Thermally-Induced Crack Evaluation in H13 Tool Steel
Abstract
:1. Introduction
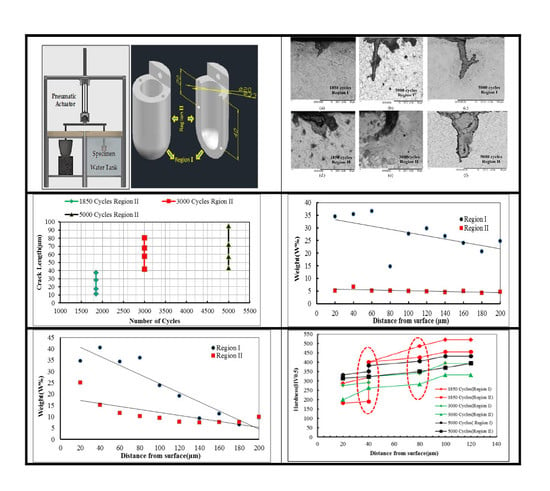
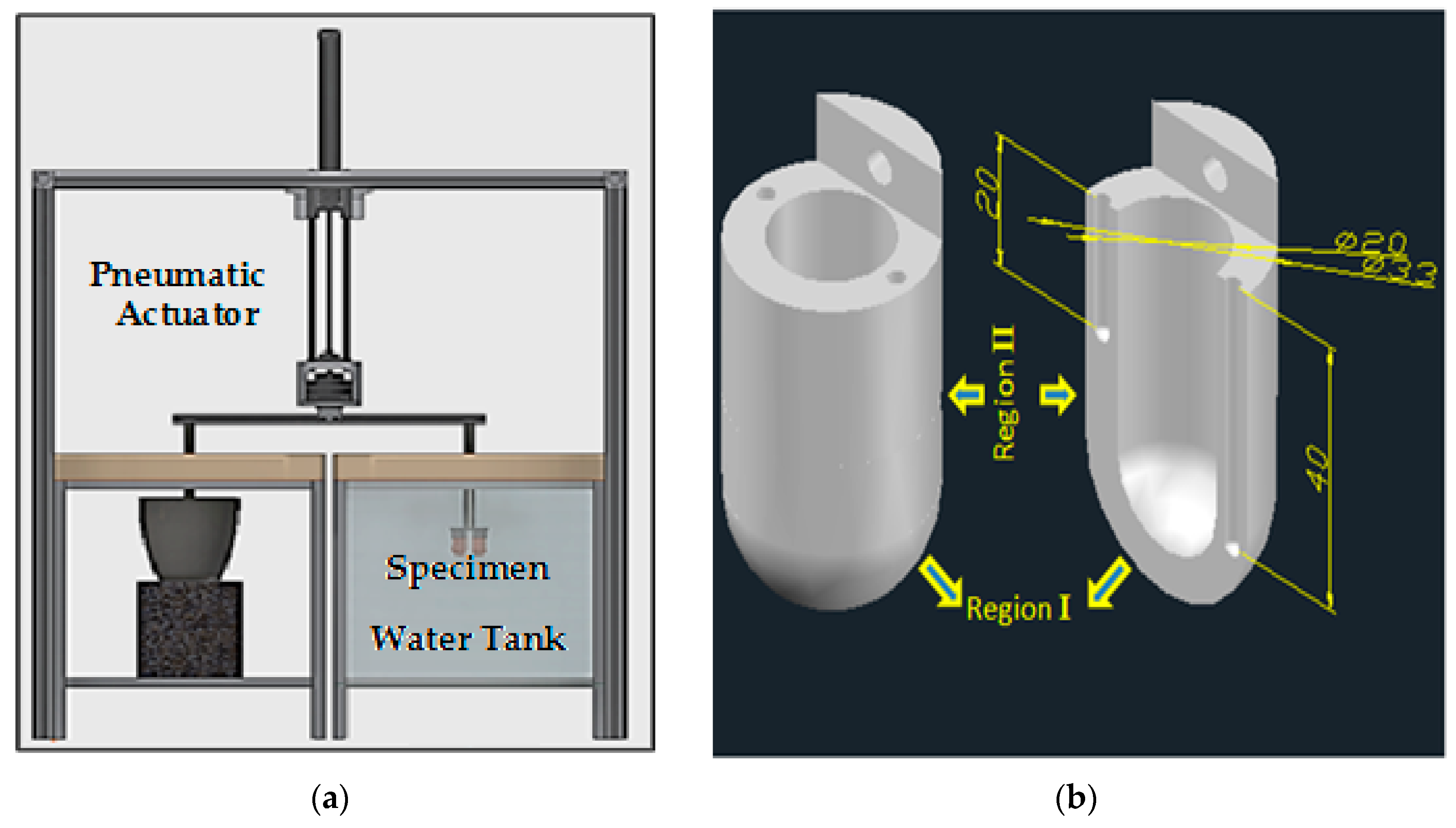
2. Materials and Methods
Material
3. Results and Discussion
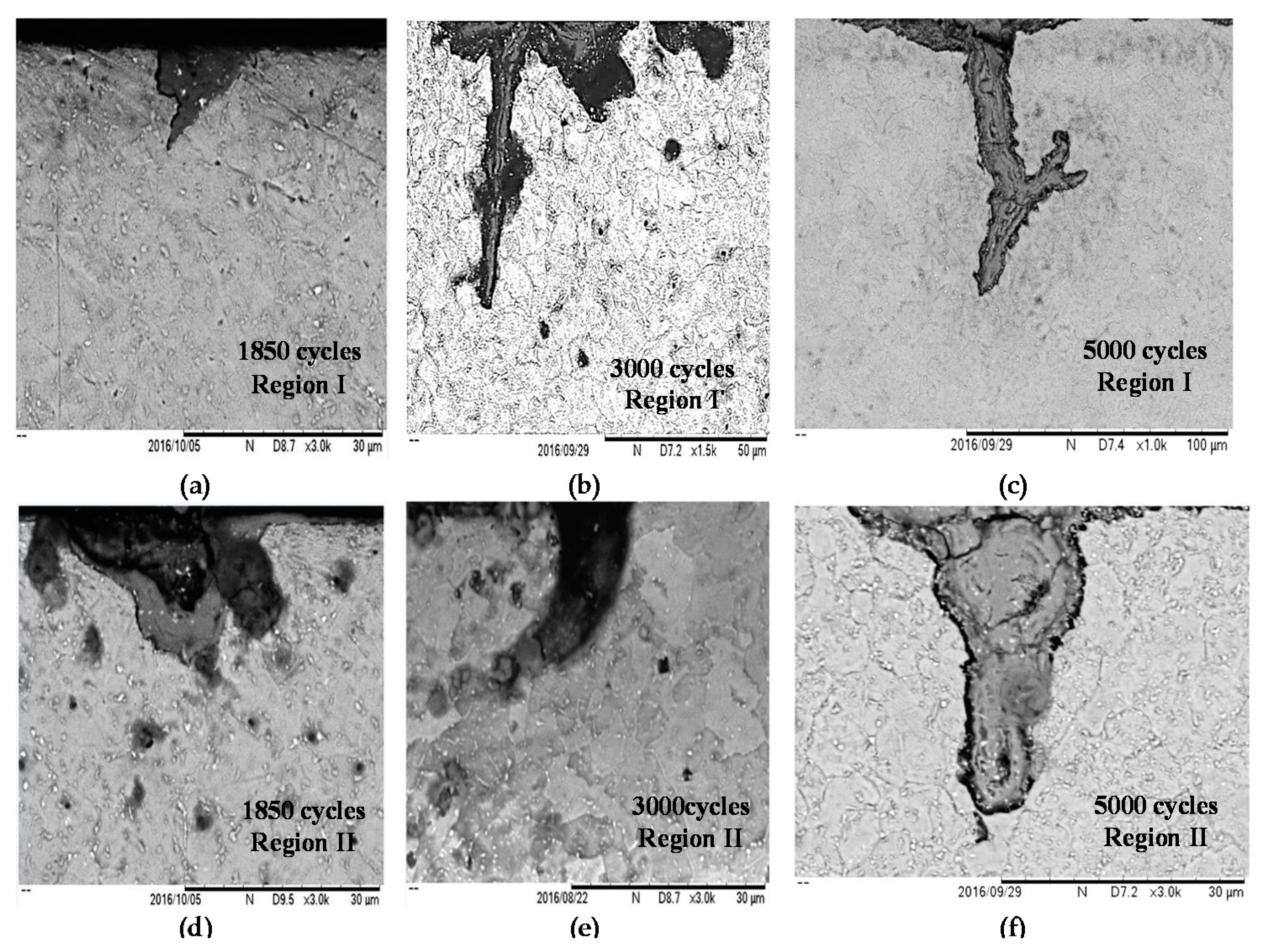
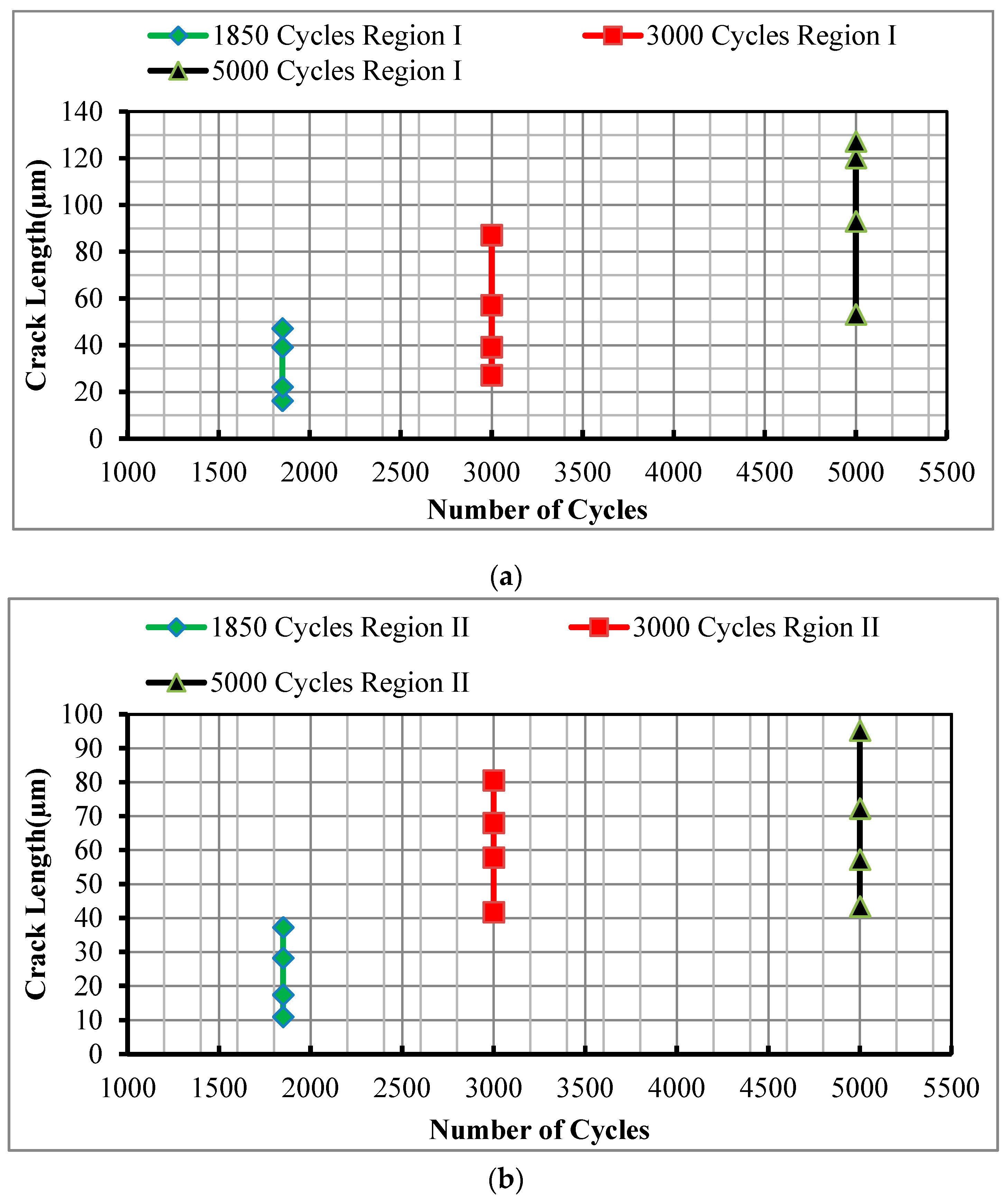
3.1. Metallographic Study
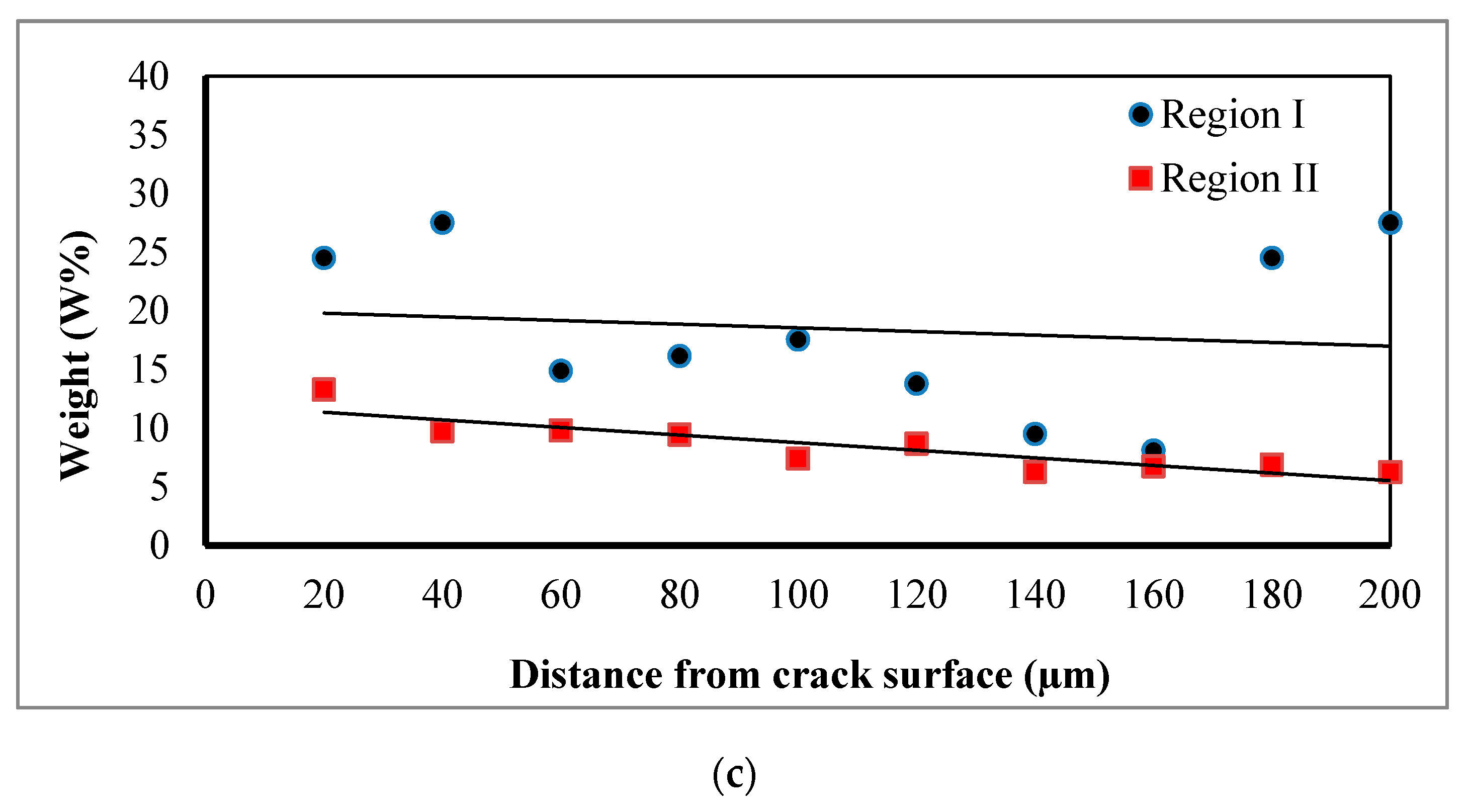
3.2. Energy Dispersive X-ray Spectroscopy (EDXS) Analysis
3.3. Hardness Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- İpek, M.; Selvi, İ.H.; Findik, F.; Torkul, O.; Cedimoğlu, I. An expert system based material selection approach to manufacturing. Mater. Des. 2013, 47, 331–340. [Google Scholar] [CrossRef]
- Liang, G.; Shi, C.; Zhou, Y.; Mao, D. Effect of ultrasonic treatment on the solidification microstructure of die-cast 35CrMo steel. Metals 2016, 6, 260. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Y.; Ou, H.; Lin, Y.J. Energy-based approach to thermal fatigue life of tool steels for die casting dies. Int. J. Fatigue 2016, 92, 166–178. [Google Scholar] [CrossRef]
- Pawłowski, B.; Bala, P.; Tokarski, T.; Krawczyk, J. Premature cracking of dies for aluminium alloy die-casting. Arch. Met. Mater. 2013, 58, 1275–1279. [Google Scholar]
