Preparation of Chitosan/Poly‐γ‐Glutamic Acid Polyelectrolyte Multilayers on Biomedical Metals for Local Antibiotic Delivery
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparing of Coating
2.3. Characterization
2.4. Biocompatibility
2.5. Antibiotic Release and Antibacterial Activity
2.6. Statistical Analysis
3. Results and Discussion
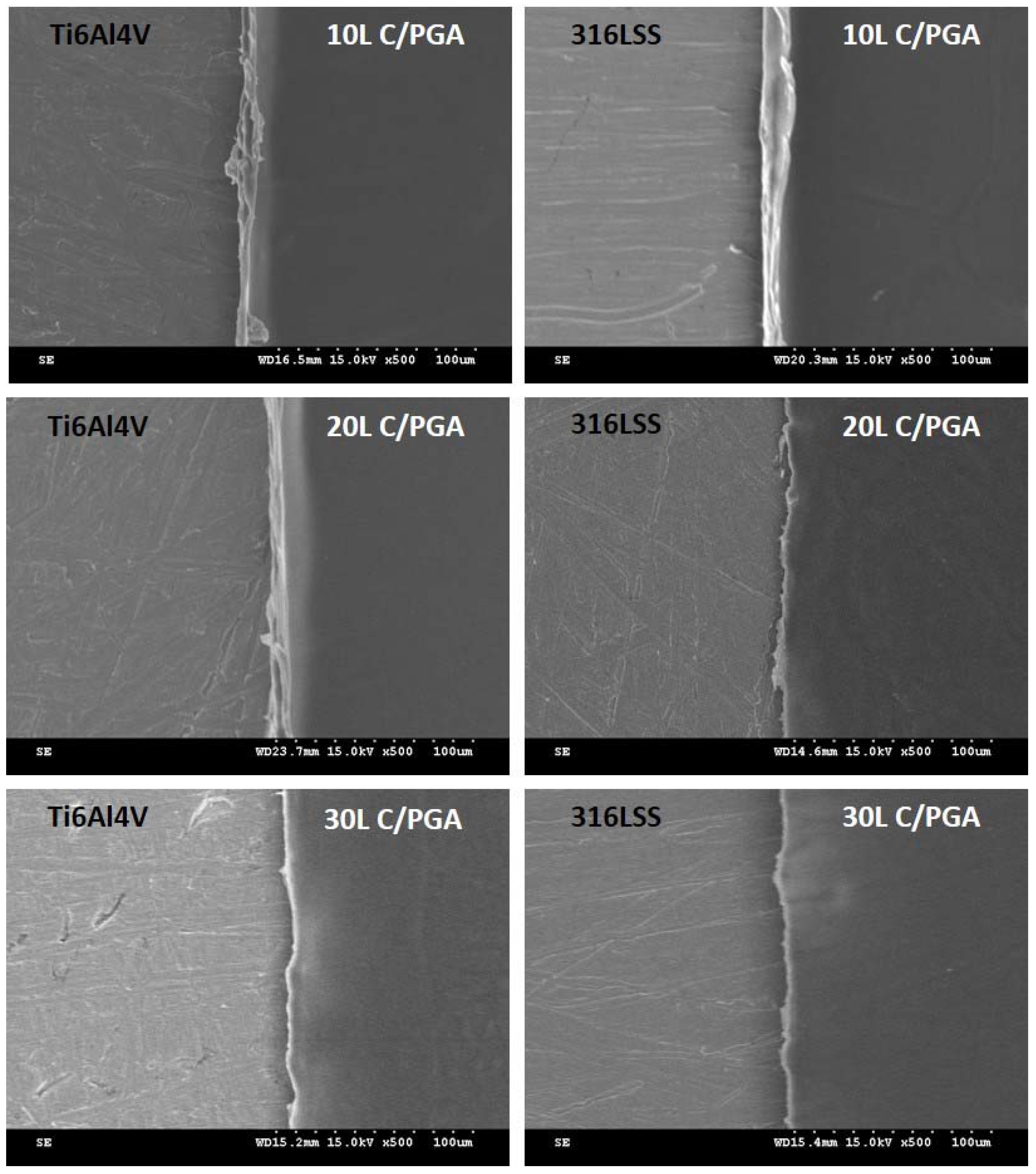
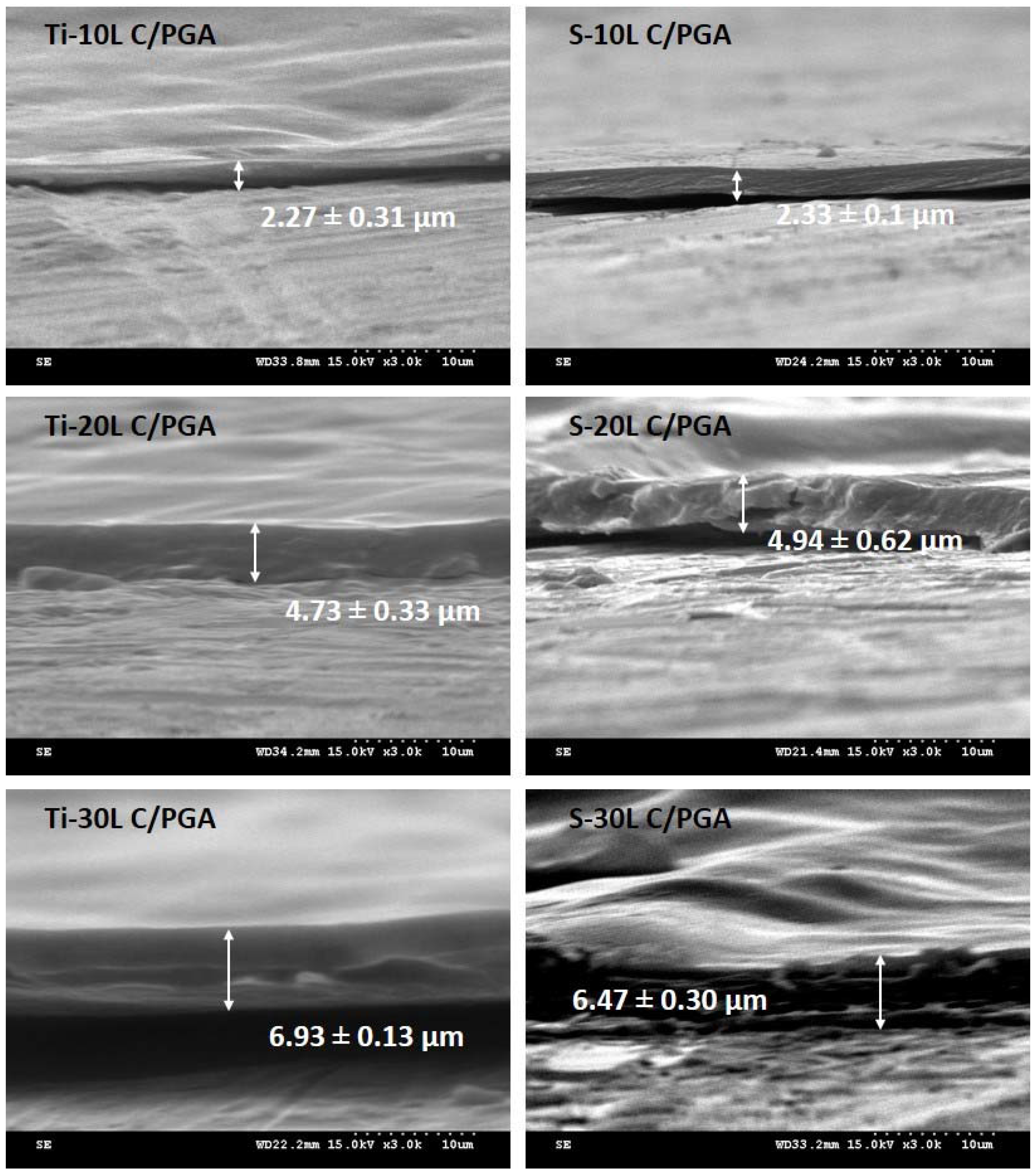
3.1. C/PGA Coating Morphology and Thickness
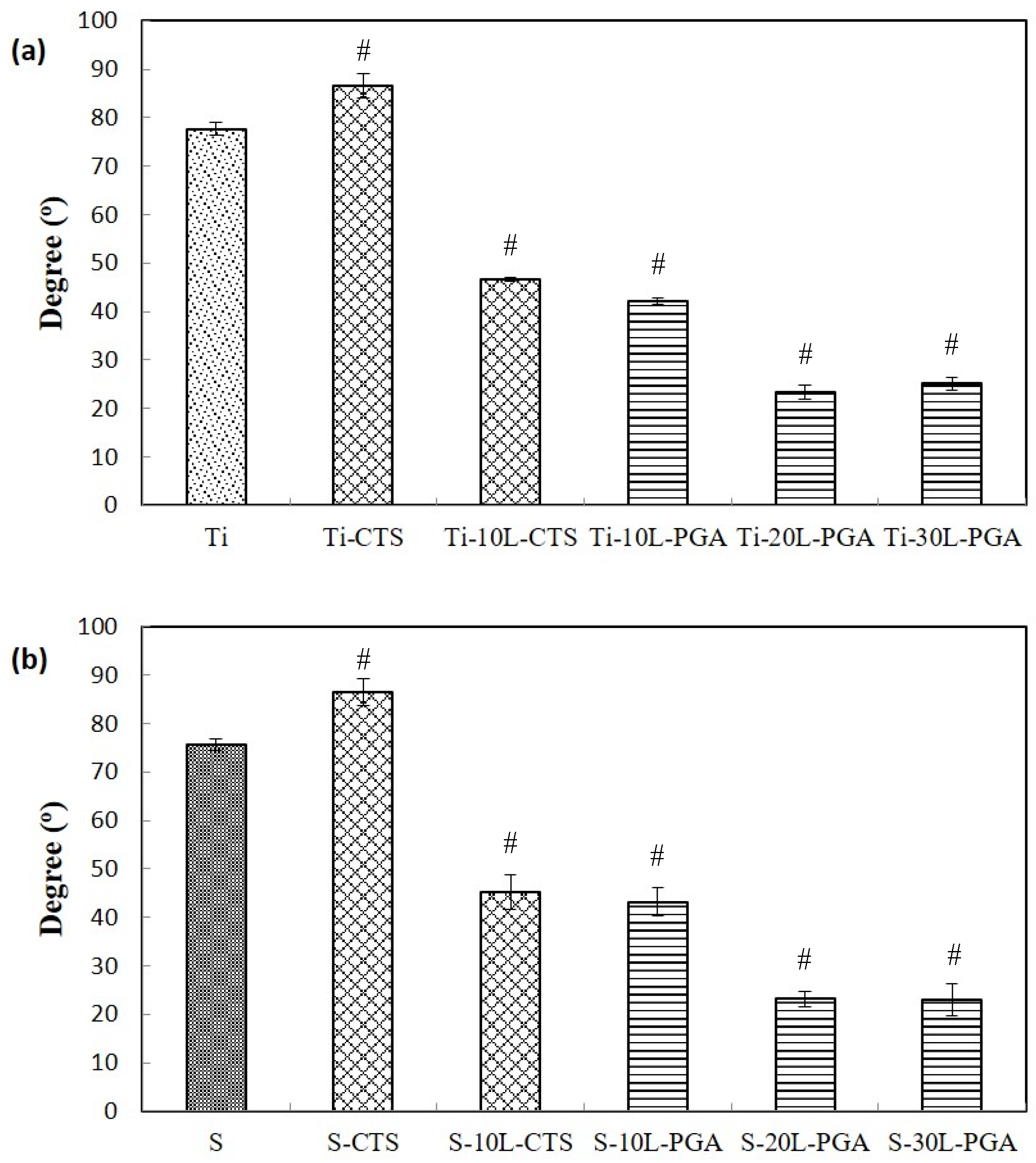
3.2. Water Contact Angle Measurement
3.3. Mechanical Properties
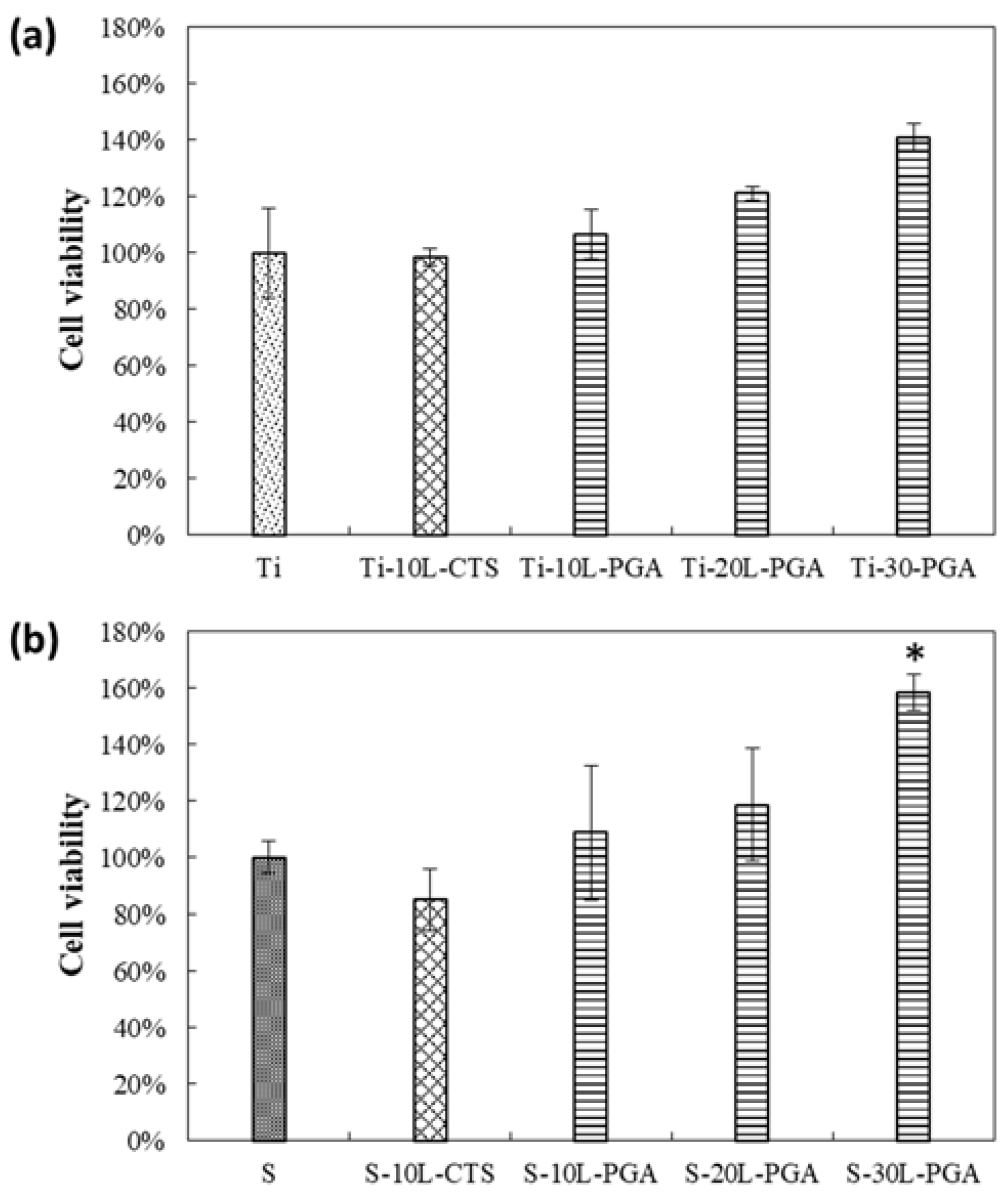
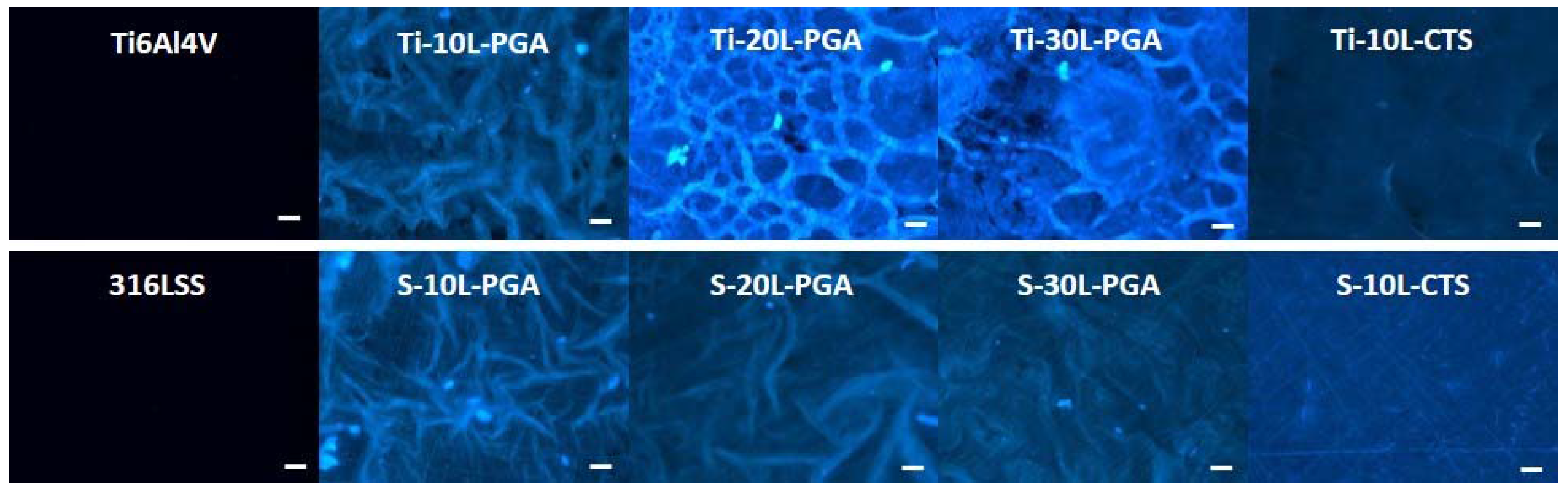
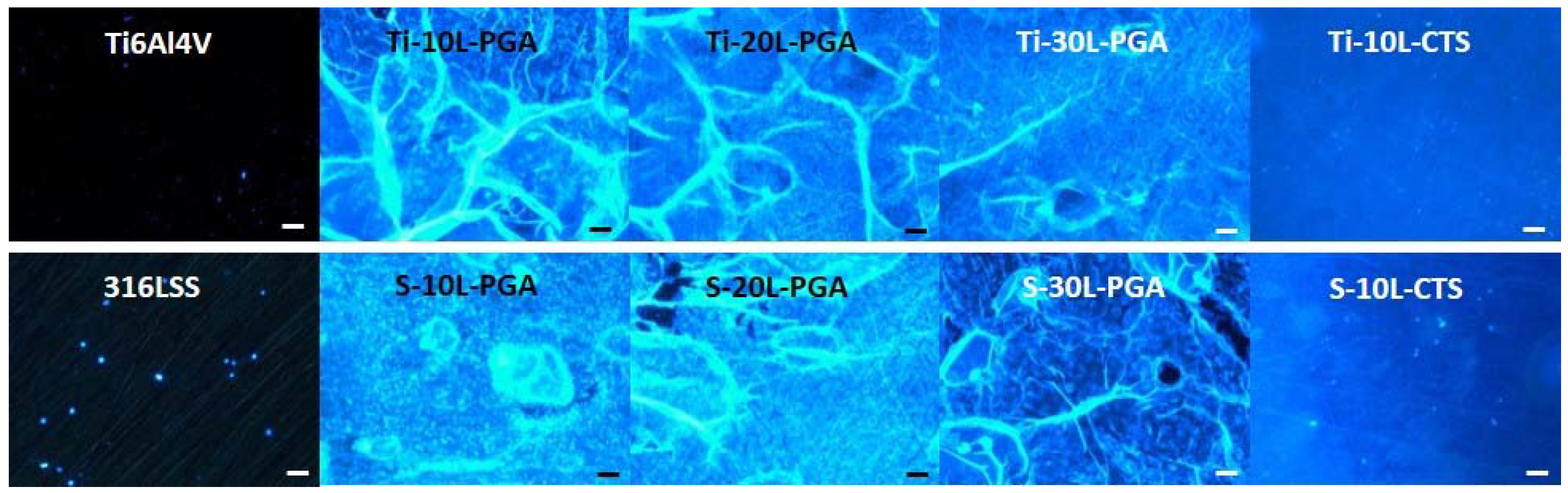
3.4. Biocompatibility
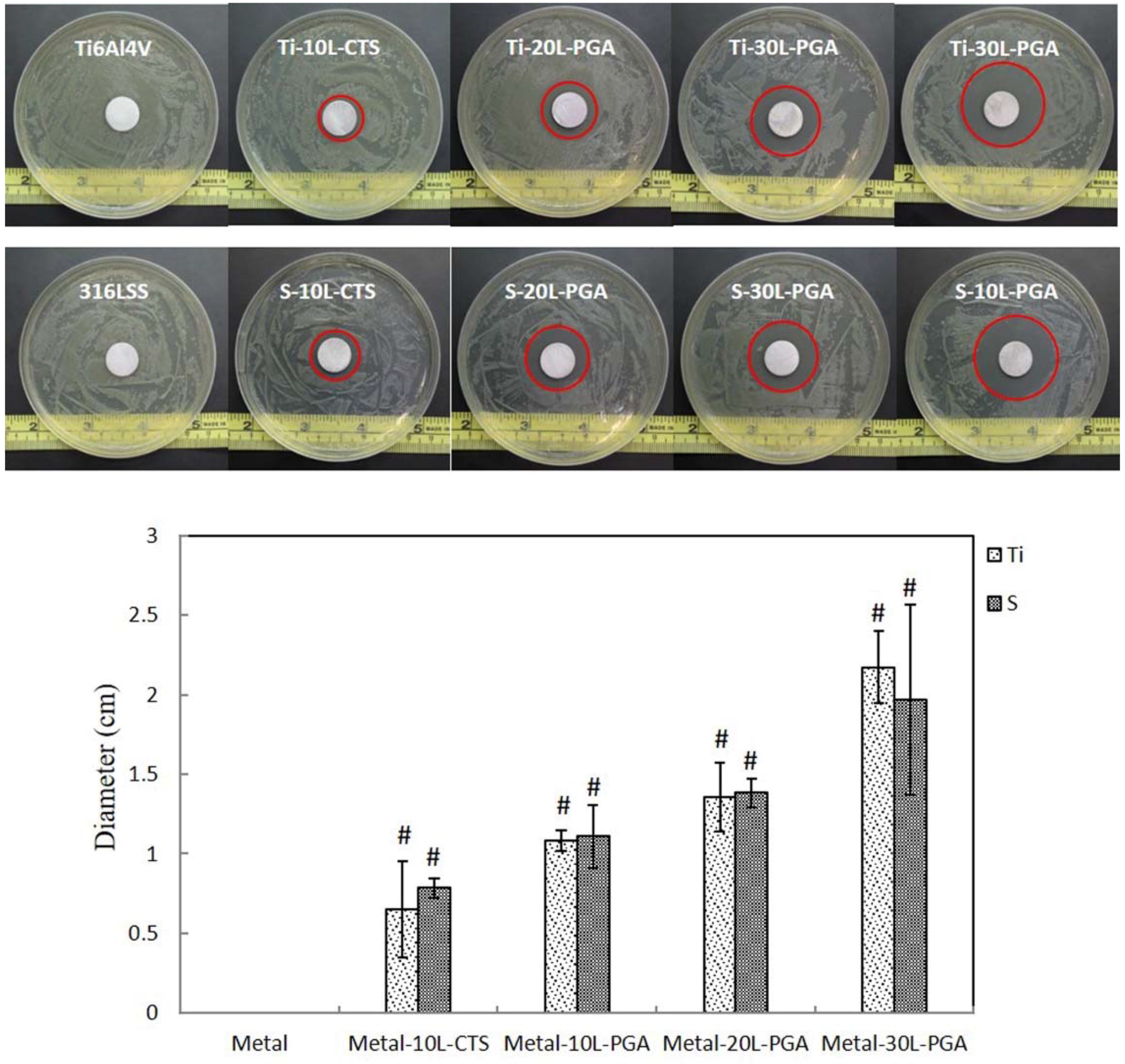
3.5. Tetracycline Release and Antibacterial Activity
4. Conclusion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Guduru, D.; Niepel, M.; Vogel, J.; Groth, T. Nanostructured material surfaces--preparation, effect on cellular behavior, and potential biomedical applications: A review. Int. J. Artif. Organs 2011, 34, 963–985. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; Mano, J. Polyelectrolyte multilayered assemblies in biomedical technologies. Chem. Soc. Rev. 2014, 43, 3453–3479. [Google Scholar] [CrossRef] [PubMed]
