1. Introduction
Magnesium is an essential element for living organisms, however, for technical purposes is the magnesium used mainly in the form of alloys. Alloying elements improve magnesium mechanical properties and it can be used to control its reactivity. Due to the suitable combination of physico-mechanical properties, biocompatibility and non-toxicity specific magnesium alloys are investigated for medical applications. In the case of orthopedic implants physical and mechanical properties of magnesium alloys are also important. These properties are similar to the properties of a human bone (e.g., density, compressive yield strength, ultimate tensile strength). Magnesium alloy implants are moreover biocompatible and biodegradable [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11]. As a result of chemical reactions with the biological environment non-toxic corrosion products are created on the surface of the implants. In the human body the magnesium alloy implants dissolve and are absorbed, which prevents surgical removal of the implants after tissue healing [
5,
6]. The disadvantage of magnesium and magnesium alloys is their high reactivity at the physiological pH (7.4–7.6) as well as in physiological media containing high concentrations of chloride ions, which could cause rapid disintegration of the implant in the biological environment [
7,
8]. Furthermore, during the corrosion process of magnesium and its alloys, the release of hydrogen gas may be too fast to be endured by the host tissues [
9].
One way to influence corrosion resistance and mechanical properties of magnesium alloys is by using high purity alloys that maintain metal impurities such as iron, nickel and copper below limits. Examples of alloying elements of magnesium alloys for biodegradable implants for improvement of the corrosion resistance of the alloys are calcium, zinc, etc. [
10,
11,
12,
13,
14,
15]. On the other hand, even the magnesium alloys for medical applications have to have good mechanical properties and they have to also contain other alloying elements. One of the basic alloying elements for magnesium mechanical and corrosion properties improvement is aluminum. Even though Al has a positive effect on magnesium alloys properties, the amount of Al added must be controlled in the case of alloys for medical applications. A high concentration of Al was considered to possibly cause neurotoxic illnesses such as dementia or Alzheimer’s disease. However, when Al is introduced into the human body in small concentrations, for instance during dietary ingestion or consumption from natural or urban water supplies, then it is naturally excreted through urine or in the form of bile [
10,
16,
17,
18,
19,
20,
21].
Hank’s balanced salt solutions (HBSSs), which are one of the options for simulating the corrosive environment of the body of living organisms are often used for analysis of the corrosion behavior of magnesium alloys that are expected to be used in medicine applications. Mainly due to higher chloride concentrations, HBSSs are more aggressive medium compared to artificial plasma. Sulfate ions contained in HBSS can also result in higher corrosion rate of magnesium and its alloys compared to other corrosion media used for material corrosion properties characterization [
22,
23,
24,
25,
26,
27,
28,
29,
30,
31].
The reactivity of material in the corrosion environment is, besides many aspects, influenced with the chemical and phase composition of the material and chemical composition of the testing solution.
Electrochemical corrosion behavior of wrought AZ31 and AZ61 alloys in HBSS characterized by Tkacz et al. in [
32] revealed different response of the materials due to their chemical and phase composition and surface finish. Based on the EIS (electrochemical impedance spectroscopy) measurement results, AZ61 magnesium alloy was considered as more corrosion resistant when compared to AZ31 magnesium alloy, while opposite conclusion can be considered based on the potentiodynamic test results. Potentiodynamic tests revealed minor influence of the surface finish in the case of AZ31 magnesium alloy represented for example by corrosion potential (
Ecorr) values. The samples with polished surface (
Ecorr = −1.676 ± 0.003 V) were characteristic with more positive value of
Ecorr comparing to the ground samples (
Ecorr = −1.701 ± 0.003 V). On the other hand, no influence of surface finish was observed in the case of AZ61 magnesium alloy (
Ecorr = −1.708 ± 0.004 V for ground sample and
Ecorr = −1.708 ± 0.003 V for polished sample). No significant influence of surface finish was observed based on the EIS test results. The increase of polarization resistance up to 24 h of immersion of the samples in the HBSS to the values of approximately 4000 Ω·cm
2 was characteristic for both the surface states of AZ31 magnesium alloy, while increasing exposure time to the corrosion environment did not have any significant influence on the polarization resistance and the value remained stable. In the case of AZ61 magnesium alloy a significant increase of the values of polarization resistance up to the 48 h of exposure of the samples to the corrosion environment was observed for both the material states, reaching the value of polarization resistance of approximately 9000 Ω·cm
2. Following an increase of immersion time resulted in an additional increase of the
Rp to the value of approximately 21,000 Ω·cm
2 for ground and 17,000 Ω·cm
2 for polished samples.
The influence of the chloride ions on corrosion behavior of Mg-Al-Zn based alloys was reported in several studies. Ambat et al. studied in [
33] the influence of chloride ion concentration and pH on the corrosion and electrochemical behavior of die-cast and ingot-cast AZ91D alloy. The effect of chloride ion concentration was studied in NaCl (0–10%) solutions at pH 7.25. The effect of pH was analyzed in solutions with the chloride ion concentration kept constant at 3.5% while the pH was varied from 1.0–12.0. Material behavior was analyzed with immersion and potentiodynamic testing. Increase in chloride ion concentration increased the corrosion rate of AZ91D magnesium alloy at pH 7.25 and 12.0 for both the material states, however, at pH 2.0, the effect of chloride ion was found to be negligible. High corrosion rate was observed for both the material states in highly acidic solutions, while the corrosion rate was found to be low in neutral pH and alkaline conditions. The corrosion rate determined from immersion tests was much higher than that obtained from electrochemical measurements. The observation was attributed to the negative difference effect and to the physical removal of β phase during corrosion. The differences between the response of material states to the corrosion environment were discussed in terms of microstructural differences.
