Effect of Rare-Earth Ce on Macrosegregation in Al-Bi Immiscible Alloys
Abstract
:1. Introduction
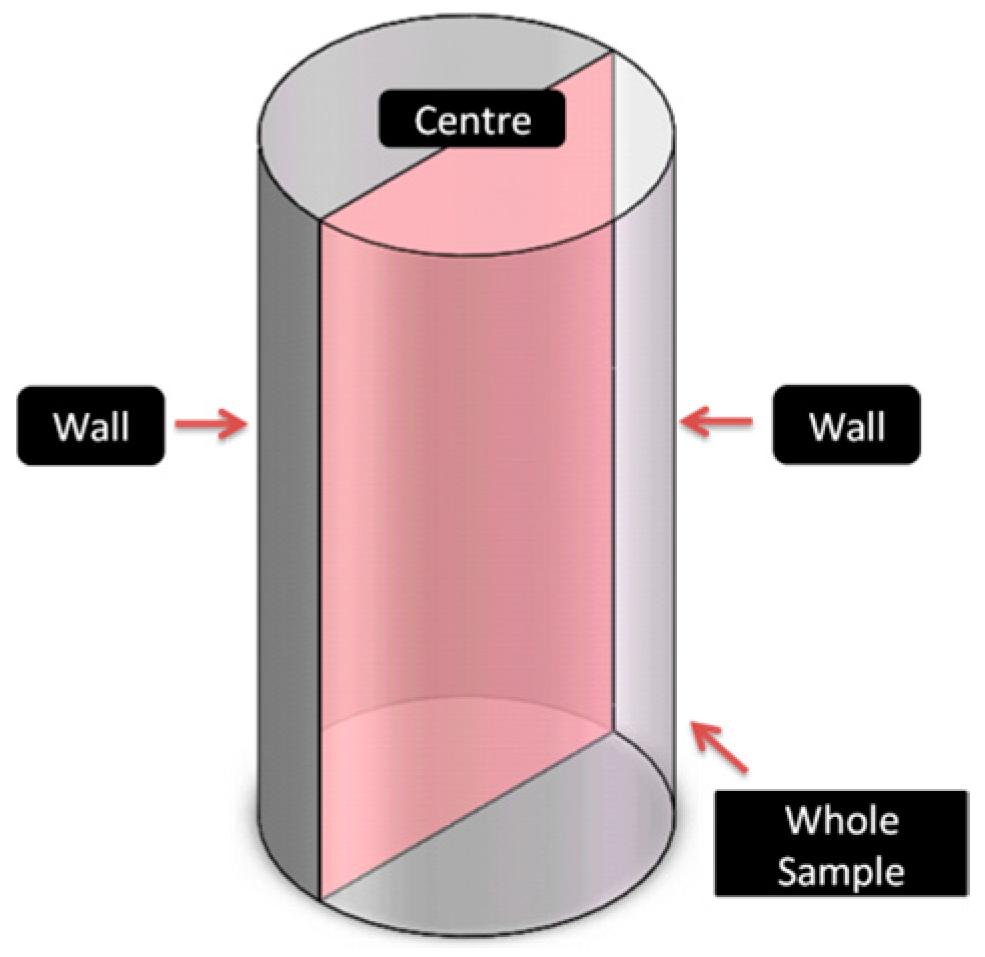
2. Experimental Procedure
3. Results and Discussion
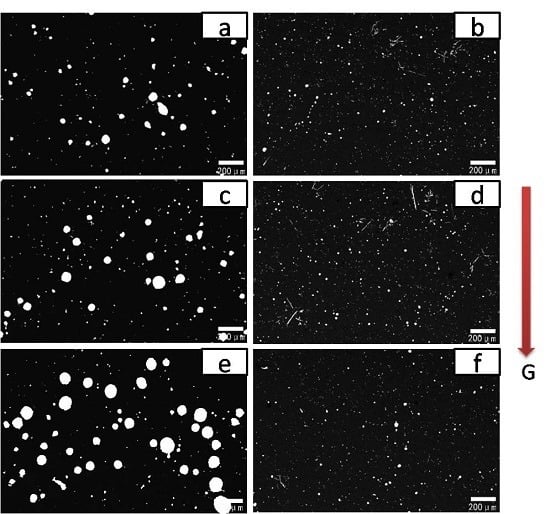
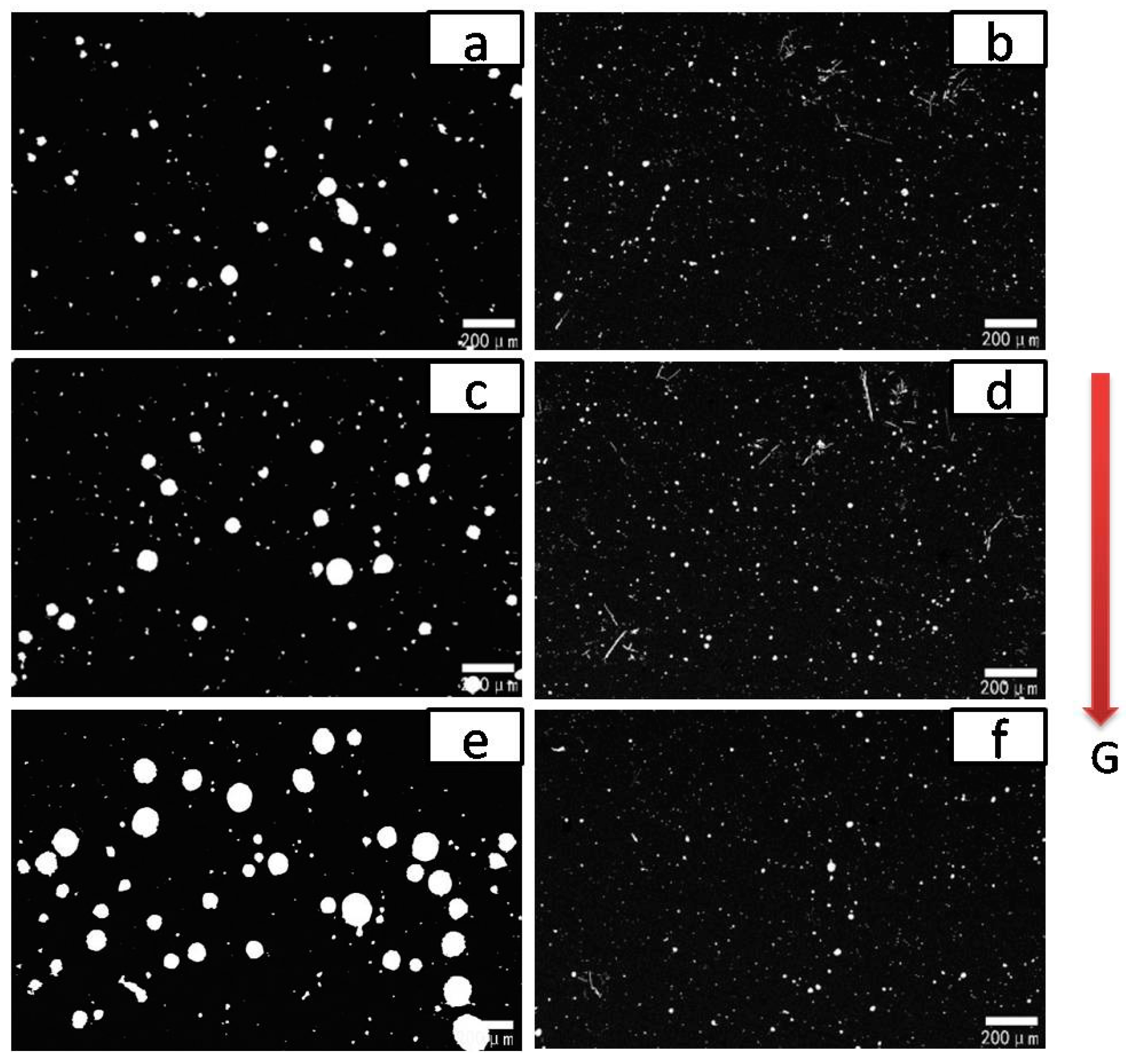
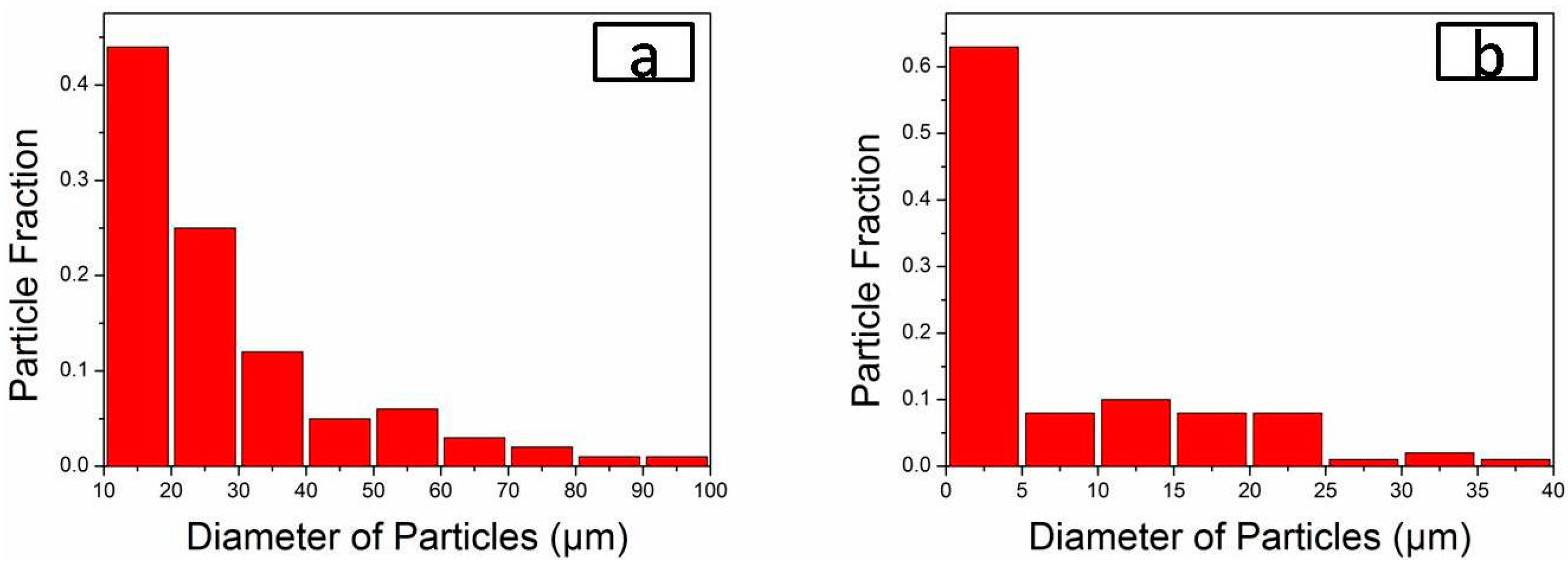
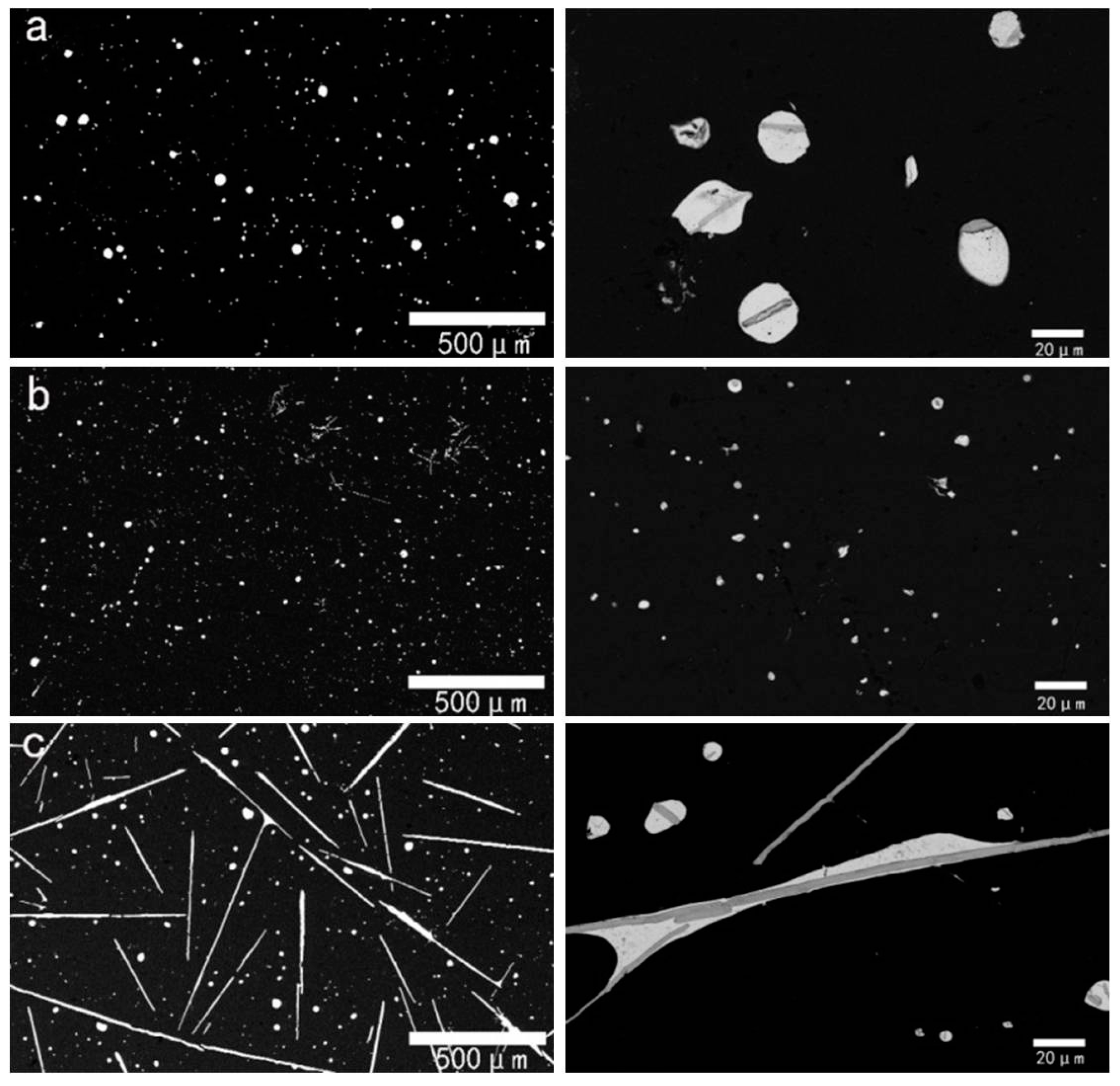
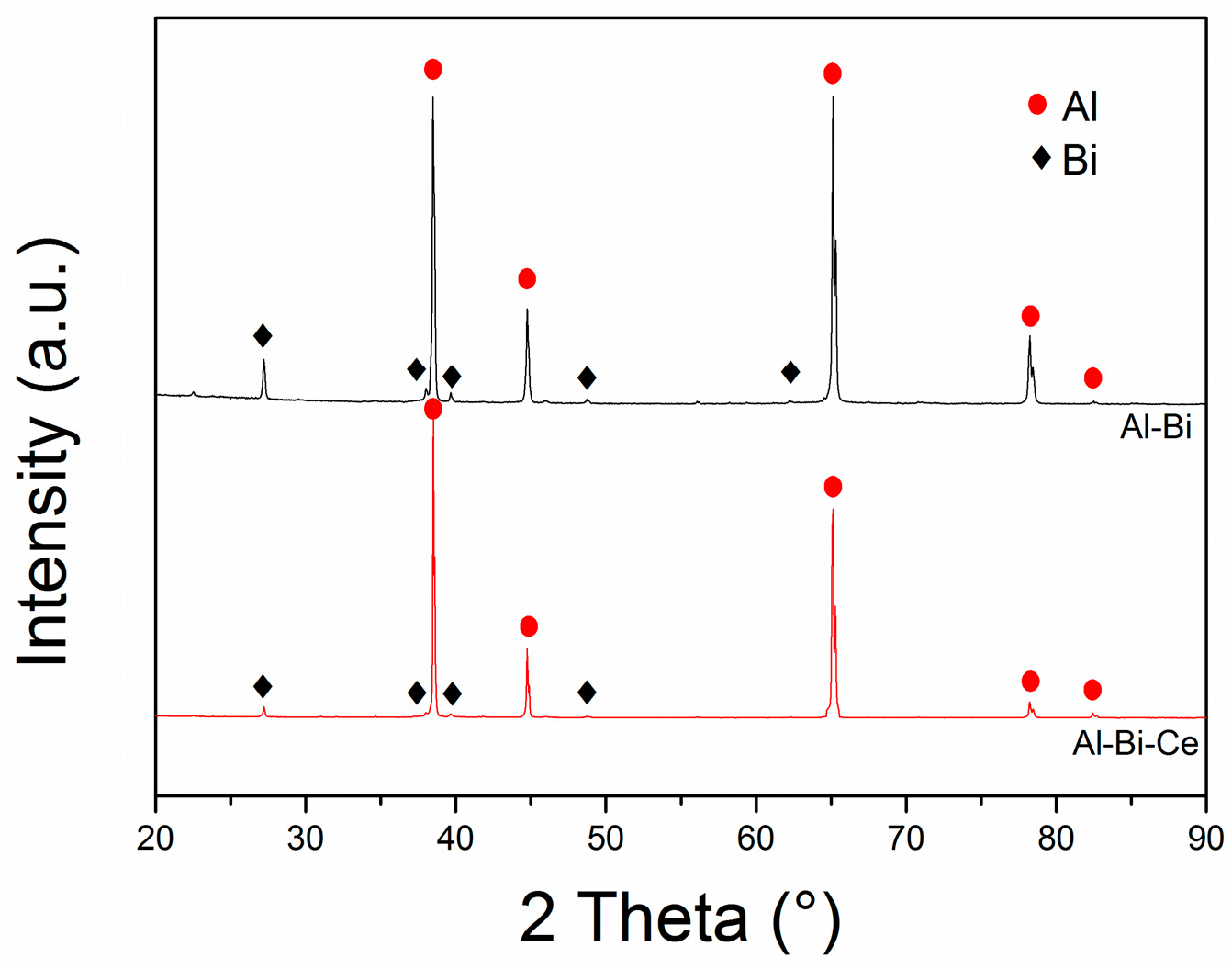
3.1. Effect of Rare-Earth Ce on the Alloy Microstructure
3.2. Effect of Rare-Earth Ce Content on the Alloy Microstructure
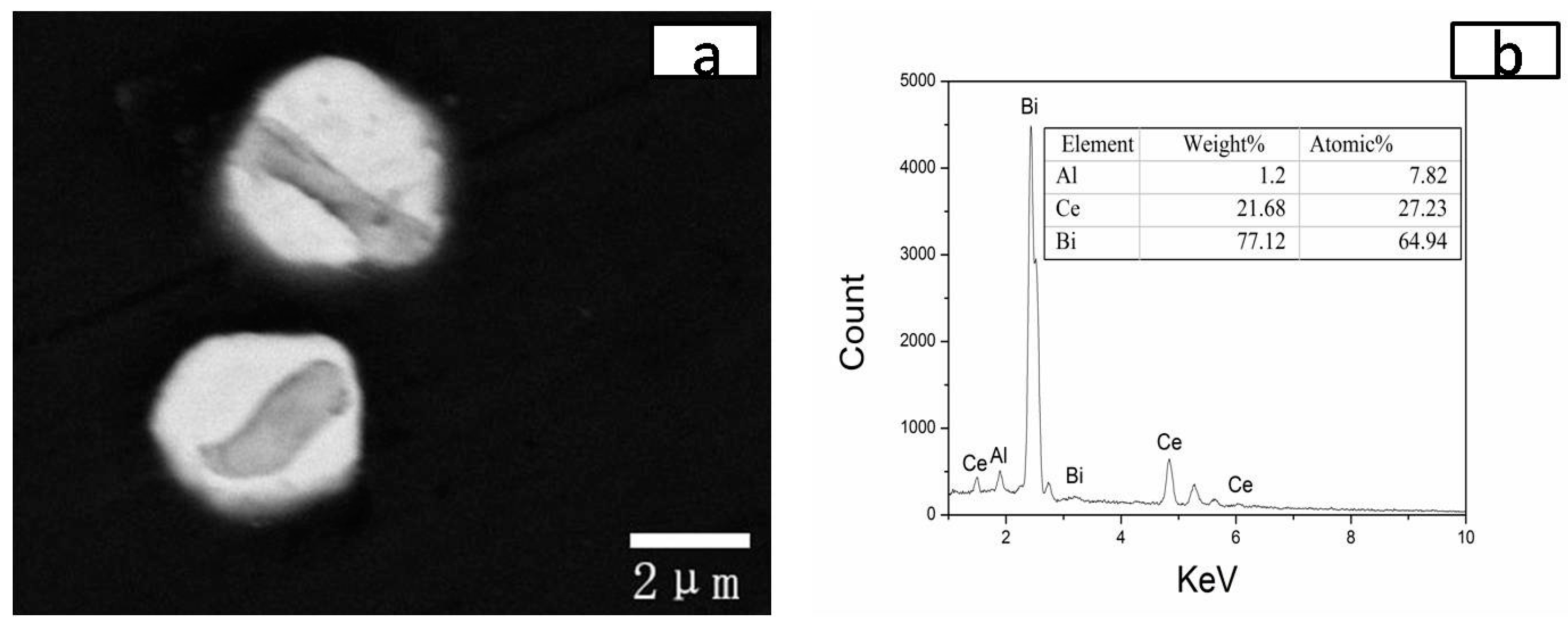
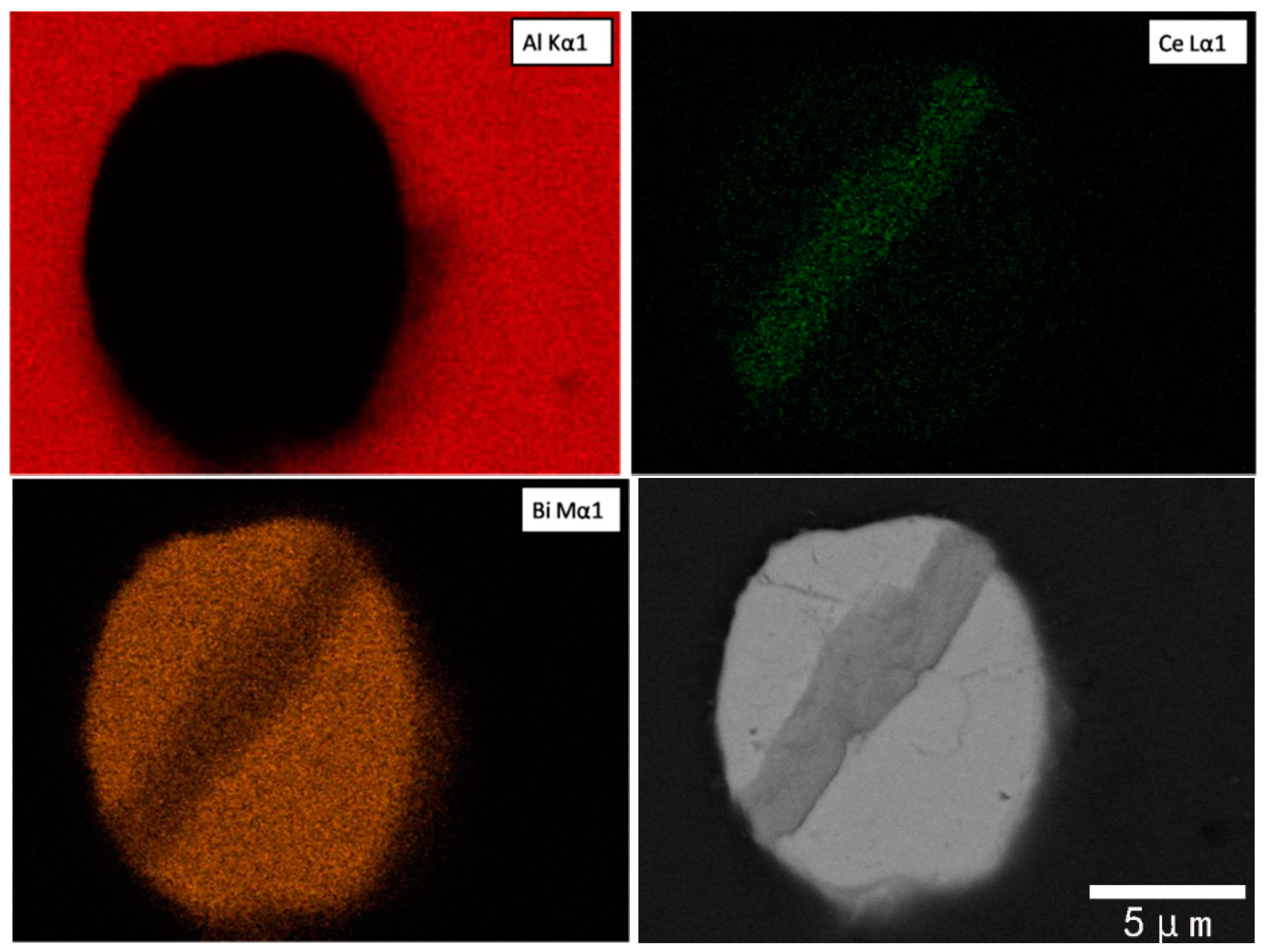
3.3. Al-Bi-Ce in Situ Compound
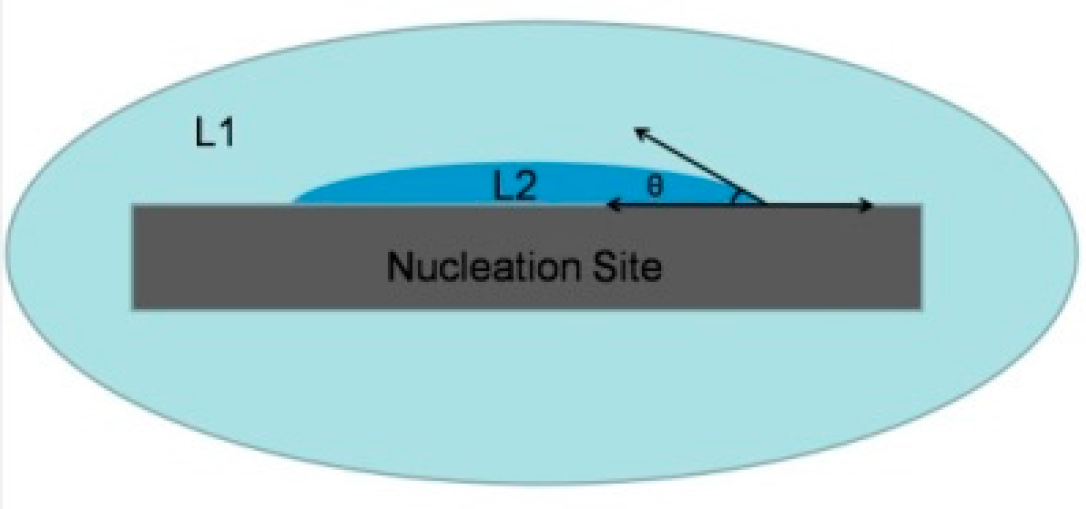
3.4. Contact Angle of CeBi2