- Mellouli, D.; Haddar, N.; Köster, A.; Ayedi, H.F. Hardness effect on thermal fatigue damage of hot-working tool steel. Eng. Fail. Anal. 2014, 45, 85–95. [Google Scholar] [CrossRef]
- Schneider, R.S.E.; Mesquita, R.A. Ifhtse global 21: Heat treatment and surface engineering in twenty-first century part 16: Advances in tool steels and their heat treatment part 2-hot work tool steels and plastic mould steels. Int. Heat Treat. Surf. Eng. 2011, 5, 94–100. [Google Scholar] [CrossRef]
- Zalaznik, A.; Nagode, M. Experimental, theoretical and numerical fatigue damage estimation using a temperature modified dirlik method. Eng. Struct. 2015, 96, 56–65. [Google Scholar] [CrossRef]
- Shaha, S.K.; Czerwinski, F.; Kasprzak, W.; Friedman, J.; Chen, D.L. Improving high-temperature tensile and low-cycle fatigue behavior of Al-Si-Cu-Mg alloys through micro-additions of Ti, V, and Zr. Metall. Mater. Trans. A 2015, 46, 3063–3078. [Google Scholar] [CrossRef]
- Maiya, P.S.; Burke, W.F. Effects of Environment on the Low-Cycle Fatigue Behavior of Type 304 Stainless Steel; Argonne National Lab.: DuPage, IL, USA, 1979. [Google Scholar]
- Visser, M.J. Evaluation of Malted Barley with Different Degrees of Fermentability Using the Rapid Visco Analyser (RVA). Master’s Thesis, University of Stellenbosch, Stellenbosch, South Africa, 2011. [Google Scholar]
