Designing of Synergistic Waste Mixtures for Multiphase Reactive Smelting
Abstract
:1. Introduction
- the optimal amount of reducing agent,
- the energy of reactions,
- the final composition of dust and slag.
2. Experimental Section
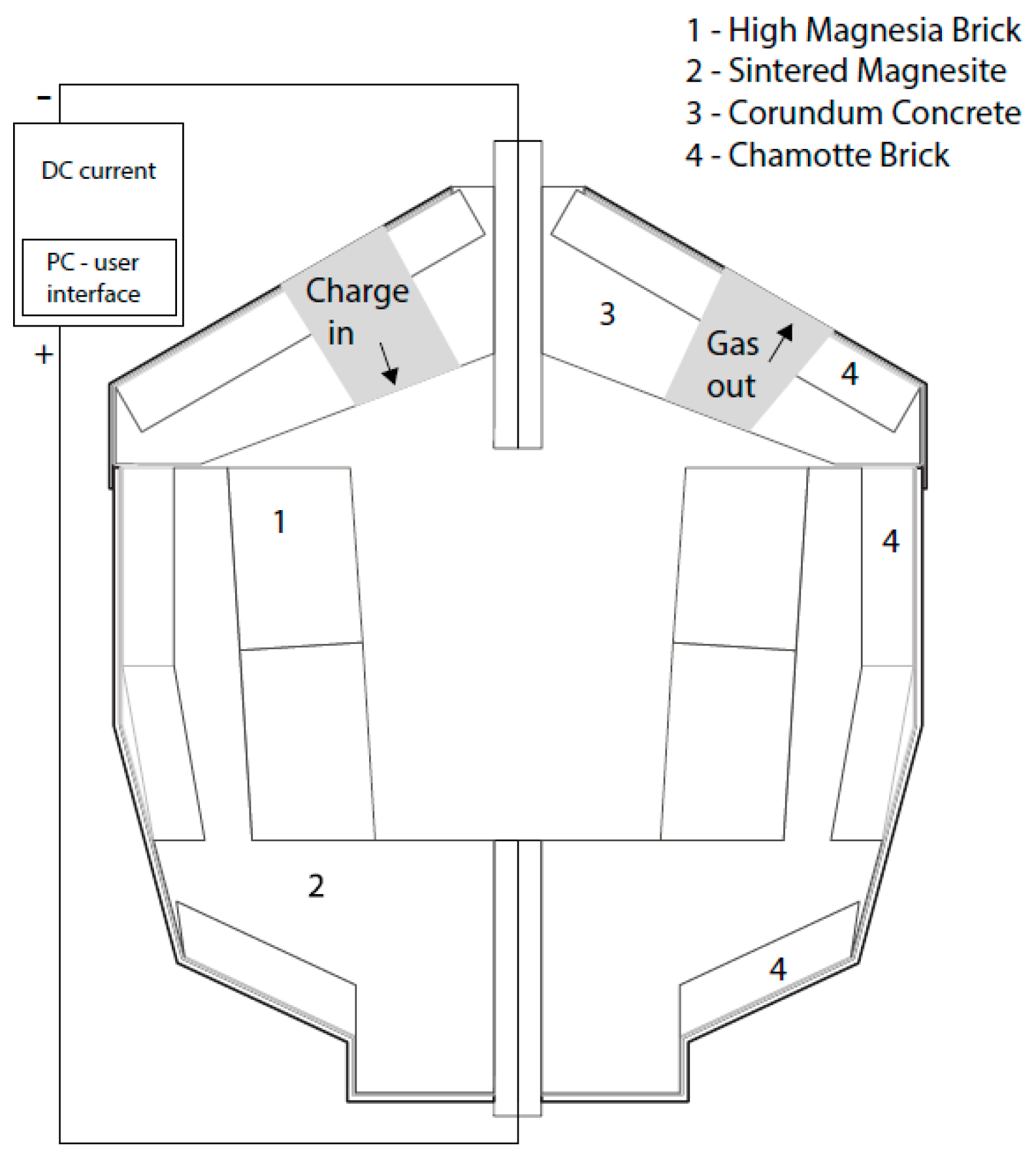
2.1. Set-up
2.2. Materials and Methods
3. Designing Mixture Composition and Energy Consumption
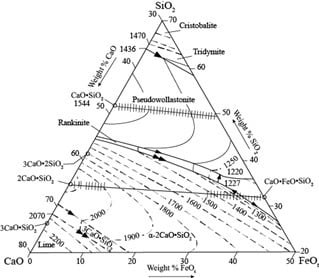
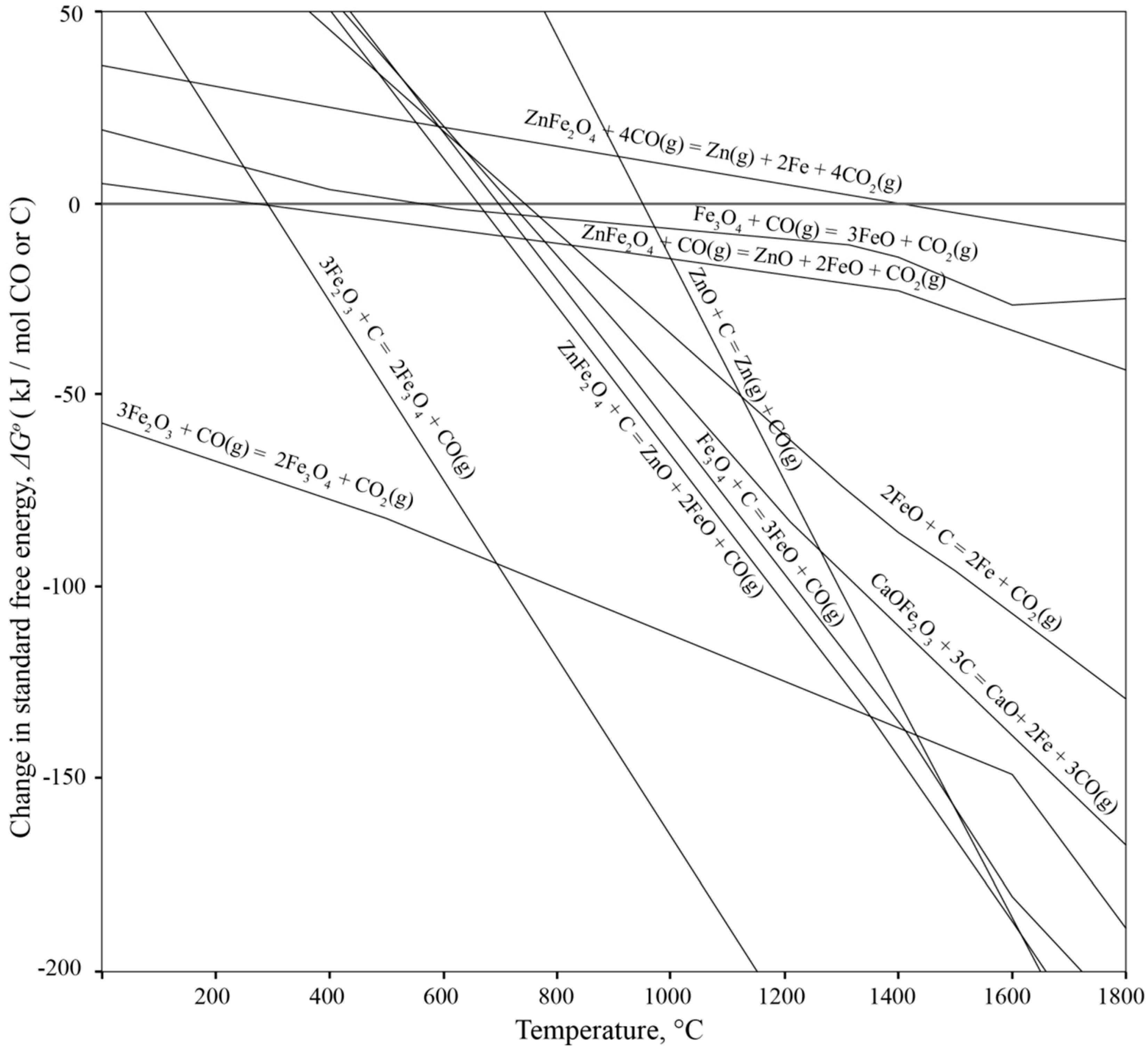
3.1. Thermo-Chemical Considerations
3.1.1. Processes inside the Furnace
3.1.2. Processes outside of the Furnace Chamber
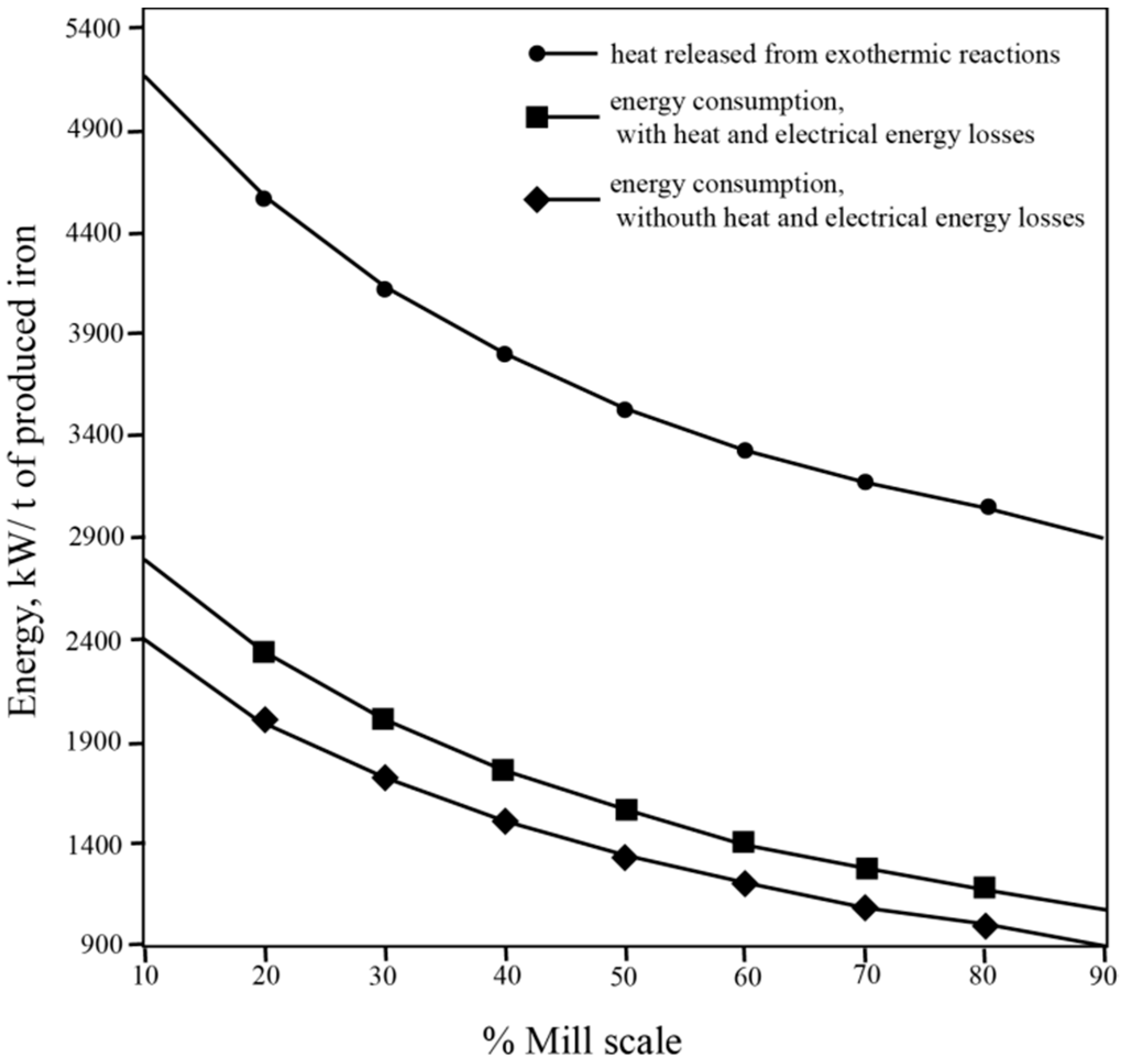
3.2. Mixture Composition and Energy Consumption
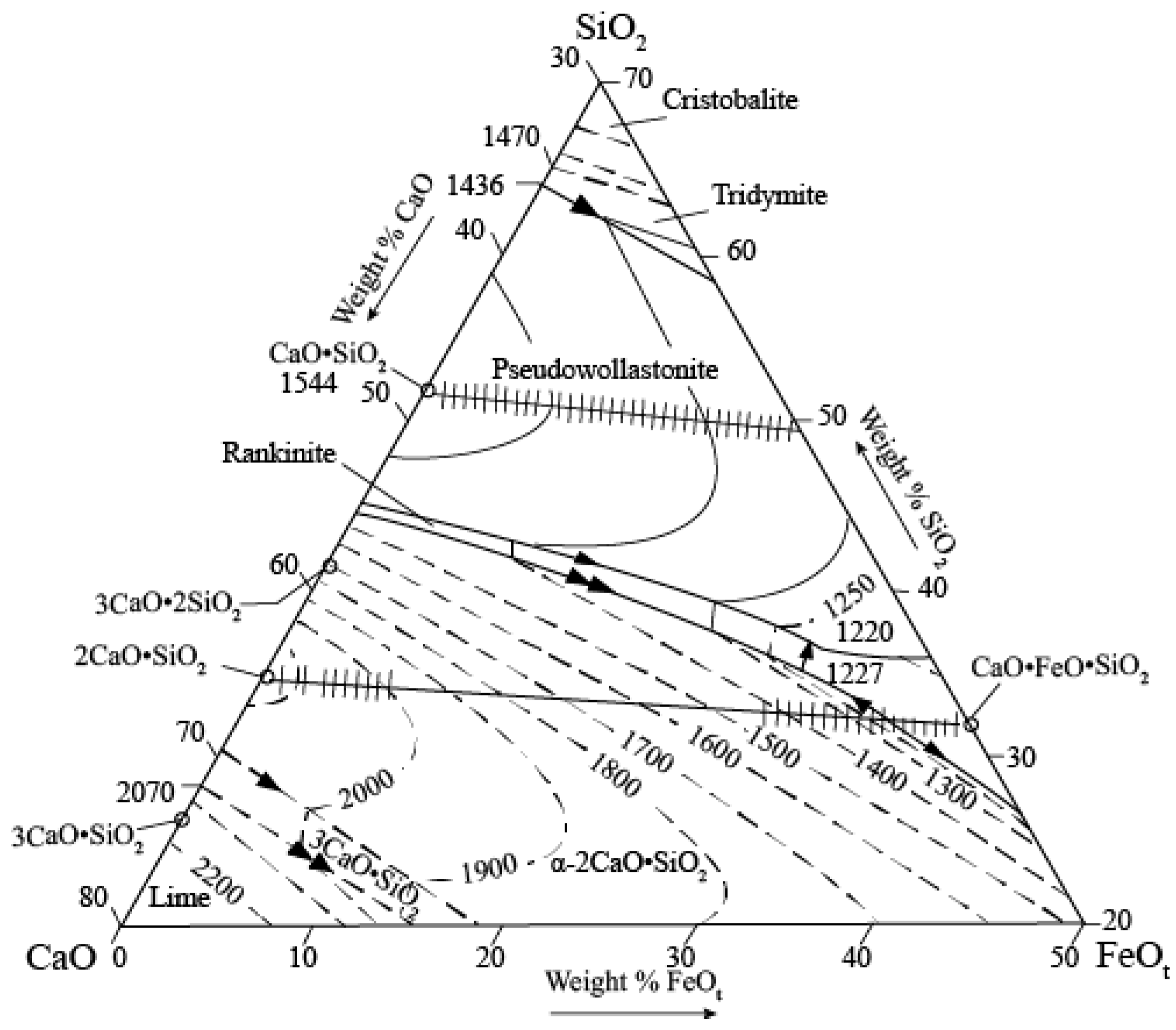
Slag Composition
4. Discussion
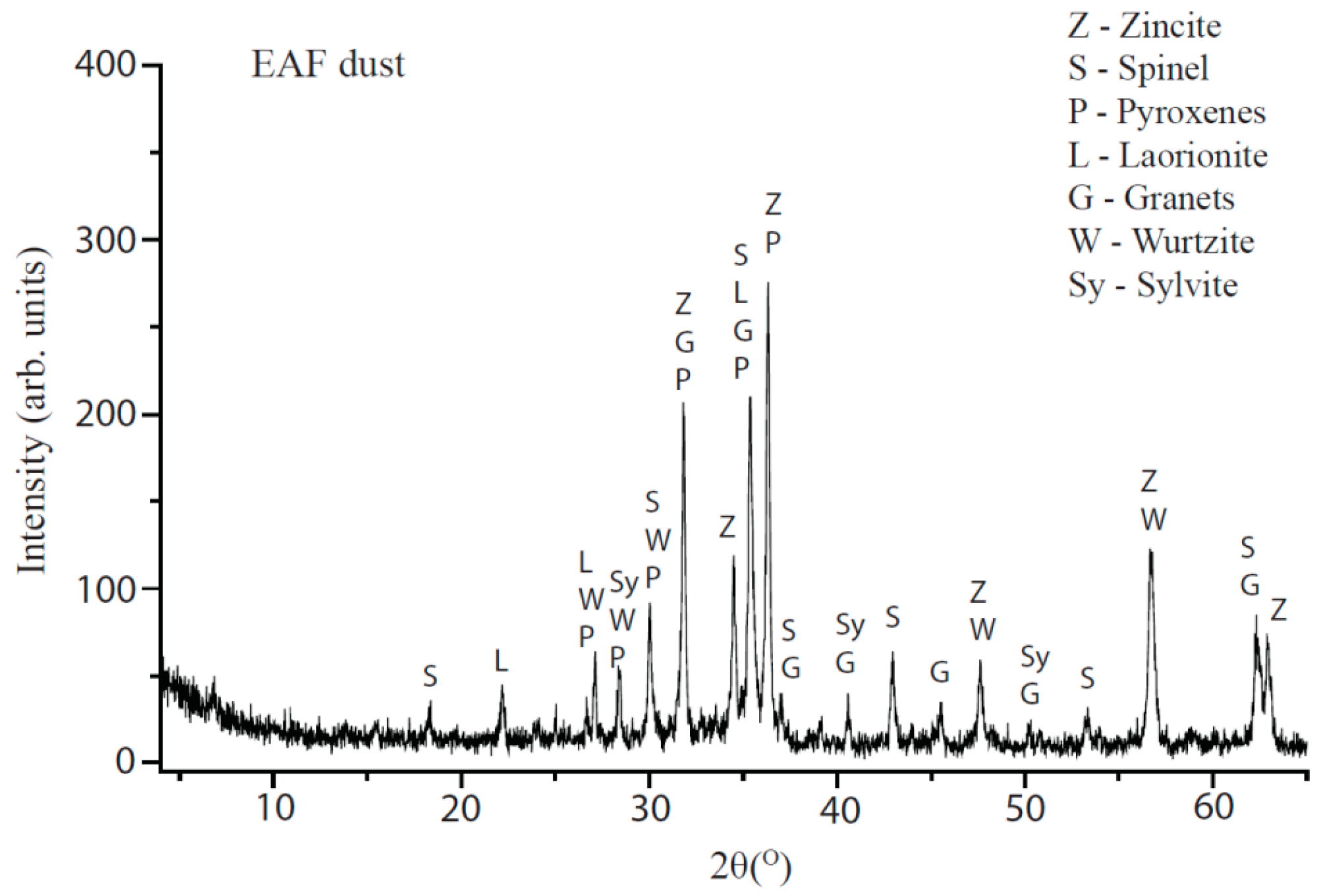
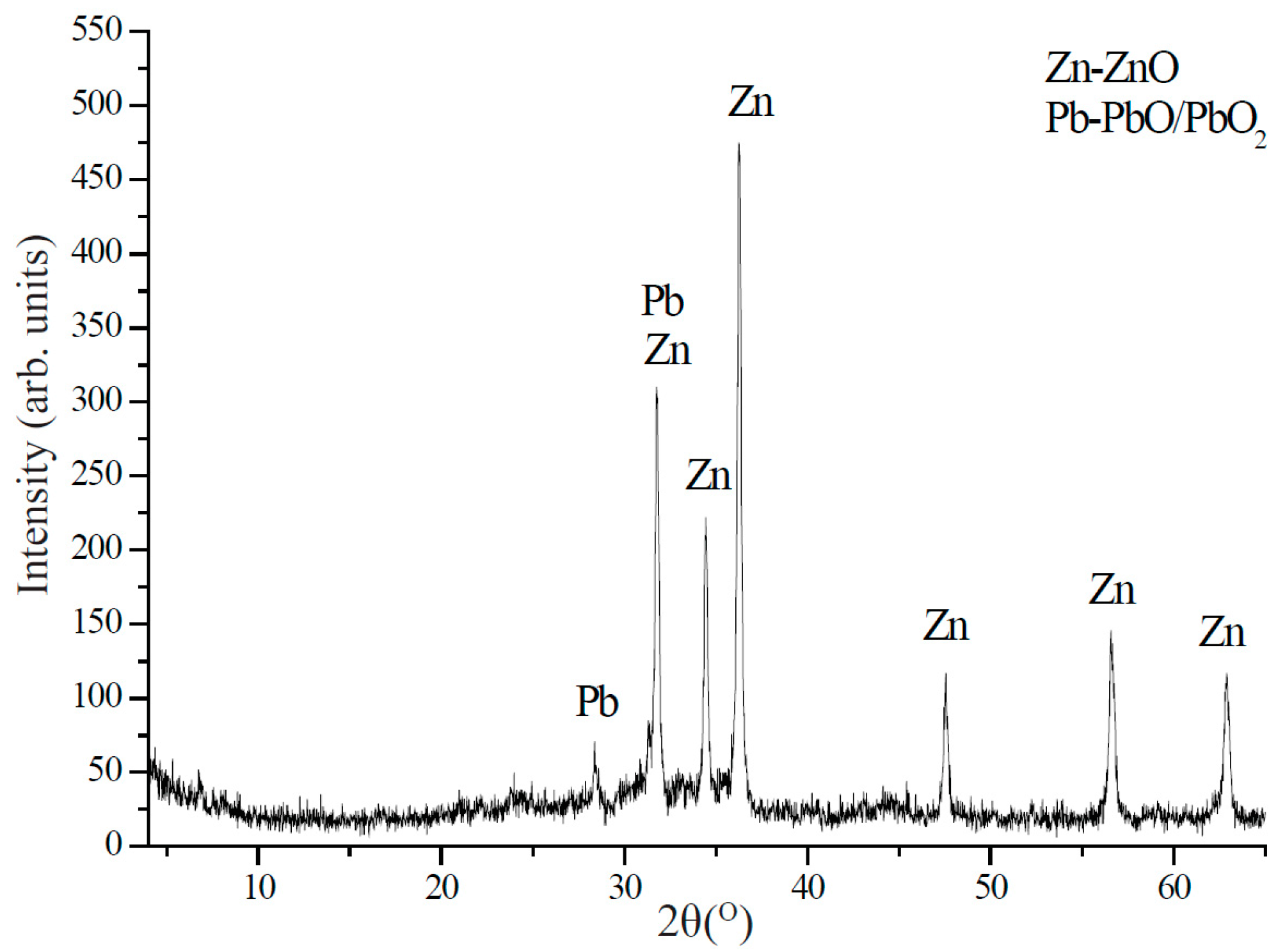
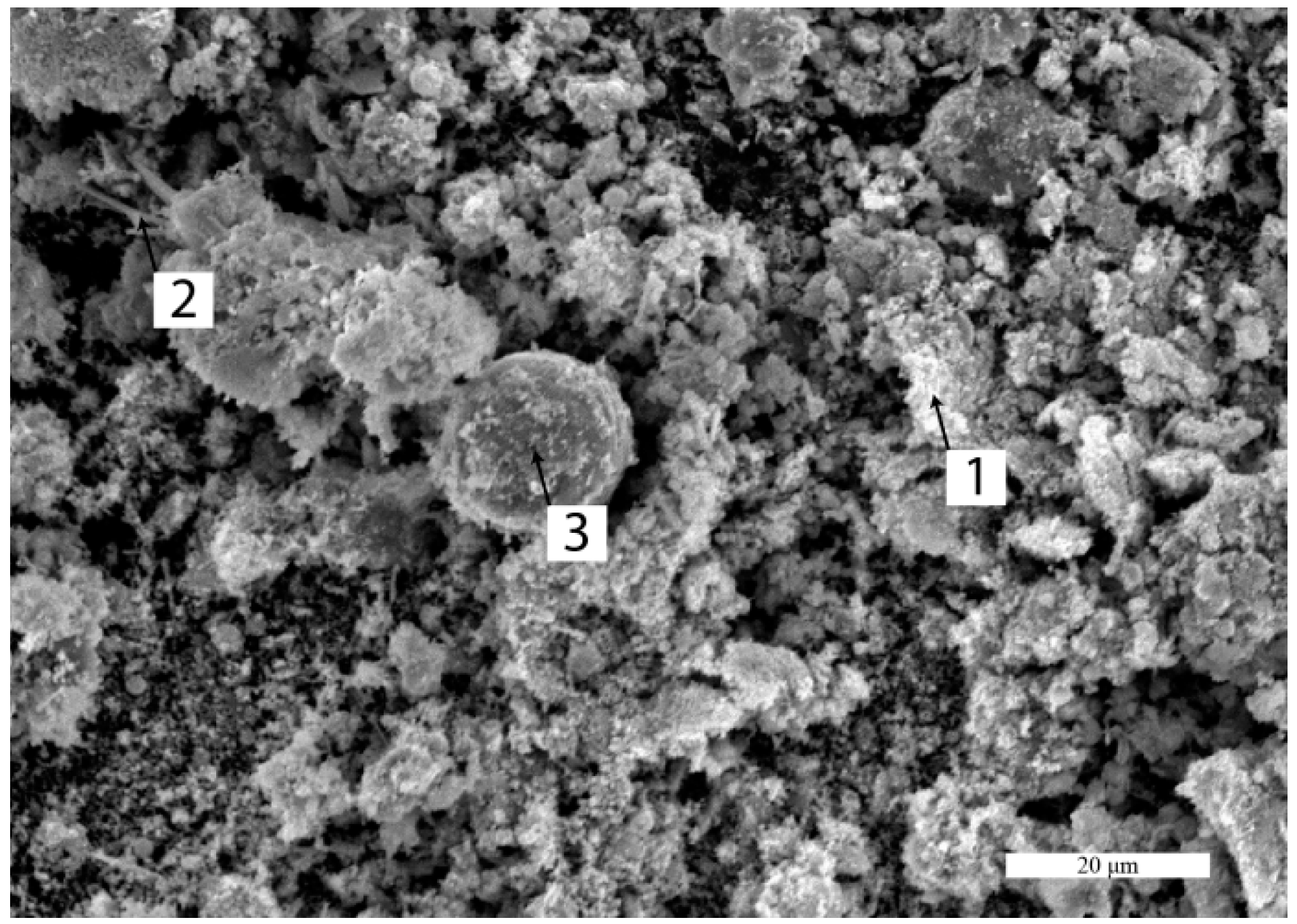
4.1. Dust Characterization
4.2. Metal Composition
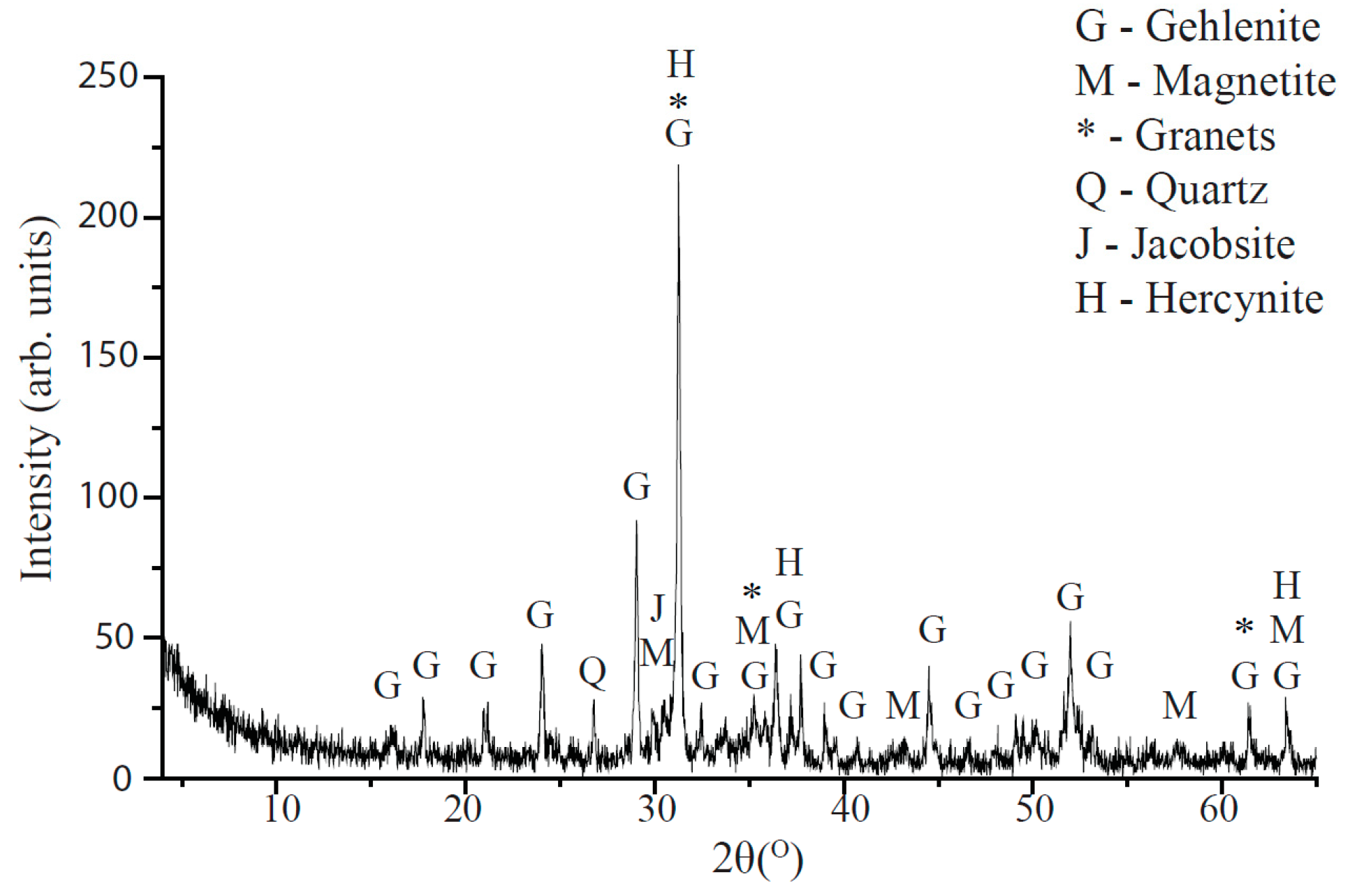
4.3. Slag Characterization
4.4. Monitoring of Exhaust Gases