- Iler, R. Multilayers of colloidal particles. J. Colloid Interface Sci. 1966, 21, 569–594. [Google Scholar] [CrossRef]
- Koylu, D.; Thapa, M.; Gumbley, P.; Thomas, S.W. Photochemical disruption of polyelectrolyte multilayers. Adv. Mater. 2012, 24, 1451–1454. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Xu, S.; Wang, J.; Liu, Y.; Chen, P.; Feng, S. Controlled loading and release of methylene blue in layer-by-layer assembled polyelectrolyte films. Mater. Sci Eng. C Mater. Biol. Appl. 2012, 32, 670–673. [Google Scholar] [CrossRef]
- De Paiva, R.; de Moraes, M.; de Godoi, F.; Beppu, M. Multilayer biopolymer membranes containing copper for antibacterial applications. J. Appl. Polym. Sci. 2012, 126, E17–E24. [Google Scholar] [CrossRef]
- Hizal, F.; Zhuk, I.; Sukhishvili, S.; Busscher, H.; van der Mei, H.; Choi, C. Impact of 3D Hierarchical Nanostructures on the Antibacterial Efficacy of a Bacteria-Triggered Self-Defensive Antibiotic Coating. ACS Appl. Mater. Interfaces 2015, 7, 20304–20313. [Google Scholar] [CrossRef] [PubMed]
- Monge, C.; Almodóvar, J.; Boudou, T.; Picart, C. Spatio-Temporal Control of LbL Films for Biomedical Applications: From 2D to 3D. Adv. Healthc. Mater. 2015, 4, 811–830. [Google Scholar] [CrossRef] [PubMed]