- Zhang, X.M.; Chen, W.P. Review on corrosion-wear resistance performance of materials in molten aluminum and its alloys. Trans. Nonferrous Met. Soc. China 2015, 25, 1715–1731. [Google Scholar] [CrossRef]
- Kusinski, J.; Kac, S.; Kopia, A.; Radziszewska, A.; Rozmus-Górnikowska, M.; Major, B.; Major, L.; Marczak, J.; Lisiecki, A. Laser modification of the materials surface layer—A review paper. Bull. Pol. Acad. Sci. Tech. Sci. 2012, 60. [Google Scholar] [CrossRef]
- Abdulhadi, H.; Ahmad, S.; Ismail, I.; Ishak, M.; Mohammed, G. Experimental investigation of thermal fatigue die casting dies by using response surface modelling. Metals 2017, 7, 191. [Google Scholar] [CrossRef]
- Cong, D.; Zhou, H.; Ren, Z.; Zhang, H.; Ren, L.; Meng, C.; Wang, C. Thermal fatigue resistance of hot work die steel repaired by partial laser surface remelting and alloying process. Opt. Lasers Eng. 2014, 54, 55–61. [Google Scholar] [CrossRef]
- Abdulhadi, H.A.; Aqida, S.N.; Ishak, M.; Mohammed, G.R. Thermal fatigue of die-casting dies: An overview. In Proceedings of the 3rd International Conference on Mechanical Engineering Research (ICMER 2015), Kuantan, Malaysia, 18–19 August 2015. [Google Scholar]
