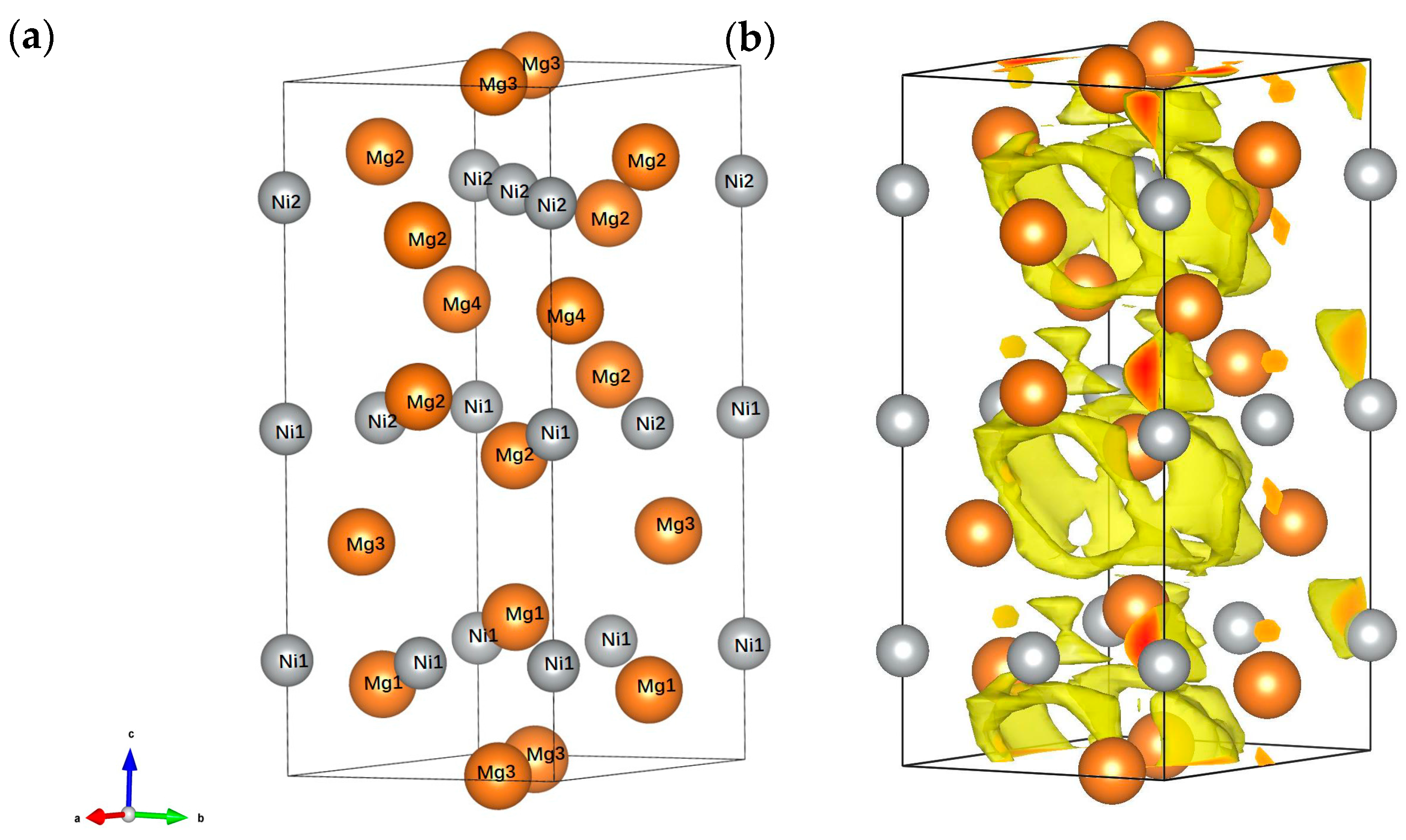
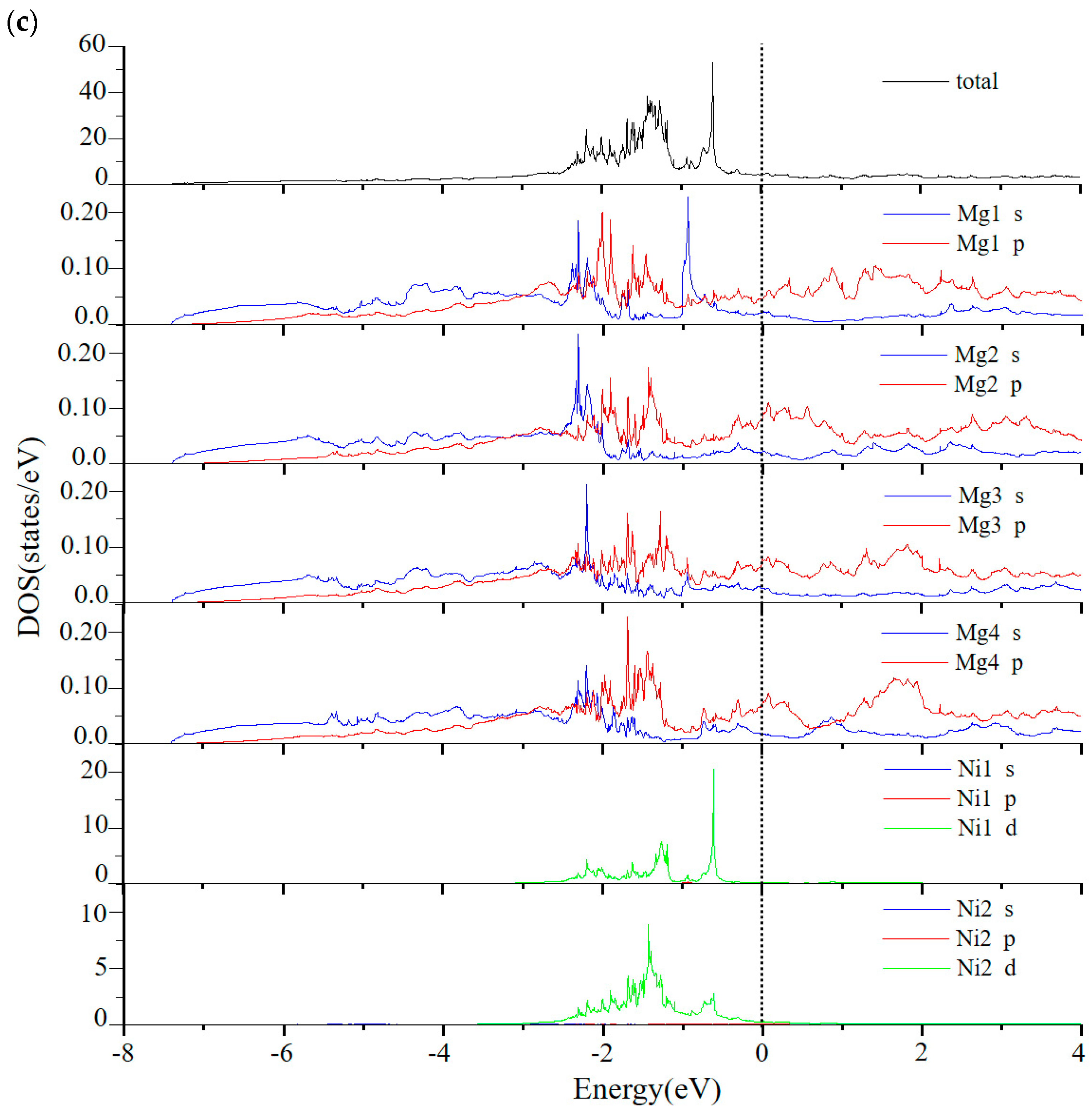
3. Results and Discussion
4. Summary and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Grey, C.P.; Tarascon, J.M. Sustainability and in situ monitoring in battery development. Nat. Mater. 2017, 16, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Hu, Y.; Chen, L. Room-temperature stationary sodium-ion batteries for large-scale electric energy storage. Energy Environ. Sci. 2013, 6, 2338–2360. [Google Scholar] [CrossRef]
- Wang, X.; Guo, X.; Chen, J.; Ge, C.; Zhang, H.; Liu, Y.; Zhao, L.; Zhang, Y.; Wang, Z.; Sun, L. Au nanoparticles decorated graphene/nickel foam nanocomposite for sensitive detection of hydrogen peroxide. J. Mater. Sci. Technol. 2017, 33, 246–250. [Google Scholar] [CrossRef]
- Kim, T.H.; Park, J.S.; Chang, S.K.; Choi, S.; Ryu, J.H.; Song, H.K. The Current Move of Lithium Ion Batteries towards the Next Phase. Adv. Energy Mater. 2012, 2, 860–872. [Google Scholar] [CrossRef]
- Armstrong, A.R.; Lyness, C.; Panchmatia, P.M.; Islam, M.S.; Bruce, P.G. The lithium intercalation process in the low-voltage lithium battery anode Li1+xV1−xO2. Nat. Mater. 