- Herron, M.; Schurr, M.; Murphy, C.; McAnulty, J.; Czuprynski, C.; Abbott, N. Interfacial Stacks of Polymeric Nanofilms on Soft Biological Surfaces that Release Multiple Agents. ACS Appl. Mater. Interfaces 2016, 8, 26541–26551. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Zhan, W.; Cao, L.; Hu, C.; Qu, Y.; Yu, Q.; Chen, H. Multifunctional and Regenerable Antibacterial Surfaces Fabricated by a Universal Strategy. ACS Appl. Mater. Interfaces 2016, 8, 30048–30057. [Google Scholar] [CrossRef] [PubMed]
- Decher, G. Fuzzy Nanoassemblies: Toward Layered Polymeric Multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Moskowitz, J.; Blaisse, M.; Samuel, R.; Hsu, H.; Harris, M.; Martin, S.; Lee, J.; Spector, M.; Hammond, P. The effectiveness of the controlled release of gentamicin from polyelectrolyte multilayers in the treatment of Staphylococcus aureus infection in a rabbit bone model. Biomaterials 2010, 31, 6019–6030. [Google Scholar] [CrossRef] [PubMed]
- Zankovych, S.; Diefenbeck, M.; Bossert, J.; Mückley, T.; Schrader, C.; Schmidt, J.; Schubert, H.; Bischoff, S.; Faucon, M.; Finger, U.; et al. The effect of polyelectrolyte multilayer coated titanium alloy surfaces on implant anchorage in rats. Acta Biomater. 