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Grabda, M.; Oleszek, S.; Shibata, E.; Nakamura, T. Study on simultaneous recycling of EAF dust and plastic waste containing TBBPA. J. Hazard. Mater. 2014, 278, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Taheri, A.M.; Saidi, A.; Nourbakhsh, A.A. The effective parameters on thermal recovery and reduction of iron oxides in EAF slag. Int. J. ISSI 2010, 7, 25–29. [Google Scholar]
- Itoh, S.; Tsubone, A.; Yokoyama, K.M.; Nakajima, K.; Nagasaka, T. New EAF Dust Treatment Process with the Aid of Strong Magnetic Field. ISIJ Int. 2008, 48, 1339–1344. [Google Scholar] [CrossRef]
- Montenegro, V.; Oustadakis, P.; Tsakiridis, P.; Leonardou, S.A. Hydrometallurgical Treatment of Steelmaking Electric Arc Furnace Dusts (EAFD). Metall. Mater. Trans. B 2013, 44B, 1058–1069. [Google Scholar] [CrossRef]
- Santos, F.; Brocchi, E.; Araujo, V.; Souza, R. Behavior of Zn and Fe Content in Electric Arc Furnace Dust as Submitted to Chlorination Methods. Metall. Mater. Trans. B 2015. [Google Scholar] [CrossRef]