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Walter, H.U. Binary systens with misciblility gap in the liquid state. In Materials Science in Space; Feuerbacher, B., Hamacher, H., Naumann, R.J., Eds.; Sprinverlag Press: Newyork, NY, USA, 1986; pp. 343–378. [Google Scholar]
- Inoue, A.; Yano, N. Microstructure and superconducting properties of melt quenched insoluble Al-Pb and Al-Pb-Bi alloys. J. Mater. Sci. 1987, 22, 123–127. [Google Scholar] [CrossRef]
- Ratke, L.; Diefenbach, S. Liquid immiscible alloys. Mater. Sci. Eng: R: Reports 1995, 15, 263–347. [Google Scholar] [CrossRef]
- Heaby, R.B.; Cahn, J.W. Experimental test of classical nucleation theory in a liquid–liquid miscibility gap system. J. Chem. Phys. 1973, 58, 896–910. [Google Scholar]
- Gránásy, L.; Ratke, L. Homogeneous nucleation within the liquid miscibility gap of Zn-Pb alloys. Scr. Metall. Mater. 1993, 28, 1329–1334. [Google Scholar]
- Uebber, N.; Ratke, L. Homogeneous nucleation within the liquid miscibility gap of Zn-Pb alloys. Scr. Metall. Mater. 1991, 25, 1133–1137. [Google Scholar]
- Sundquist, B.E.; Oriani, R.A. Homegeneous nucleation in a miscibility gap system: A Critical Test of Nucleation Theory. J. Chem. Phys. 1962, 36, 2604–2615. [Google Scholar] [CrossRef]
- Ikeda, T.; Nishi, S. On the structure of Al-Pb alloys solidified by rapid cooling. J. Jpn. Inst. Metal. Mater. 1982, 46, 645–651. [Google Scholar]
- Battezzati, L.; Curiotto, S.; Johnson, E.; Pryds, N. Undercooling and demixing in rapidly solidified Cu-Co alloys. Mater. Sci. Eng. A 2007, 449, 7–11. [Google Scholar] [CrossRef]