2011, 10, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Sarkar, A.D.; Maark, T.A.; Jiang, X.; Deshpande, M.D.; Bououdina, M.; Ahuja, R. Pure and Li-doped NiTiH: potential anode materials for Li-ion rechargeable batteries. Appl. Phys. Lett. 2013, 103, 033902. [Google Scholar] [CrossRef]
- Obrovac, M.N.; Christensen, L.; Le, D.B.; Dinh, J.R. Alloy design for lithium-ion battery anodes. J. Electrochem. Soc. 2007, 154, A849–A855. [Google Scholar] [CrossRef]
- Tarascon, J.-M.; Grugeon, S.; Morcrette, M.; Laruelle, S.; Rozier, P.; Poizot, P. New concepts for the search of better electrode materials for rechargeable lithium batteries. C. R. Chim. 2005, 8, 9–15. [Google Scholar] [CrossRef]
- Nazri, G.-A.; Pistoia, G. Lithium Batteries: Science and Technology; Springer: New York, NY, USA, 2009. [Google Scholar]
- Poizot, P.; Laruelle, S.; Grugeon, S.; Dupont, L.; Tarascon, J.-M. Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries. Nature 2000, 407, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Jiang, X.; Sarkar, A.D.; Maark, T.A.; Deshpande, M.D.; Bououdina, M.; Johansson, B.; Ahuja, R. Screening study of light-metal and transition-metal-doped NiTiH hydrides as Li-ion battery anode materials. Solid State Ion. 2014, 258, 88–91. [Google Scholar] [CrossRef]