The effect of chloride ion concentration and pH on the corrosion (immersion tests) and electrochemical behavior (potentiodynamic tests) of AZ63 alloy were studied in NaCl solutions at different concentrations (0.01, 0.2, 0.6, 1 and 2 M) and pH values (2, 3, 8, 11 and 11.5) were studied by Altun and Sen in [
34]. Authors observed that the corrosion rate increased with the increase in concentration of NaCl solution. However, it was observed, that with the increase in chloride ion concentration, the rising rate of corrosion rate decreased. The corrosion rate increase with increasing chloride ion concentration was attributed to the participation of chloride ions in the dissolution reaction. Chloride ions are aggressive for both magnesium and aluminum. The adsorption of chloride ions to oxide covered magnesium surface transforms Mg(OH)
2 to easily soluble MgCl
2. Authors also observed a shift of the corrosion potential to more negative (more active) values with the increase in chloride ion concentration. The explanation for this behavior was found in adsorption of these ions on the alloy surface at weak parts of the oxide film. Corrosion potential was observed to be shifted to more negative (more active) values with the decrease in pH value of the solution. Higher pH values were discussed to favor the formation of Mg(OH)
2 which protects the alloy from corrosion.
Merino et al. studied in [
35] the influence of chloride ion concentration and temperature on the corrosion of Mg-Al alloys in a salt fog with the focus on the effect of Al content in the alloy. The results of their investigation showed that the corrosion attack of Mg, AZ31, AZ80 and AZ91D materials under the salt fog test increases with increasing temperature and chloride anion concentration, while the effect of temperature was considered to be more noticeable than that of chloride concentration. Authors also analyzed the influence of the Al content and resulting presence of intermetallic phases on material corrosion behavior. In the case of the wrought AZ31 only negligible influence of the present AlMn based phase was observed, while in the case of cast AZ80 and AZ91 alloys the creation of galvanic couples between Al-Mn and β-Mg
17Al
12 phases with the Mg matrix resulted in more pronounced corrosion attack. Authors also observed the influence of the distribution, size and morphology of the β phase and resulting Al-rich corrosion products layer created on the material surface during the corrosion.
Influence of sulfate anion concentration and pH on the corrosion of Mg-Al-Zn-Mn (GA9) magnesium alloy was investigated by Shetty et al. [
36]. The studies were carried out in sodium sulfate solutions with concentrations range of 0.1–2 M; and at different temperatures of 30–50 °C and pH of 3.0–12.0. According to the experimental data, the corrosion rate of the alloy increased with the increase in temperature, and also with the increase in the concentration of sodium sulfate in the medium. It was observed that the rate of corrosion decreased with the increase in pH.
Even thought, magnesium alloys corrosion properties are widely investigated, most of the studies are performed in NaCl and Na
2SO
4 solutions simulating corrosion environment in engineering applications [
33,
34,
35,
36]. Corrosion behavior of magnesium alloys was studied in several types of HBSS [
22,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32], however, the influence of the chemical composition of HBSS on corrosion processes is not available in the literature according to the authors’ knowledge.
Corrosion characteristics of metallic materials can be analyzed in different ways. In this work, electrochemical methods have been used to investigate the corrosion behavior of magnesium alloys. Potentiodynamic tests and electrochemical impedance spectroscopy were used for the description of the material response to the HBSS and enriched HBSS+. Obtained data were discussed with the aim to identify the influence of material chemical and phase composition, surface roughness and composition of the corrosion solution on corrosion behavior. Values of corrosion potential (Ecorr) expressing thermodynamics of the corrosion process and corrosion current density values (icorr) expressing the kinetic of the corrosion process were obtained by potentiodynamic measurements. Corrosion potential characteristic for the material expresses the thermodynamic stability of the system and the conditions for material corrosion and its resistance against corrosion process. Kinetic of the corrosion process can be shown by the evolution of the corrosion rate (vcorr) which can be calculated from the icorr. Polarization resistance (Rp) values were obtained by electrochemical impedance spectroscopy (EIS). Evolution of the corrosion products on the specimen surface was analyzed in terms of scanning electron microscopy and correlated with the composition of the used corrosion solution.
The presented results show differences in electrochemical corrosion behavior of AZ31 and AZ61 alloys in HBSS and enriched HBSS+ with the aim to characterize material behavior and different response of ground and polished materials on the different chemical composition of the corrosion solution.