- Morcali, M.H.; Yucel, O.; Aydin, A.; Verin, B. Carbothermic reduction of electric arc furnace dust and calcination of waelz oxide by semi-pilot scale rotary furnace. J. Min. Metall. Sect. B Metall. 2012, 48, 173–184. [Google Scholar] [CrossRef]
- Shatokhaa, V.I.; Gogenko, O.O.; Kripak, S.M. Utilising of the oiled rolling mills scale in iron ore sintering process. Resour. Conserv. Recycl. 2011, 55, 435–440. [Google Scholar] [CrossRef]
- Gavrilovski, M.; Manojlovic, V.; Kamberovic, Z.; Korac, M.; Sokic, M. Semi-empirical software for the aluminothermic and carbothermic reactions. Metall. Mater. Eng. 2014, 20, 199–206. [Google Scholar] [CrossRef]
- Zivanovic, P.; Gavrilovski, M.; Stopic, S.; Matic, S.; Lajic, Z.; Tiano, A. The calculation and analysis of the adiabatic temperature of self-propagating high-temperature synthesis in CaWO4 + Mg(Al) system. Metall. Mater. Eng. 2005, 11, 339–344. [Google Scholar]
- Wu, C.C.; Chang, F.C.; Chen, W.S.; Tsai, M.S.; Wang, Y.N. Reduction behavior of zinc ferrite in EAF-dust recycling with CO gas as a reducing agent. J. Environ. Manag. 2014, 143, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Machadoa, J.G.M.S.; Brehm, F.A.; Moraes, C.A.M.; Santos, C.A.; Vilela, A.C.F.; Cunha, J.B.M. Chemical, physical, structural and morphological characterization of the electric arc furnace dust. J. Hazard. Mater. 2006, 136, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.S.; Song, Y.J. Recycling EAF dust by heat treatment with PVC. Miner. Eng. 2007, 20, 739–746. [Google Scholar] [CrossRef]
- Yoo, J.M.; Kim, B.S.; Lee, J.C.; Kim, M.S.; Nam, C.W. Kinetics of the Volatilization Removal of Lead in Electric Arc Furnace Dust. Mater. Trans. 2005, 46, 323–328. [Google Scholar] [CrossRef]
- Issa, H.; Kamberovic, Z.; Gavrilovski, M.; Korac, M.; Andjic, Z. Modeling of metallurgical properties of sinter mixtures of nonstandard raw iron-bearing materials. Met. Int. 2013, 18, 5–8. [Google Scholar]
- Kim, B.S.; Yoo, J.M.; Park, J.T.; Lee, J.C. A kinetic study of the carbothermic reduction of zinc oxide with various additives. Mater. Trans. 2006, 47, 2421–2426. [Google Scholar] [CrossRef]