- Mohammed, G.; Ishak, M.; Aqida, S.; Abdulhadi, H. Effects of heat input on microstructure, corrosion and mechanical characteristics of welded austenitic and duplex stainless steels: A review. Metals 2017, 7, 39. [Google Scholar] [CrossRef]
- Alimi, A.; Fajoui, J.; Kchaou, M.; Branchu, S.; Elleuch, R.; Jacquemin, F. Multi-scale hot working tool damage (X40CrMoV5-1) analysis in relation to the forging process. Eng. Fail. Anal. 2016, 62, 142–155. [Google Scholar] [CrossRef]
- Jia, Z.X.; Liu, Y.W.; Li, J.Q.; Liu, L.J.; Li, H.L. Crack growth behavior at thermal fatigue of H13 tool steel processed by laser surface melting. Int. J. Fatigue 2015, 78, 61–71. [Google Scholar] [CrossRef]
- Krupp, U. Modeling crack propagation accounting for microstructural features. In Fatigue Crack Propagation in Metals and Alloys; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar]
- Klobčar, D.; Kosec, L.; Kosec, B.; Tušek, J. Thermo fatigue cracking of die casting dies. Eng. Failure Anal. 2012, 20, 43–53. [Google Scholar] [CrossRef]
- Syarifah Nur Aqida, S.A.; Naher, S.; Brabazon, D. Thermal simulation of laser surface modification of H13 die steel. Key Eng. Mater. 2012, 504–506, 351–356. [Google Scholar] [CrossRef]
- Mohammed, G.R.; Ishak, M.; Aqida, S.N.; Abdulhadi, H.A. The effect of fiber laser parameters on microhardness and microstructure of duplex stainless steel. MATEC Web Conf. 2017, 90, 01024. [Google Scholar] [CrossRef]