4. Discussion
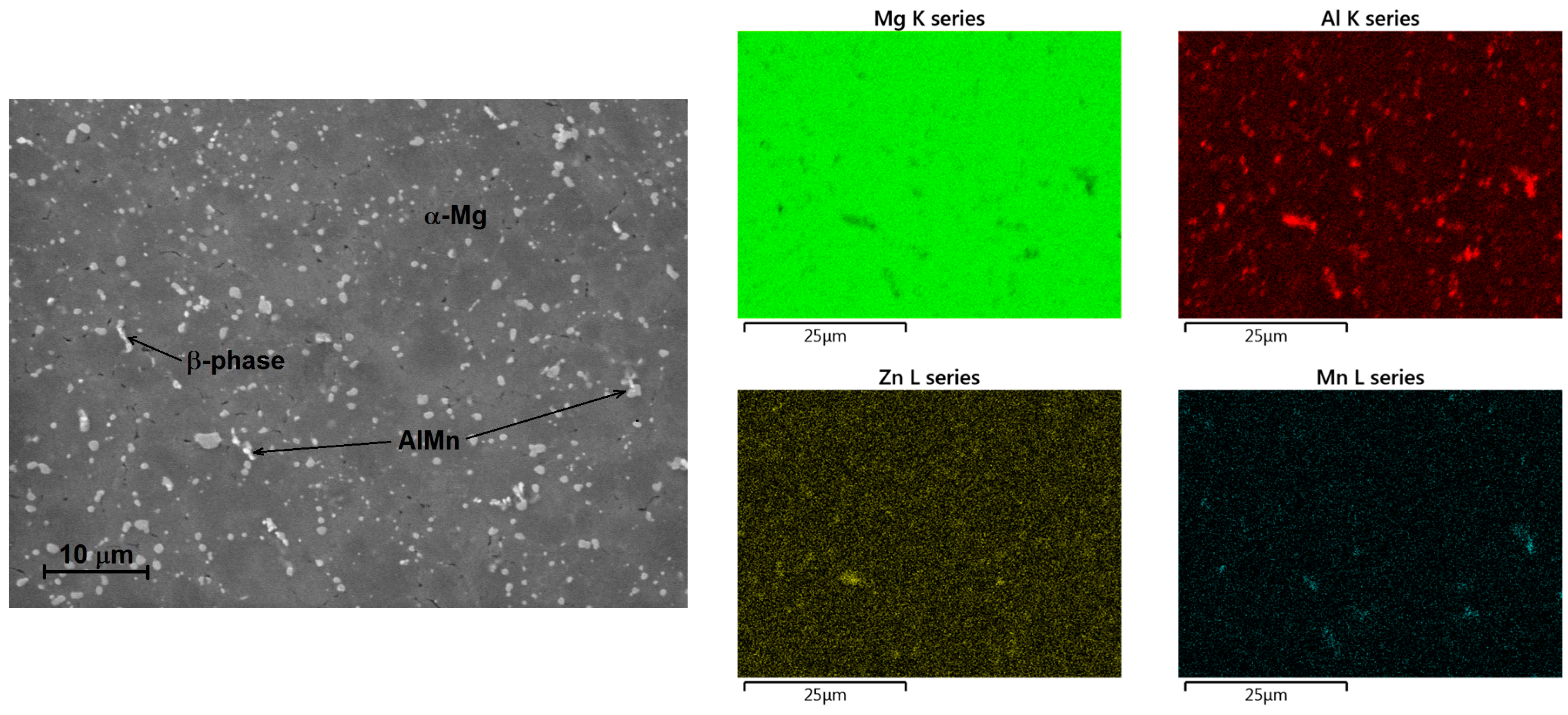
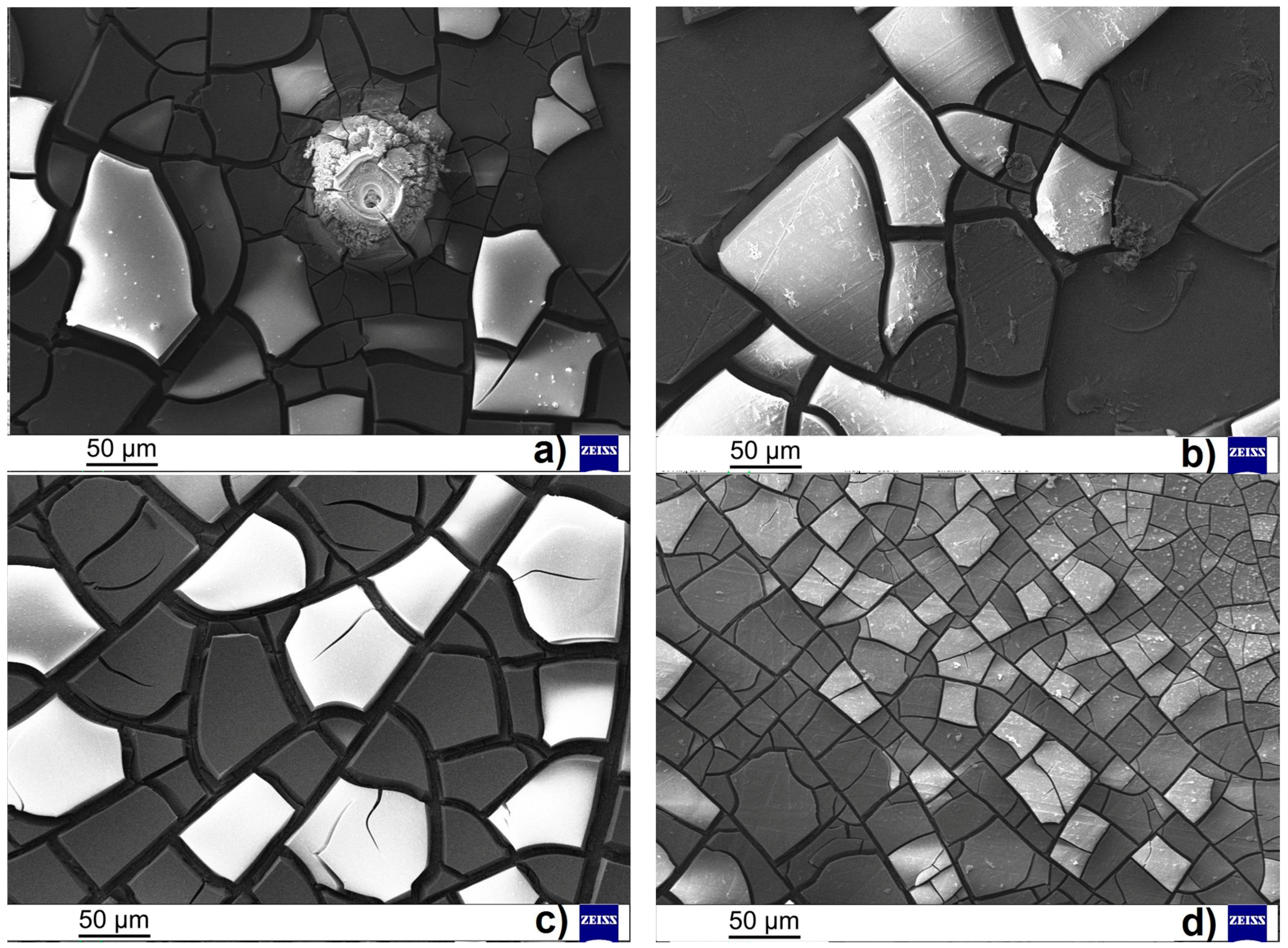
The effect of the surface treatment (ground vs. polished surface) was observed during potentiodynamic measurements on both types of tested magnesium alloys. In both the cases, more positive value of corrosion potential,
Ecorr (
Table 4), was determined for the polished samples (0.25 µm) when compared to the ground samples (#1200). The microstructure of the AZ61 magnesium alloy contains a higher number of intermetallic phases (Mg
17Al
12 and AlMn,
Figure 2) than the microstructure of the AZ31 magnesium alloy containing only AlMn particles (
Figure 1). All of these intermetallic phase particles have more positive potential [
46,
47,
48] than the substitution solid solution (α-Mg) which caused the formation of microcells which usually result in the acceleration of the corrosion process. The observations are in agreement with [
