Rapid Degradation of Azo Dyes by Melt-Spun Mg-Zn-Ca Metallic Glass in Artificial Seawater
Abstract
:1. Introduction
2. Materials and Methods
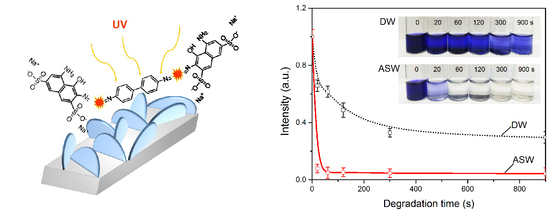
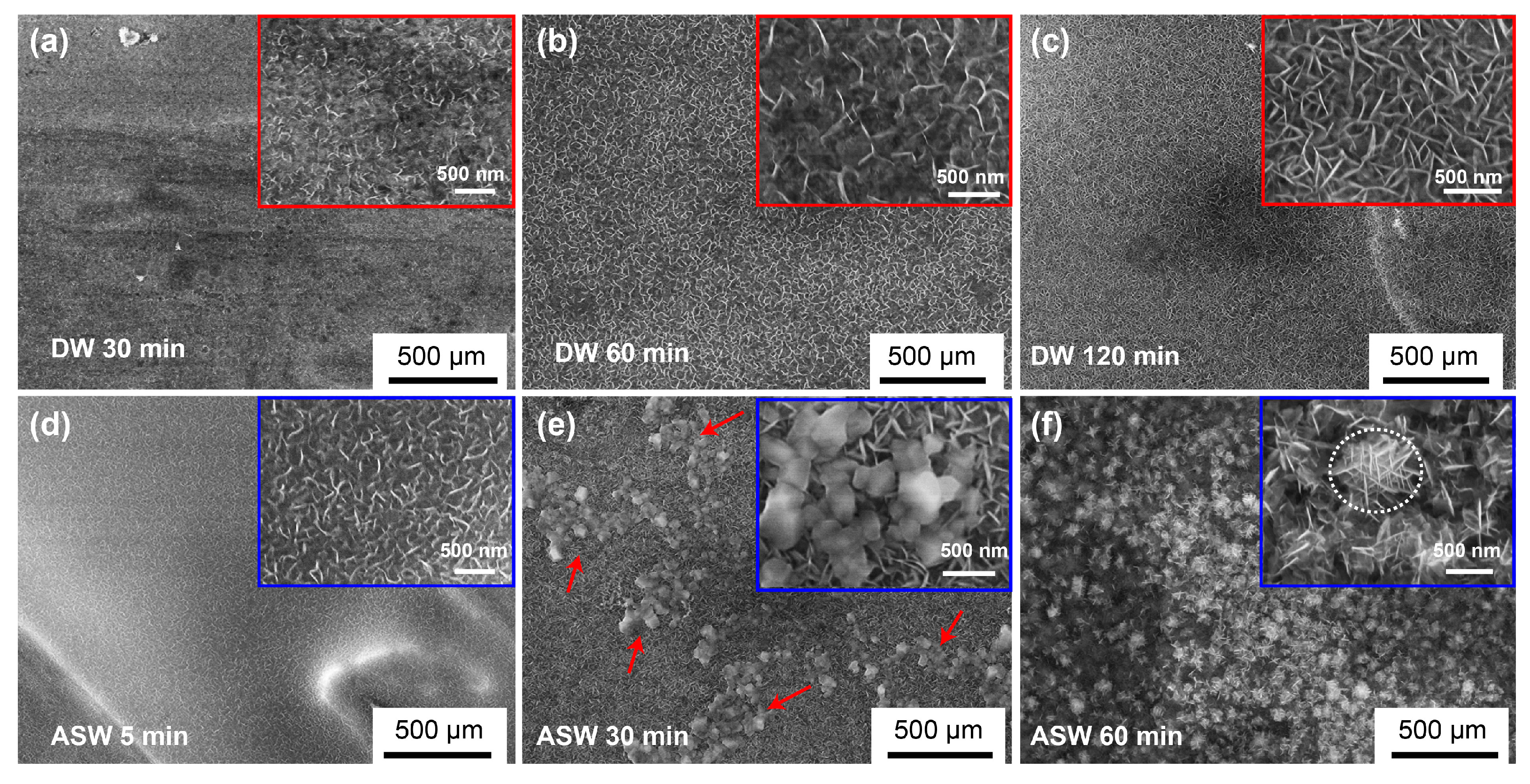
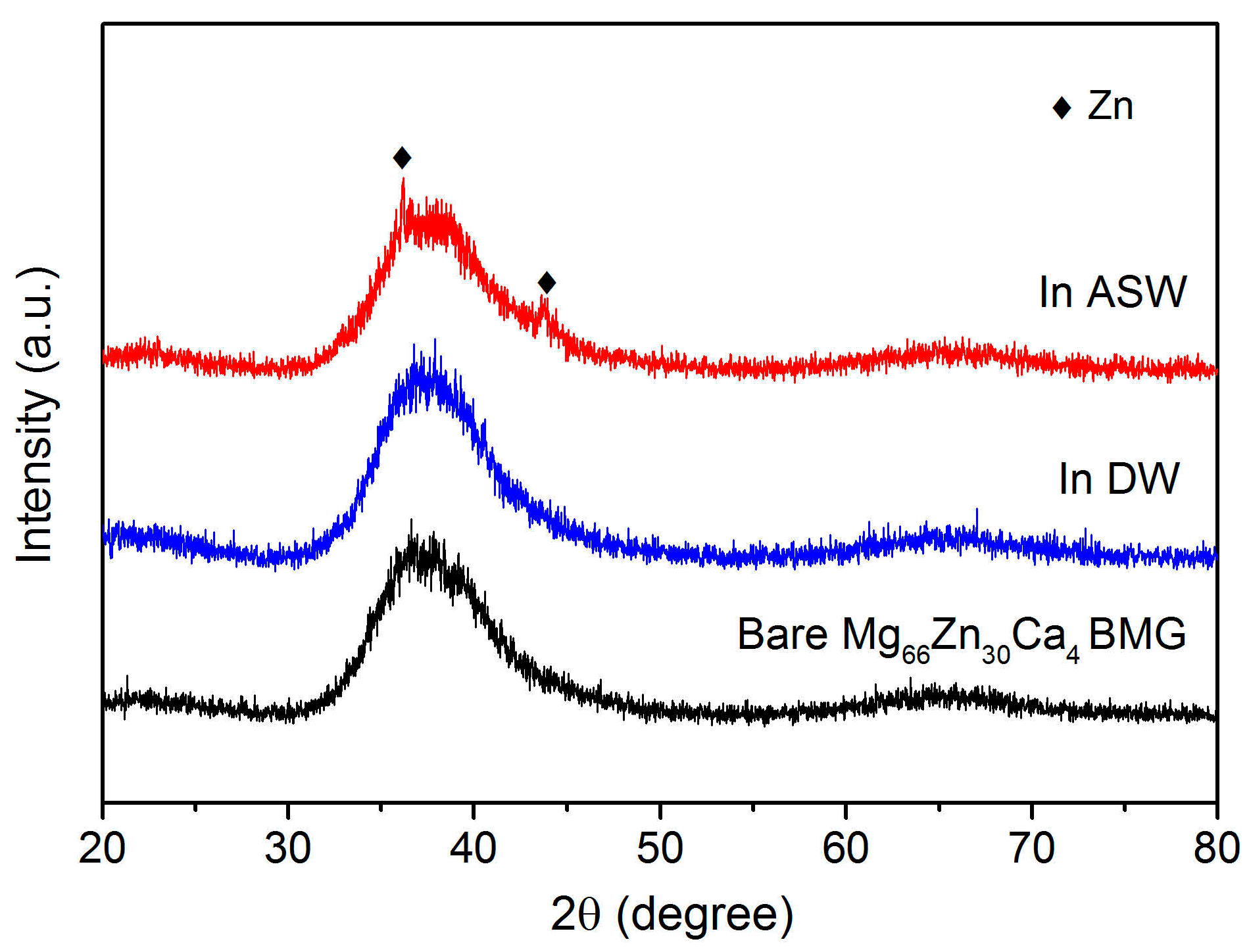
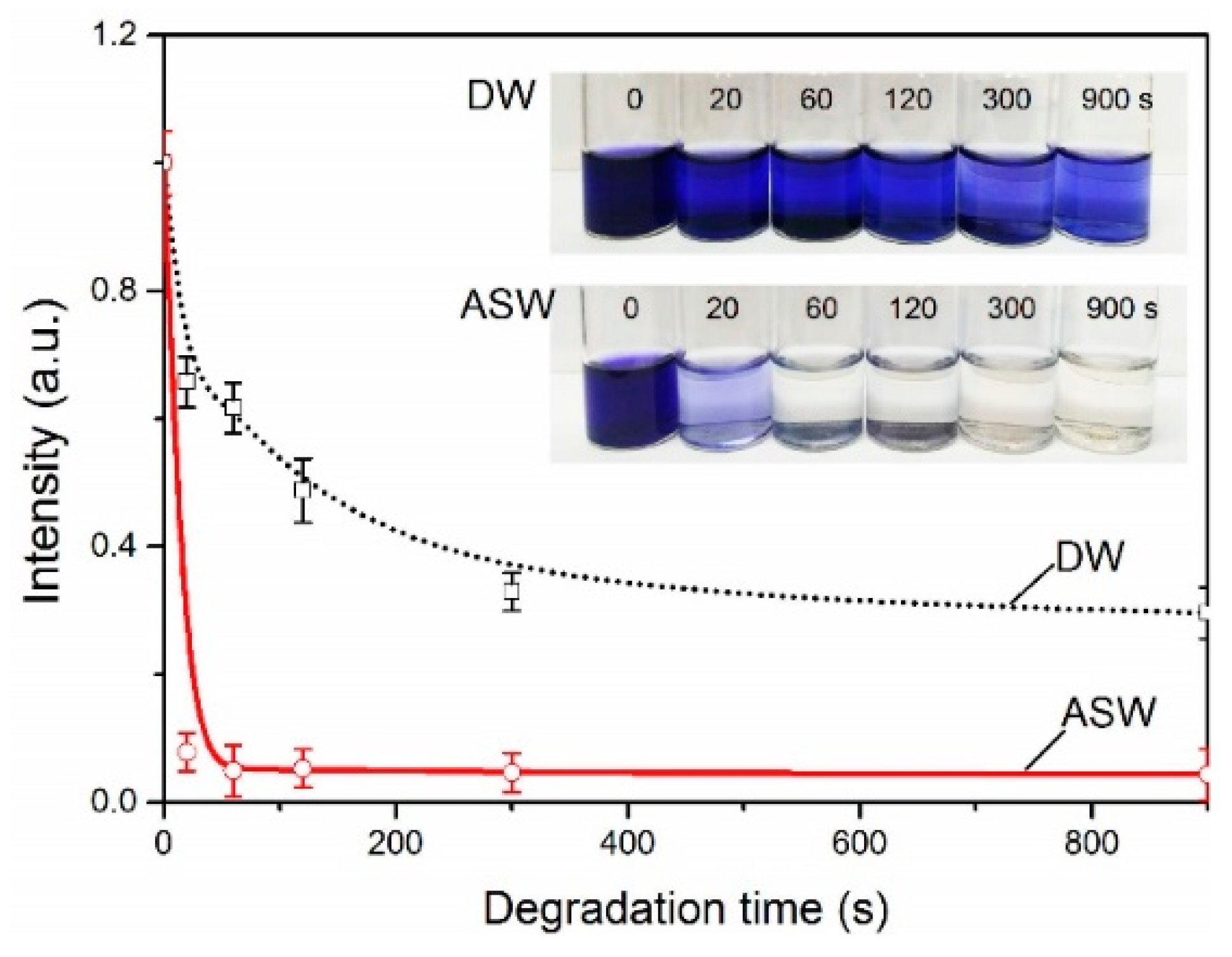
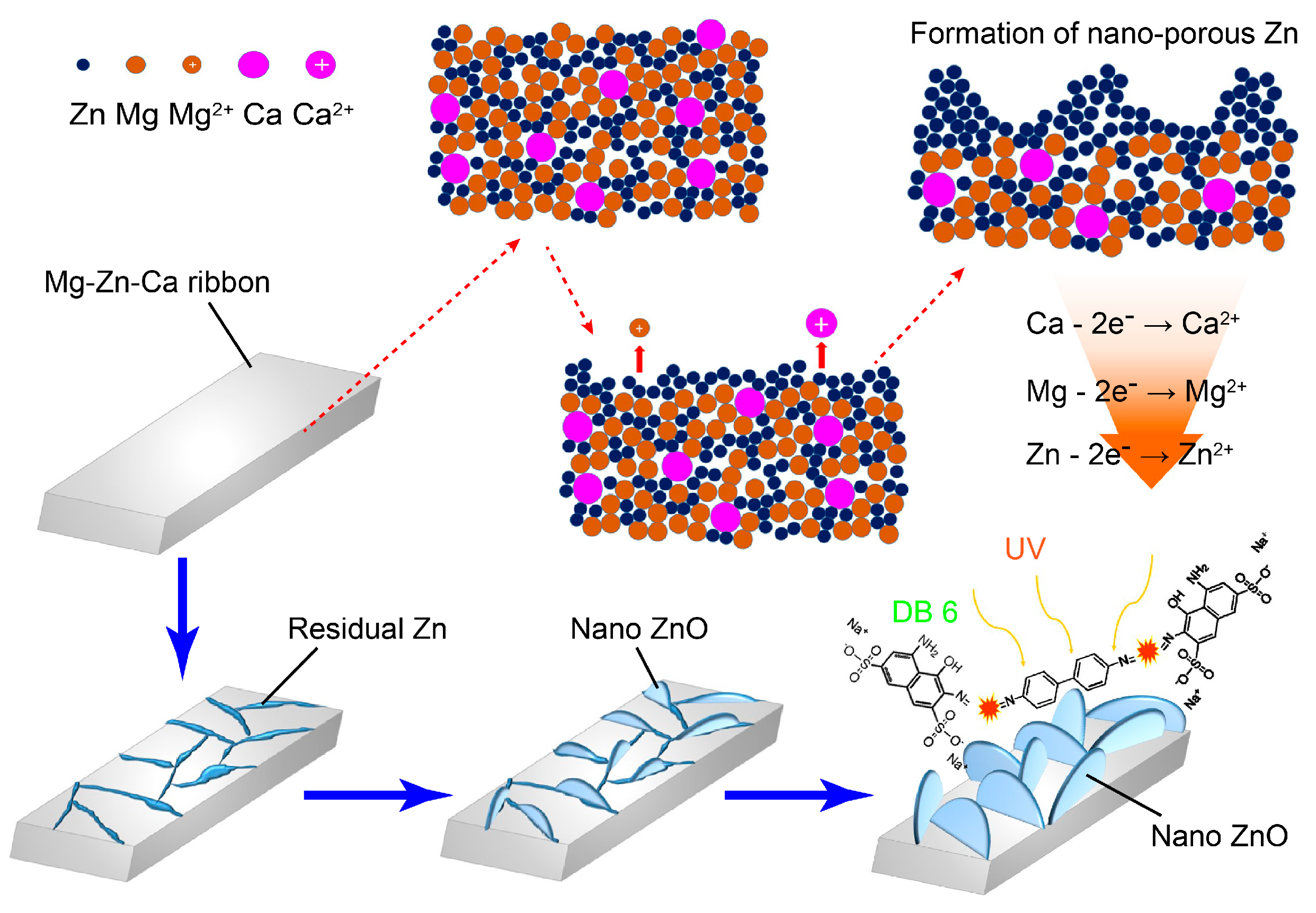
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, J.Q.; Liu, Y.H.; Chen, M.W.; Louzguine-Luzgin, D.V.; Inoue, A.; Perepezko, J.H. Excellent capability in degrading azo dyes by MgZn-based metallic glass powders. Sci. Rep. 2012, 2, 418. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.F.; Si, J.J.; Song, J.G.; Yang, Q.; Hui, X.D. Synthesis of Mg-Zn-Ca metallic glasses by gas-atomization and their excellent capability in degrading azo dyes. Mater. Sci. Eng. B 2014, 181, 46–55. [Google Scholar] [CrossRef]
- Ramya, M.; Karthika, M.; Selvakumar, R.; Raj, B.; Ravi, K.R. A facile and efficient single step ball milling process for synthesis of partially amorphous Mg-Zn-Ca alloy powders for dye degradation. J. Alloys Compd. 2017, 696, 185–192. [Google Scholar] [CrossRef]