- Oumellal, Y.; Rougier, A.; Nazri, G.A.; Tarascon, J.-M.; Aymard, L. Metal hydrides for lithium-ion batteries. Nat. Mater. 2008, 7, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Oumellal, Y.; Rougier, A.; Tarascon, J.-M.; Aymard, L. 2LiH + M (M = Mg, Ti): New concept of negative electrode for rechargeable lithium-ion batteries. J. Power Sources 2009, 192, 698–702. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous electron gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for open-shell transition metals. Phys. Rev. B 1993, 48, 13115–13118. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, Z.; Guo, W.; Jiang, G.; Xu, S.; Ahuja, R.; Liu, X. Revisiting Mg–Mg2Ni System from Electronic Perspective. Metals 2017, 7, 489. https://doi.org/10.3390/met7110489
Qian Z, Guo W, Jiang G, Xu S, Ahuja R, Liu X. Revisiting Mg–Mg2Ni System from Electronic Perspective. Metals. 2017; 7(11):489. https://doi.org/10.3390/met7110489
Chicago/Turabian StyleQian, Zhao, Weimin Guo, Guanzhong Jiang, Shaokun Xu, Rajeev Ahuja, and Xiangfa Liu. 2017. "Revisiting Mg–Mg2Ni System from Electronic Perspective" Metals 7, no. 11: 489. https://doi.org/10.3390/met7110489