- .Svečko, R.; Kusić, D.; Kek, T.; Sarjaš, A.; Hančič, A.; Grum, J. Acoustic emission detection of macro-cracks on engraving tool steel inserts during the injection molding cycle using PZT sensors. Sensors 2013, 13, 6365–6379. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wong, C. Recent advances of conductive adhesives as a lead-free alternative in electronic packaging: Materials, processing, reliability and applications. Mater. Sci. Eng. R 2006, 51, 1–35. [Google Scholar] [CrossRef]
- Čerče, L.; Pušavec, F.; Kopač, J. A new approach to spatial tool wear analysis and monitoring. J. Mech. Eng. 2015, 61, 489–497. [Google Scholar] [CrossRef]
- Muhič, M.; Kosel, F.; Pukšič, A.; Klobčar, D. A new approach to monitoring thermal fatigue cracks in die casting moulds. Int. J. Mater. Res. 2011, 102, 69–75. [Google Scholar] [CrossRef]
- Muhič, M.; Tušek, J.; Kosel, F.; Klobčar, D. Analysis of die casting tool material. J. Mech. Eng. 2010, 56, 351–356. [Google Scholar]
- Alekseev, V.V.; Orlova, E.A.; Kozlov, F.A.; Varseev, E.V. Evolution of a two-layer oxide coating on the steel surface of the primary coolant circuit in the course of nuclear power plant operation. J. Eng. Phys. Thermophys. 2016, 89, 272–279. [Google Scholar] [CrossRef]