- Ghidelli, M.; Gravier, S.; Blandin, J.J.; Pardoen, T.; Raskin, J.P.; Mompiou, F. Compositional-induced structural change in ZrXNi100−X thin film metallic glasses. J. Alloys Compd. 2014, 615, S348–S351. [Google Scholar] [CrossRef]
- Tang, Y.; Shao, Y.; Chen, N.; Yao, K.F. Rapid decomposition of Direct Blue 6 in neutral solution by Fe-B amorphous alloys. RSC Adv. 2015, 5, 6215–6221. [Google Scholar] [CrossRef]
- Tang, Y.; Shao, Y.; Chen, N.; Liu, X.; Chen, S.Q.; Yao, K.F. Insight into the high reactivity of commercial Fe-Si-B amorphous zero-valent iron in degrading azo dye solutions. RSC Adv. 2015, 43, 34032–34039. [Google Scholar] [CrossRef]
- Chen, S.Q.; Yang, G.N.; Luo, S.T.; Yin, S.J.; Jia, J.L.; Li, Z.; Gao, S.G.; Shao, Y.; Yao, K.F. Unexpected high performance of Fe-based nanocrystallized ribbons for azo dye decomposition. J. Mater. Chem. A 2017, 27, 14230–14240. [Google Scholar] [CrossRef]
- Liu, S.; Cui, C.; Wang, X.; Li, N.; Shi, J.; Cui, S.; Chen, P. Effect of cooling rate on microstructure and grain refining behavior of in situ CeB6/Al composite inoculant in aluminum. Metals 2017, 7, 204. [Google Scholar]
- Liu, S.; Wang, X.; Cui, C.; Zhao, L.; Li, N.; Zhang, Z.; Ding, J.; Sha, D. Enhanced grain refinement of in situ CeB6/Al composite inoculant on pure aluminum by microstructure control. J. Alloys Compd. 2017, 701, 926–934. [Google Scholar] [CrossRef]