- Wang, J.L.; Ding, F.; Shen, W.F.; Li, T.X.; Wang, G.H.; Zhao, J.J. Thermal behavior of Cu-Co bimetallic clusters. Sol. State Commun. 2001, 119, 13–18. [Google Scholar] [CrossRef]
- Derby, B.; Favier, J.J. A criterion for the determination of monotectic structrue. Acta Metall. 1983, 31, 1123–1130. [Google Scholar] [CrossRef]
- Majumdar, B.; Chattopadhyay, K. Aligned monotectic growth in unidirectionally solidified Zn-Bi alloys. Metall. Mater. Trans. A 2000, 31, 1833–1842. [Google Scholar] [CrossRef]
- Jun, J.; Zhao, Z.J.; Zhang, J.H. The solidification of immiscible alloy in erthogonal electromagnetic field. Chin. J. Mech.Eng. 1991, 4, 90–94. [Google Scholar]
- Zheng, T.X.; Zhong, Y.B.; Lei, Z.S.; Ren, W.L.; Ren, Z.M.; Debray, F.; Beaugnon, E.; Fautrelle, Y. Effects of high static magnetic field on distribution of solid particles in BiZn immiscible alloys with metastable miscibility gap. J. Alloy. Compd. 2015, 623, 36–41. [Google Scholar] [CrossRef]
- Fang, F.; Zhu, M.; Deng, H.Q.; Shu, X.L.; Hu, X.Y. Self-diffusion Al and Bi atom in Al-Pb immiscible alloys system. Mater. Sci. Eng. B 2004, 108, 253–257. [Google Scholar] [CrossRef]
- Zhao, J.Z.; Li, H.L.; Li, H.Q.; Xing, C.Y.; Zhang, X.F.; Wang, Q.L.; He, J. Microstructure formation in centrifugally cast Al-Bi alloys. Comput. Mater. Sci. 2010, 49, 121–125. [Google Scholar] [CrossRef]
- Kaban, I.; Hoyer, W. Effect of Cu and Sn on liquid–liquid interfacial energy in ternary and quaternary Al–Bi-based monotectic alloys. Mater. Sci. Eng. A 2008, 495, 3–7. [Google Scholar] [CrossRef]