49] where a positive effect of decreasing surface roughness on AZ91 alloy electrochemical corrosion properties was presented. However, authors in [
49] performed the EIS measurement only 2 h of exposure of the material in 0.5 wt % NaCl solution and cannot detect the influence of evolution of the layer of corrosion products and its adhesion to the material surface.
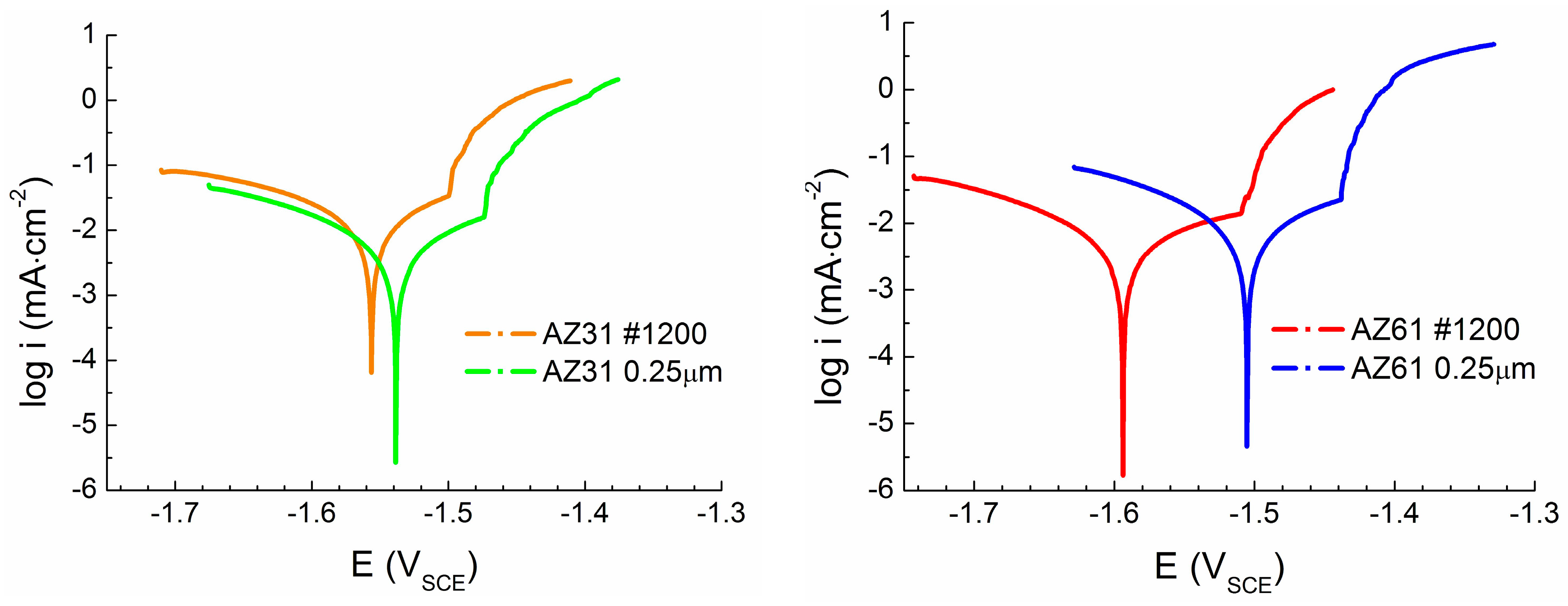
The difference in the
Ecorr values determined for ground and polished surface was more significant in the case of AZ61 magnesium alloy (
Figure 8). The grinding process results in the higher roughness of the treated surface comparing to the polished surface resulting in a larger real surface area exposed to the corrosion environment [
49].