- Ghidelli, M.; Idrissi, H.; Gravier, S.; Blandin, J.-J.; Raskin, J.-P.; Schryvers, D.; Pardoen, T. Homogeneous flow and size dependent mechanical behavior in highly ductile Zr65Ni35 metallic glass films. Acta Mater. 2017, 131, 246–259. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, Z.; Zhang, H. Effects of the addition of Co, Ni or Cr on the decolorization properties of Fe-Si-B amorphous alloys. J. Phys. Chem. Solids 2017, 110, 152–160. [Google Scholar] [CrossRef]
- Zhang, C.; Sun, Q. Annealing-induced different decolorization performances of Fe-Mo-Si-B amorphous alloys. J. Non-Cryst. Solids 2017, 470, 93–98. [Google Scholar] [CrossRef]
- Xie, S.H.; Huang, P.; Kruzic, J.J.; Zeng, X.R.; Qian, H.X. A highly efficient degradation mechanism of methyl orange using Fe-based metallic glass powders. Sci. Rep. 2016, 6, 21947. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, N.; Cheng, M.; Luo, S.; Shao, Y.; Yao, K. Multi-phase nanocrystallization induced fast degradation of methyl orange by annealing Fe-based amorphous ribbons. Intermetallics 2017, 90, 30–35. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Ma, E.; Xu, J. Reliability of compressive fracture strength of Mg-Zn-Ca bulk metallic glasses: Flaw sensitivity and Weibull statistics. Scr. Mater. 2008, 58, 496–499. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Cui, C.; Zhao, L.; Liu, S.; Chen, C. Fabrication, microstructure and refining mechanism of in situ CeB6/Al inoculant in aluminum. Mater. Des. 2015, 65, 432–437. [Google Scholar] [CrossRef]
- Gu, X.; Shiflet, G.J.; Guo, F.Q.; Poon, S.J. Mg-Ca-Zn bulk metallic glasses with high strength and significant ductility. J. Mater. Res. 2011, 20, 1935–1938. [Google Scholar] [CrossRef]
- Li, H.; Pang, S.; Liu, Y.; Sun, L.; Liaw, P.K.; Zhang, T. Biodegradable Mg-Zn-Ca-Sr bulk metallic glasses with enhanced corrosion performance for biomedical applications. Mater. Des. 2015, 67, 9–19. [Google Scholar] [CrossRef]
- Greer, J.R.; De Hosson, J.T.M. Plasticity in small-sized metallic systems: Intrinsic versus extrinsic size effect. Prog. Mater. Sci. 2011, 56, 654–724. [Google Scholar] [CrossRef]
- Ghidelli, M.; Sebastiani, M.; Johanns, K.E.; Pharr, G.M. Effects of indenter angle on micro-scale fracture toughness measurement by pillar splitting. J. Am. Ceram. Soc. 2017, 100, 5731–5738. [Google Scholar] [CrossRef]