- Bhadeshia, H.; Honeycombe, R. Nanostructured steels. In Steels: Microstructure and Properties, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Fabas, A.; Monceau, D.; Josse, C.; Lamesle, P.; Rouaix-Vande Put, A. Mechanism of metal dusting corrosion by pitting of a chromia-forming alloy at atmospheric pressure and low gas velocity. Corros. Sci. 2016, 107, 204–210. [Google Scholar] [CrossRef]
- Albertsen, J.Z.; Grong, Ø.; Walmsley, J.C.; Mathiesen, R.H.; Van Beek, W. A model for high-temperature pitting corrosion in nickel-based alloys involving internal precipitation of carbides, oxides, and graphite. Metall. Mater. Trans. A 2008, 39, 1258–1276. [Google Scholar] [CrossRef]
- Kennedy, F.E. Contact temperature of a moving solid surface. Encycl. Tribol. 2013, 544–548. [Google Scholar] [CrossRef]
- Shoji, T.; Lu, Z.; Murakami, H. Formulating stress corrosion cracking growth rates by combination of crack tip mechanics and crack tip oxidation kinetics. Corros. Sci. 2010, 52, 769–779. [Google Scholar] [CrossRef]
- Hanno, M.E. Influence of Ferrite Content on Fatigue Strength of Quenched and Tempered 42crmos4 Steel. Master’s Thesis, KTH Royal Institute of Technology in Stockholm, Stockholm, Sweden, 2012. [Google Scholar]