2013, 9, 4926–4934. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and Chitosan: Properties and Applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Lin, Q.; Ren, K.; Ji, J. Hyaluronic acid and chitosan-DNA complex multilayered thin film as surface-mediated nonviral gene delivery system. Colloids Surf. B Biointerfaces 2009, 74, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, C.; Liu, C.; Meng, X.; Lee, C.; Park, H. Preparation and biocompatibility of chitosan microcarriers as biomaterial. Biochem. Eng. J. 2006, 27, 269–274. [Google Scholar] [CrossRef]
- Sung, M.; Park, C.; Kim, C.; Poo, H.; Soda, K.; Ashiuchi, M. Natural and edible biopolymer poly-gamma-glutamic acid: Synthesis, production, and applications. Chem. Rec. 2005, 5, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Guo, Y.; Liu, J.; Qiu, H.; Zhao, M.; Zou, W.; Li, S. Microbial synthesis of poly-γ-glutamic acid: Current progress, challenges, and future perspectives. Biotechnol. Biofuels 2016, 9, 134. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.; Ho, T.; Hsieh, K.; Su, Y.; Lin, P.; Yang, J.; Yang, K.; Yang, S. γ-Polyglutamic Acid Produced by Bacillus Subtilis (Natto): Structural Characteristics, Chemical Properties and Biological Functionalities. J. Chin. Chem. Soc. 2006, 53, 1363–1384. [Google Scholar] [CrossRef]