- Bafghi, M.S.; Karimi, M.; Adeli, M. A kinetic study on the carbothermic reduction of zinc oxide from electric arc furnace dust. Iranian J. Mater. Sci. Eng. 2013, 10, 18–30. [Google Scholar]
- Seetharaman, S.; McLean, A.; Guthrie, R.; Sridhar, S. Treatise on Process Metallurgy, Volume 1: Process Fundamentals; Elsevier: Oxford, UK, 2014. [Google Scholar]
- Lopez, F.A.; Delgado, A.L. Enhancement of electric arc furnace dust by recycling to electric arc furnace. J. Environ. Eng. 2002, 128, 1169–1174. [Google Scholar] [CrossRef]
- Pickles, C.A. Thermodynamic analysis of the selective carbothermic reduction of electric arc furnace dust. J. Hazard. Mater. 2008, 150, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, J.; Xu, A.; Yang, Q.; He, D.; Tian, N. Carbothermic reduction of zinc and iron oxides in electric arc furnace dust. J. Iron. Steel Res. Int. 2014, 21, 427–432. [Google Scholar] [CrossRef]
- Li, X.; Xiao, W.; Liu, W.; Liu, G.; Peng, Z.; Zhou, Q.; Qi, T. Recovery of alumina and ferric oxide from Bayer red mud rich in iron by reduction sintering. Trans. Nonferrous Met. Soc. China 2009, 19, 1342–1347. [Google Scholar] [CrossRef]
- Pickels, C.A. Thermodynamic modelling of the multiphase pyrometallurgical processing of electric arc furnace dust. Miner. Eng. 2009, 22, 977–985. [Google Scholar] [CrossRef]
- Seetharaman, S.; McLean, A.; Guthrie, R.; Sridhar, S. Electric Furnace Steelmaking. In Treatise on Process Metallurgy, Volume 3: Industrial Processes, Part A; Elsevier: Oxford, UK, 2014. [Google Scholar]
- Ramakrishna, G.; Kadrolkar, A.; Srikakulapu, N.G. Exergy and its efficiency calculations in ferrochrome production. Metall. Mater. Trans. B 2015, 46B, 1073–1081. [Google Scholar] [CrossRef]
- Wang, H.; Yu, H.; Teng, L.; Seetharaman, S. Evaluation on Material and Heat Balance of EAF Processes with Introduction of CO2. J. Min. Metall. Sect. B Metall. 2016, 52, 01–08. [Google Scholar] [CrossRef]