Ecorr reaches more negative value comparing to the polished surface and therefore the ground surface can be considered as less stable from a thermodynamic point of view than the polished surface. While only a small amount of intermetallic phase particles was present in the case of the AZ31 magnesium alloy, the effect of the chemical heterogeneity and surface roughness was smaller compared to the AZ61 magnesium alloy samples. Corrosion potential
Ecorr of the AZ31 and AZ61 alloys determined from measurements in HBSS without Mg
2+ and Ca
2+ ions reported by authors in [
32] have more negative values than data obtained in enriched HBSS+ with Mg
2+ and Ca
2+ ions (
Figure 8). In the [
32] was not observed pitting corrosion attack of the samples (no pitting potential
Epit was observed on the curves) which indicate higher reactivity of tested magnesium alloys in enriched HBSS+ probably caused by the content of Mg
2+, Ca
2+ and sulphate ions.
While in the case of AZ31 magnesium alloy a positive effect of polishing on the material response of the corrosion environment from the kinetic point of view can be considered, opposite behavior was observed in the case of AZ61 magnesium alloy. Polishing of the surface of AZ31 alloy resulted in 50% decrease in the corrosion current density (icorr). However, the same treatment resulted in 50% increase of the icorr in the case of the AZ61 alloy. However, this behavior can be also explained by the presence on a large amount of intermetallic phase particles in the microstructure of AZ61 alloy and higher real surface area after grinding process.
Corrosion current density (
icorr) and the resulting corrosion rate (
vcorr) were higher in the corrosion environment of HBSS without Mg
2+ and Ca
2+ ions reported in [
32] (
Figure 8). Comparing to the presented data the differences in the values were mostly about ten times. This effect could be caused by the presence or absence of especially Mg
2+ ions in the solution. In the case of HBSS without Mg
2+ and Ca
2+ ions could play a role the concentration gradient [
50,
51,
52]. Magnesium in the alloys reacts with the corrosion environment to produce Mg
2+ ions. In the beginning of the immersion of the samples no Mg
2+ ions are present in the solution. Thanks to the concentration gradient the Mg
2+ ions migrate from the place with high concentration place of the ions (the surface of the samples) to the place with low concentration of the ions (HBSS without Mg
2+ and Ca
2+ ions). This migration of the Mg
2+ ions supports reactions of the corrosion environment with the surface of AZ31 and AZ61 alloys. On the other hand, the concentration gradient of the enriched HBSS+ with Mg
2+ and Ca
2+ ions should be lower compared to the solution without the ions because of the primary presence of the Mg
2+ ions in the corrosion environment. The migration of the Mg
2+ ions from the surface of the alloys to the corrosion environment is not so fast (like in HBSS without Mg
2+ and Ca
2+ ions) which reduces a number of the amount of reactions of the corrosion environment with the surface of the magnesium alloys.
This theory is supported by the results presented in [
33,
34,
36]. The authors describe the effect of the chlorides and sulphate ions concentration on the corrosion resistance of magnesium alloys. With the increasing content of these ions, the
icorr values increased and
vcorr, respectively. However, when comparing
icorr values (
Figure 8) in HBSS and enriched HBSS+, this phenomenon did not occur. Enriched HBSS+ contains more chloride and sulphate ions (
Table 1) but
icorr values are lower. Corrosion behavior of magnesium alloys in corrosion environment with sulphate ions is usually measured in sodium sulphate solution [
36] where the absence of Mg
2+ ions could cause higher reactivity of the alloys.
Potentiodynamic measurements are only short-time measurements. All the measurements take approximately 10 min (5 min of stabilization time and 5 min of measurement itself). Therefore, the potentiodynamic measurements are affected by conditions at the beginning of the measurement like the concentration of specific ions in the solution, concentration gradient, etc. Moreover, if the corrosion potential (
Epit) appear, no Tafel region could be observed in the anodic branch of the polarization curves [
38].