- Paul, N.; Paul, A.; Steitz, R.; Kreuzer, M.; Lux-Steiner, M. Selective self assembly of glutamate molecules on polyelectrolyte multilayers. J. Phys. Chem. B 2012, 116, 4492–4499. [Google Scholar] [CrossRef] [PubMed]
- Ogunleye, A.; Bhat, A.; Irorere, V.; Hill, D.; Williams, C.; Radecka, I. Poly-γ-glutamic acid: Production, properties and applications. Microbiology 2015, 161, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Philippova, O.; Korchagina, E. Chitosan and its hydrophobic derivatives: Preparation and aggregation in dilute aqueous solutions. Polym. Sci. Ser. A 2012, 54, 552–572. [Google Scholar] [CrossRef]
- Kasemo, B. Biological surface science. Surf. Sci. 2002, 500, 656–677. [Google Scholar] [CrossRef]
- Discher, D.; Janmey, P.; Wang, Y. Tissue cells feel and respond to the stiffness of their substrate. Science 2002, 310, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Zysset, P.; Guo, X.; Hoffler, C.; Moore, K.; Goldstein, S. Elastic modulus and hardness of cortical and trabecular bone lamellae measured by nanoindentation in the human femur. J. Biomech. 1999, 32, 1005–1012. [Google Scholar] [CrossRef]