- Kowalski, M.; Spencer, P.J.; Neuschutz, D. Phase Diagrams. In Verein Deutscher Eisenhuttenleute, Slag Atlas, 2nd ed.; Verlag Stahleisen GmbH: Dusseldorf, Germany, 1995; pp. 21–214. [Google Scholar]
- Muller, J.; Zietsman, J.H.; Pistorius, P.C. Modeling of Manganese Ferroalloy Slag Properties and Flow During Tapping. Metall. Mater. Trans. B 2015, 46B, 2015–2639. [Google Scholar] [CrossRef]
- Mills, K.C. Viscosities of Molten Slags. In Verein Deutscher Eisenhuttenleute, Slag Atlas, 2nd ed.; Verlag Stahleisen GmbH: Dusseldorf, Country, 1995; pp. 349–402. [Google Scholar]
- Degterov, S.A.; Jak, E.; Hayes, P.C.; Pelton, A.D. Experimental Study of Phase Equilibria and Thermodynamic Optimization of the Fe-Zn-O System. Metall. Mater. Trans. B 2001, 32B, 643–657. [Google Scholar] [CrossRef]
- Katayama, H.; Sano, N.; Sasabe, M.; Matsuoka, S. Research Activities on Removal of Residual Elements from Steel Scrap in Japan. Mem. Fac. Sci. Eng. Shimane Univ. 1997, 30, 99–144. [Google Scholar]