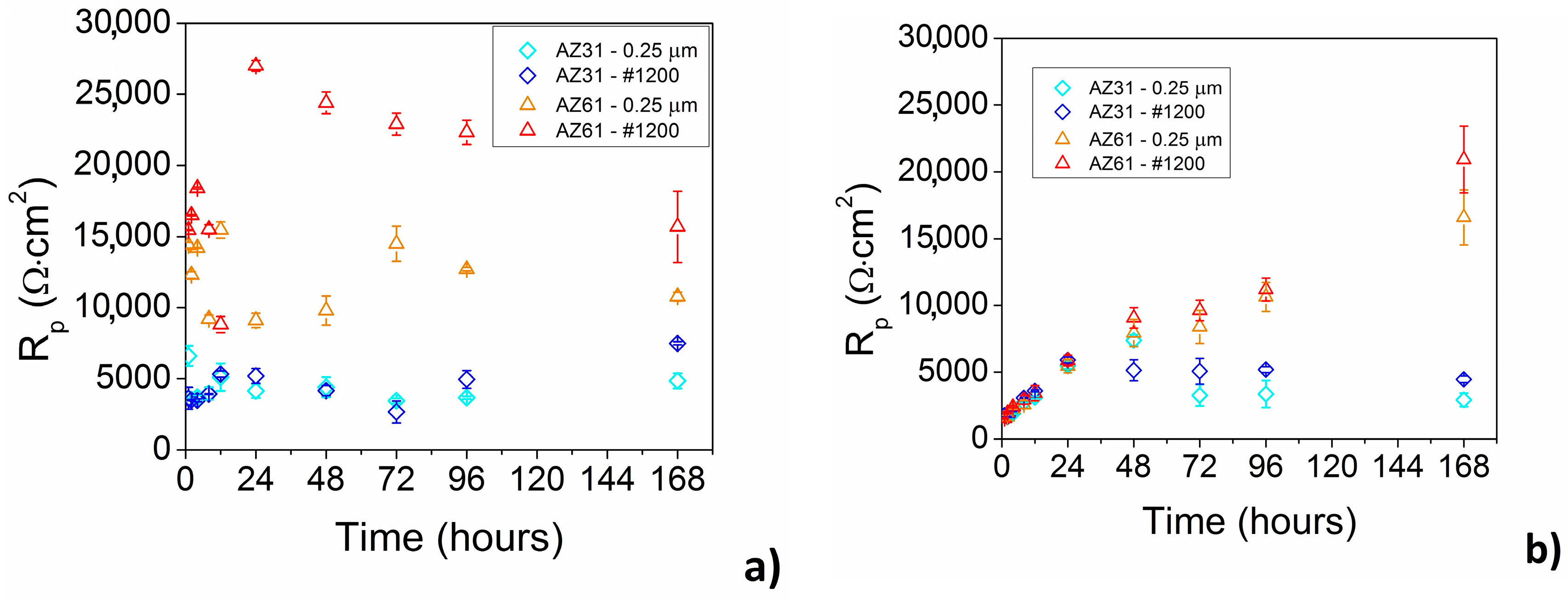
From the long-time point of view the corrosion behavior of AZ31 and AZ61 alloys can be characterized by EIS. Electrochemical corrosion behavior of the magnesium alloys in enriched HBSS+ with Mg
2+ and Ca
2+ ions represented by the evolution of polarization resistance is shown in
Figure 9a.
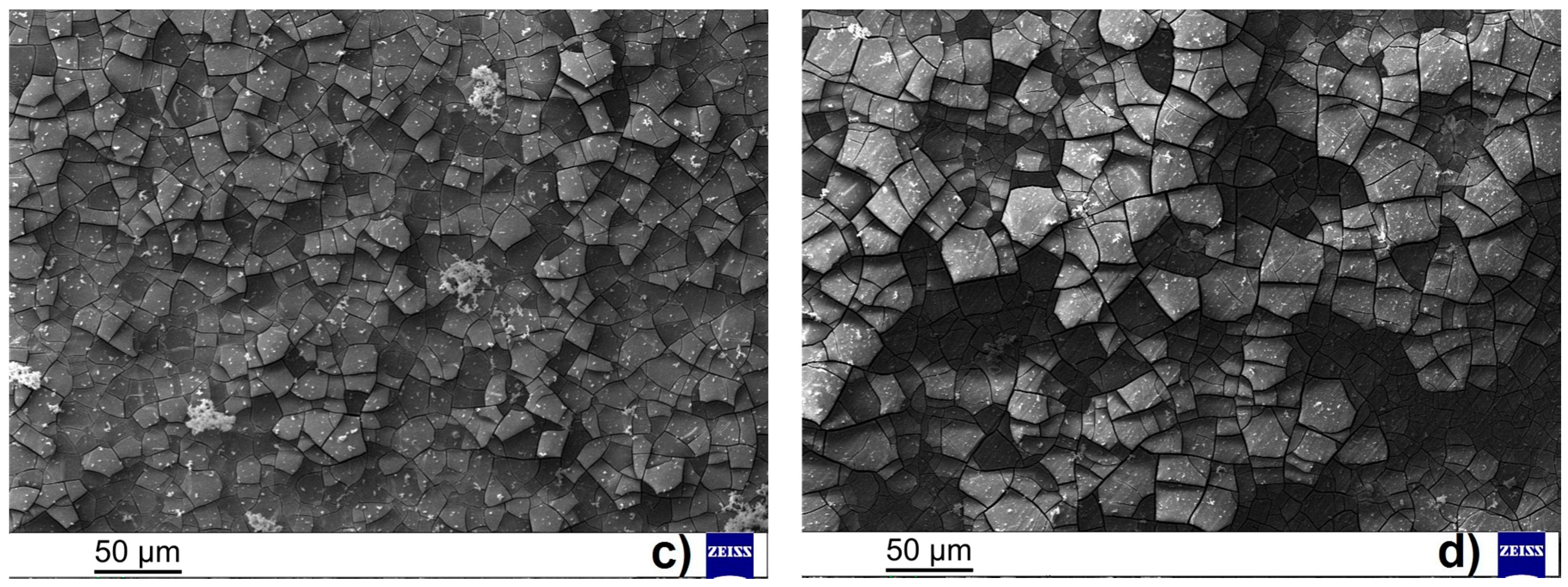
While almost no influence of the surface finish was observed in the case of AZ31 magnesium alloy, higher values of polarization resistance were observed for ground AZ61 magnesium alloy comparing to the polished material state.