- Mathieu, V.; Vayron, R.; Richard, G.; Lambert, G.; Naili, S.; Meningaud, J.; Haiat, G. Biomechanical determinants of the stability of dental implants: influence of the bone-implant interface properties. J. Biomech. 2014, 47, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Chesnutt, B.; Wright, L.; Haggard, W.; Bumgardner, J. Mechanical property, degradation rate, and bone cell growth of chitosan coated titanium influenced by degree of deacetylation of chitosan. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 86, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Renoud, P.; Toury, B.; Benayoun, S.; Attik, G.; Grosgogeat, B. Functionalization of titanium with chitosan via silanation: Evaluation of biological and mechanical performances. PLoS ONE 2012, 7, e39367. [Google Scholar] [CrossRef] [PubMed]
- Fang, K.; Song, W.; Wang, L.; Jia, S.; Wei, H.; Ren, S.; Xu, X.; Song, Y. Immobilization of chitosan film containing semaphorin 3A onto a microarc oxidized titanium implant surface via silane reaction to improve MG63 osteogenic differentiation. Int. J. Nanomed. 2014, 9, 4649–4657. [Google Scholar]
- Yan, S.; Zhang, K.; Liu, Z.; Zhang, X.; Gan, L.; Cao, B.; Chen, X.; Cui, L.; Yin, J. Fabrication of poly(l-glutamic acid)/chitosan polyelectrolyte complex porous scaffolds for tissue engineering. J. Mater. Chem. B 2013, 1, 1541–1551. [Google Scholar] [CrossRef]
- Song, Z.; Yin, J.; Luo, K.; Zheng, Y.; Yang, Y.; Li, Q.; Yan, S.; Chen, X. Layer-by-layer buildup of poly(l-glutamic acid)/chitosan film for biologically active coating. Macromol. Biosci. 2009, 9, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yang, J.; Tsai, T.; Ji, D.; Chang, W.; Chen, C.; Lee, S. Development of a chitosan–polyglutamate based injectable polyelectrolyte complex scaffold. Carbohydr. Polym. 2011, 85, 318–324. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, Y.; Yan, S.; Liu, Z.; He, S.; Cui, L.; Yin, J. Poly(l-glutamic acid)/chitosan polyelectrolyte complex porous microspheres as cell microcarriers for cartilage regeneration. Acta Biomater. 2014, 10, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Jaafar, I.; LeBlon, C.; Wei, M.; Ou-Yang, D.; Coulter, J.; Jedlicka, S. Improving fluorescence imaging of biological cells on biomedical polymers. Acta Biomater. 2011, 7, 1588–1598. [Google Scholar] [CrossRef] [PubMed]
- Rabea, E.; Badawy, M.; Stevens, C.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]


| Group | Reduced Young’s Modulus (GPa) | Hardness (MPa) |