- Liu, X.; Chen, N.; Gu, J.L.; Du, J.; Yao, K.F. Novel Cu-Ag bimetallic porous nanomembrane prepared from a multi-component metallic glass. RSC Adv. 2015, 62, 50565–50571. [Google Scholar] [CrossRef]
- Li, R.; Wu, N.; Liu, J.; Jin, Y.; Chen, X.-B.; Zhang, T. Formation and evolution of nanoporous bimetallic Ag-Cu alloy by electrochemically dealloying Mg-(Ag-Cu)-Y metallic glass. Corros. Sci. 2017, 119, 23–32. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Mahmoudian, M.R.; Basirun, W.J.; Alias, Y.; Ebadi, M. Facile fabrication of Zn/Zn-5(OH)(8)Cl-2·H2O flower-like nanostructure on the surface of Zn coated with poly (N-methyl pyrrole). Appl. Surf. Sci. 2011, 257, 10539–10544. [Google Scholar] [CrossRef]
- Su, P.P.; Zhao, J.; Rong, F.; Li, C.; Yang, Q.H. Fabrication of ZnO with tunable morphology through a facile treatment of Zn-based coordination polymers. Sci. China Chem. 2015, 58, 411–416. [Google Scholar] [CrossRef]
- Daneshvar, N.; Salari, D.; Khataee, A.R. Photocatalytic degradation of azo dye acid red 14 in water on ZnO as an alternative catalyst to TiO2. J. Photochem. Photobiol. A 2004, 162, 317–322. [Google Scholar] [CrossRef]