In the case of AZ31 magnesium alloy the values of polarization resistance oscillated around 4000 Ω·cm
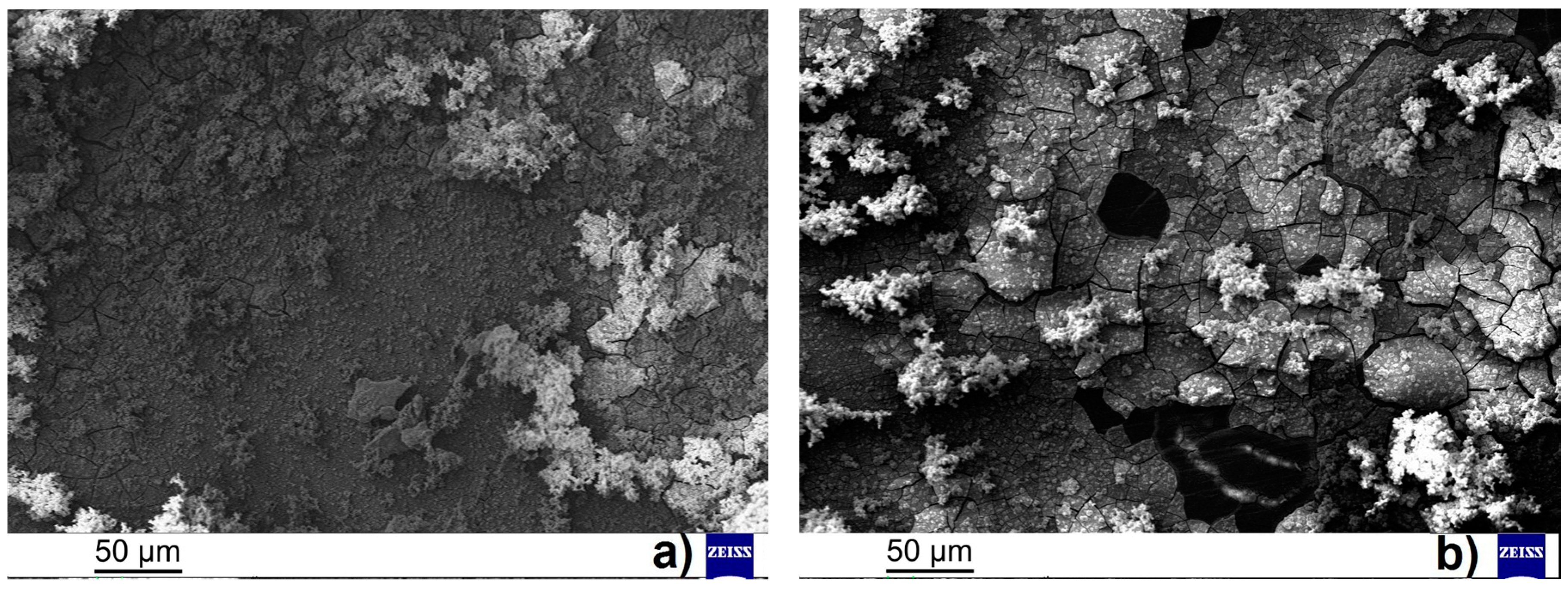
2 for all the times of measurement for both the material states. The similar resistivity of both the material states can be connected with similar corrosion products (amount and character) observed on material surfaces (
Figure 6a,b). On the surfaces of both the analyzed samples was observed the presence of a layer of magnesium phosphate (Mg
3(PO
4)
2) and hydroxiapatite (Ca
10(PO
4)
6(OH)
2) primarily created on the sample surface and covered by large number of clusters of MgO and Mg(OH)
2 products [
45,
53]. The assumption that the layer of the magnesium phosphate (Mg
3(PO
4)
2) and hydroxiapatite (Ca
10(PO
4)
6(OH)
2) is thicker (
Figure 6) in the case of ground sample correlate with slightly higher values of
Rp determined for the material state (
Table 5). However, material surface finish did not show any significant influence on AZ31 magnesium alloy corrosion resistivity in enriched HBSS+ with Mg
2+ and Ca
2+ ions from the long-time point of view.