|---|---|---|
| Ti6Al4V (Ti) | 39.5 ± 5.5 | 4610 ± 39 |
| Ti-10L-CTS | 4.5 ± 0.2 | 234 ± 4 |
| Ti-10L-PGA | 3.6 ± 0.2 | 203 ± 4 |
| Ti-20L-PGA | 3.9 ± 0.2 | 201 ± 4 |
| Ti-30L-PGA | 3.9 ± 0.2 | 224 ± 4 |
| 316LSS (S) | 53.9 ± 3.6 | 3113 ± 54 |
| S-10L-CTS | 4.7 ± 0.2 | 244 ± 5 |
| S-10L-PGA | 3.0 ±0.1 | 215 ± 3 |
| S-20L-PGA | 3.4 ± 0.1 | 244 ± 3 |
| S-30L-PGA | 3.9 ± 0.1 | 238 ± 4 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.-C.; Wang, H.-Y.; Wang, A.-N.; Tseng, C.-H.; Liu, H.-L.; Chung, R.-J. Preparation of Chitosan/Poly‐γ‐Glutamic Acid Polyelectrolyte Multilayers on Biomedical Metals for Local Antibiotic Delivery. Metals 2017, 7, 418. https://doi.org/10.3390/met7100418
Liu W-C, Wang H-Y, Wang A-N, Tseng C-H, Liu H-L, Chung R-J. Preparation of Chitosan/Poly‐γ‐Glutamic Acid Polyelectrolyte Multilayers on Biomedical Metals for Local Antibiotic Delivery. Metals. 2017; 7(10):418. https://doi.org/10.3390/met7100418
Chicago/Turabian StyleLiu, Wai-Ching, Huey-Yuan Wang, An-Ni Wang, Chih-Hsien Tseng, Hsuan-Liang Liu, and Ren-Jei Chung. 2017. "Preparation of Chitosan/Poly‐γ‐Glutamic Acid Polyelectrolyte Multilayers on Biomedical Metals for Local Antibiotic Delivery" Metals 7, no. 10: 418. https://doi.org/10.3390/met7100418