- Schwarz, B.; Mattern, N.; Shuleshova, O.; Eckert, J. Liquid–liquid demixing and microstructure of Co-Cu-Zr alloys with low Zr content. Intermetallics 2013, 32, 250–258. [Google Scholar] [CrossRef]
- Zhang, K.; Bian, X.F.; Li, Y.M.; Yang, C.C.; Yang, H.B.; Zhang, Y. High-efficiency control of phase separation in Al-based immiscible alloys by TiC particles. J. Alloy. Compd. 2015, 639, 563–570. [Google Scholar] [CrossRef]
- Chen, L.Y.; Xu, J.Q.; Choi, H.; Konishi, H.; Jin, S.; Li, X.C. Rapid control of phase growth by nanoparticles. Nat. Commun. 2014, 5, 3879. [Google Scholar] [CrossRef] [PubMed]
- Budai, I.; Kaptay, G. Monotectic Al/Cd alloys with homogeneously dispersed Cd-droplets stabilized by strontium aluminide precipitates. Intermetallics 2011, 19, 423–425. [Google Scholar] [CrossRef]
- Nagy, O.Z.; Szabo, J.T.; Kaptay, G. Stabilization of metallic emulsions by in situ precipitating intermetallic layers. Intermetallics 2012, 26, 26–30. [Google Scholar] [CrossRef]
- Sun, Q.; Jiang, H.X.; Zhao, Z.J.; He, J. Effect of TiC particles on the liquid–liquid decomposition of Al-Pb alloys. Mater. Des. 2016, 91, 361–367. [Google Scholar] [CrossRef]
- Gshneidner, K.A.; Calderwood, F.W. The Bi-Ce system. Bull. Alloy Ph. Diagr. 1989, 10, 427–430. [Google Scholar] [CrossRef]
- Cai, Y.; Tan, M.J.; Shen, G.J.; Su, H.Q. Microstructure and heterogeneous nucleation phenomena in cast SiC particles reinforced magnesium composite. Mater. Sci. Eng. A 2000, 282, 232–239. [Google Scholar]
- Kaban, I.; Kohler, M.; Ratke, L.; Hoyer, W.; Mattern, N.; Eckert, J.; Greer, A.L. Surfaces, interfaces and phase transitions in Al-In monotectic alloys. Acta Mater. 2011, 59, 6880–6889. [Google Scholar] [CrossRef]



© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Man, T.; Zhang, L.; Xu, N.; Wang, W.; Xiang, Z.; Wang, E. Effect of Rare-Earth Ce on Macrosegregation in Al-Bi Immiscible Alloys. Metals 2016, 6, 177. https://doi.org/10.3390/met6080177
Man T, Zhang L, Xu N, Wang W, Xiang Z, Wang E. Effect of Rare-Earth Ce on Macrosegregation in Al-Bi Immiscible Alloys. Metals. 2016; 6(8):177. https://doi.org/10.3390/met6080177
Chicago/Turabian StyleMan, Tiannan, Lin Zhang, Naikang Xu, Wenbin Wang, Zhaolong Xiang, and Engang Wang. 2016. "Effect of Rare-Earth Ce on Macrosegregation in Al-Bi Immiscible Alloys" Metals 6, no. 8: 177. https://doi.org/10.3390/met6080177