- Kansal, S.K.; Singh, M.; Sud, D. Studies on photodegradation of two commercial dyes in aqueous phase using different photocatalysts. J. Hazard. Mater. 2007, 141, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.C.; Cui, C.X.; Wang, X.; Liu, S.J.; Bu, S.J.; Wang, Q.Z.; Qi, Y.M. Corrosion resistance and calcium-phosphorus precipitation of micro-arc oxidized magnesium for biomedical applications. Appl. Surf. Sci. 2015, 330, 431–438. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.; Hu, X.; Qi, Y.; Wang, X.; Li, Z.; Zhao, L.; Liu, S.; Cui, C. Rapid Degradation of Azo Dyes by Melt-Spun Mg-Zn-Ca Metallic Glass in Artificial Seawater. Metals 2017, 7, 485. https://doi.org/10.3390/met7110485
Chen P, Hu X, Qi Y, Wang X, Li Z, Zhao L, Liu S, Cui C. Rapid Degradation of Azo Dyes by Melt-Spun Mg-Zn-Ca Metallic Glass in Artificial Seawater. Metals. 2017; 7(11):485. https://doi.org/10.3390/met7110485
Chicago/Turabian StyleChen, Peng, Ximei Hu, Yumin Qi, Xin Wang, Zongjia Li, Lichen Zhao, Shuiqing Liu, and Chunxiang Cui. 2017. "Rapid Degradation of Azo Dyes by Melt-Spun Mg-Zn-Ca Metallic Glass in Artificial Seawater" Metals 7, no. 11: 485. https://doi.org/10.3390/met7110485