In the case of ground AZ61 magnesium alloy an increase of
Rp up to 24 h of exposure followed by its decrease back to the value characteristic for the beginning of the experiment was observed (
Table 5 and
Figure 8a). In the case of polished AZ61 magnesium alloy a decrease of
Rp up to 24 h of exposure, it follows an increase up to 72 h of exposure followed by an additional decrease to the value lower than the resistance of material on the beginning of the exposure was observed (
Table 5 and
Figure 8). Higher values of
Rp comparing to the AZ31 magnesium alloy can be explained by material higher chemical heterogeneity and thus faster evolution of corrosion products on the material surface. On the other hand, the differences between the polarization resistance of the ground and polished AZ61 magnesium alloy can be corresponding to the higher material surface roughness. Higher real surface area of the rough ground samples can be connected by faster growth of the layer of magnesium phosphate (Mg
3(PO
4)
2) and hydroxiapatite (Ca
10(PO
4)
6(OH)
2) on sample surface comparing to the polished sample surface. This was also observed in
Figure 6c,d, where the thicker layer of corrosion products was assumed for the ground sample when compared to the polished sample. The not stable evolution of the
Rp in time can correlate with the thicker layer of corrosion products and its cracking, corrosion or remove from the samples surface, which was observed only in a minor form in the case of AZ31 magnesium alloy with the stable evolution of
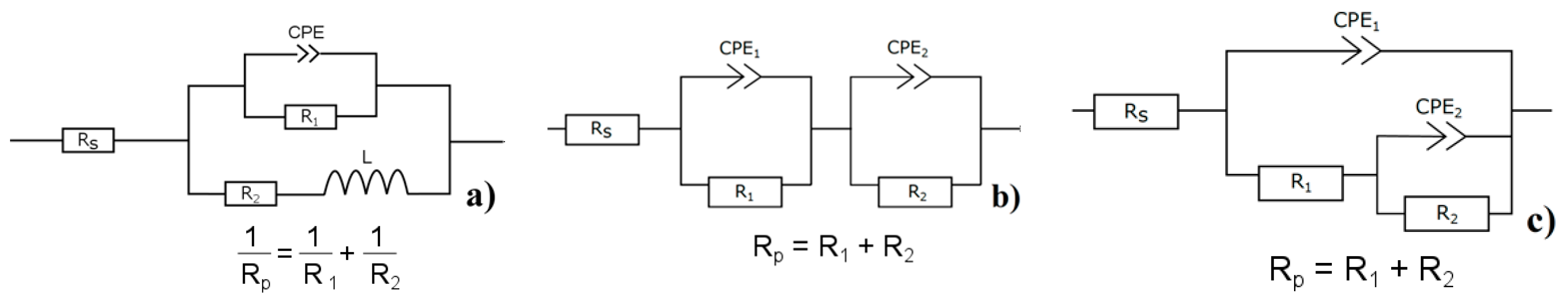
Rp in time. The presence of inductive loop L [
54,
55] in the equivalent circuit implies the occurrence of pitting corrosion on the magnesium alloy’s surface.
The values of polarization resistance Rp are quite high at the beginning of exposition of the samples in the corrosion environment. The values of Rp support the theory of Mg2+ ions concentration gradient as well as in the case of explanation for icorr change (vcorr respectively). Magnesium reacts more slowly when Mg2+ ions are present in the corrosion environments as itself (enriched HBSS+ with Mg2+ and Ca2+ ions). Helmholtz double layer created on the interface magnesium alloy/corrosion environment is more stable as in the case of HBSS without Mg2+ ions and the resistance of the material is high.
The opposite phenomenon can be observed in the case of HBSS without Mg
2+ and Ca
2+ ions. At the beginning of the exposition of the samples to this corrosion environment the
Rp values are lower (
Figure 9b) than in the case of the enriched HBSS+ with Mg
2+ and Ca
2+ ions and the value increase with increasing time of exposure to the corrosion environment.
Up to 24 h of exposure comparable values of
Rp were determined for both the alloys and for both the material states in HBSS without Mg
2+ and Ca
2+ ions (
Figure 9b). With increasing exposure time the values of
Rp slowly decreased for AZ31 magnesium alloy, while slightly higher values of
Rp were determined for the ground samples. This observation corresponds to the smaller number of cracks in the layer of corrosion products present on the surface of ground samples comparing to the polished samples (
Figure 7a,b). An increase of
Rp above 24 h of exposure to the corrosion environment was observed for AZ61 magnesium alloy, while the highest values were determined for 168 h of exposure to the HBSS without Mg
2+ and Ca
2+ ions (
Figure 9b). Same as in the case of the AZ31 alloy, also in the case of AZ61 alloy slightly higher values of R
p were determined for the ground samples.
Figure 7c,d showing the surface of the samples after the EIS measurements can exhibit the explanation for this material behavior. While the smaller number of finer cracks were observed on the surface of the layer of corrosion products created on the ground sample compared to the deep and large cracks observed on the surface of the polished sample.
In the both corrosion environments, the influence of corrosion products must be taken into account when predicting material corrosion behavior, because magnesium and its alloys are very reactive by themselves. The corrosion layer behaves as a barrier against the further access of corrosion environment to the surface of the magnesium alloys. The layers of the corrosion products are composed mainly of the oxides, hydroxides, phosphates and chlorides [
45]. However, the layer is porous and uncompacted (cracks are present in
Figure 6 and
Figure 7) which leads to other reactions in the corrosion environments of the samples. Even though, different corrosion products are created on the surface of AZ31 and AZ61 alloys due to the chemical composition of the used corrosion solution, more stable material behavior was observed for the AZ31 magnesium alloy samples in both of the solutions. AZ61 magnesium alloy exhibited more stable corrosion response in the HBSS without Mg
2+ and Ca
2+ ions [
32] comparing to its behavior in enriched HBSS+ with Mg
2+ and Ca
2+ ions.