- Muojekwu, C.; Samarasekera, I.; Brimacombe, J. Heat transfer and microstructure during the early stages of metal solidification. Metall. Mater. Trans. B 1995, 26, 361–382. [Google Scholar] [CrossRef]
- Ng, H.; Douguet, E.; Bettles, C.; Muddle, B. Age-hardening behaviour of two metastable beta-titanium alloys. Mater. Sci. Eng. A 2010, 527, 7017–7026. [Google Scholar] [CrossRef]



| H13 Tool Steel | |||||||
| Element | C | Si | Mn | Cr | Mo | V | W |
| wt % | 0.51 | 1.26 | 0.413 | 5.5 | 1.52 | 1.0 | 0.02 |
| Al 356 | |||||||
| Element | Cu | Mg | Mn | Si | Zn | Ti | Fe |
| wt % | 0.25 | 0.45 | 0.35 | 7.5 | 0.35 | 0.25 | 0.2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdulhadi, H.A.; Ahmad, S.N.A.S.; Ismail, I.; Ishak, M.; Mohammed, G.R. Thermally-Induced Crack Evaluation in H13 Tool Steel. Metals 2017, 7, 475. https://doi.org/10.3390/met7110475
Abdulhadi HA, Ahmad SNAS, Ismail I, Ishak M, Mohammed GR. Thermally-Induced Crack Evaluation in H13 Tool Steel. Metals. 2017; 7(11):475. https://doi.org/10.3390/met7110475
Chicago/Turabian StyleAbdulhadi, Hassan Abdulrssoul, Syarifah Nur Aqida Syed Ahmad, Izwan Ismail, Mahadzir Ishak, and Ghusoon Ridha Mohammed. 2017. "Thermally-Induced Crack Evaluation in H13 Tool Steel" Metals 7, no. 11: 475. https://doi.org/10.3390/met7110475