- Mombelli, D.; Mapelli, C.; Barella, S.; Gruttadauria, A.; le Saout, G.; Gracia-Diaz, E. The efficiency of quartz addition on electric arc furnace (EAF) carbonsteel slag stability. J. Hazard. Mater. 2014, 279, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Joint Research Centre. Best Available Techniques (BAT) Reference Document for the Non-Ferrous Metals Industries; European Commission: Seville, Spain, 2014; pp. 700–704. [Google Scholar]



| CaO | SiO2 | FeO | MgO | Al2O3 | MnO | Na2O | K2O | Cu | S | Zn | Ni |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 36.9 | 26.6 | 12.5 | 9.6 | 9.8 | 1.6 | 0.60 | 0.60 | 0.10 | 0.20 | 0.10 | 0.30 |
| Fe | Si | Zn | C | Cd | Mn | Ca | Al | Na | K | Pb | Mg | Cu |
| 20.78 | 1.65 | 30.68 | 3.11 | 0.22 | 7.12 | 3.06 | 0.25 | 0.53 | 1.44 | 3.68 | 1.43 | 0.45 |
| O (oxides) | F (fluorides) | Cl (chlorides) | S (sulfides) | Others | ||||||||
| 20.52 | 0.08 | 2.69 | 1.63 | 0.68 | ||||||||
| Fe | Si | Cr | Sn | Ni | C | P | S | Mn | Ca | Al | Mg | Cu | O | LOI | Others |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 70.87 | 0.80 | 0.04 | 0.01 | 0.03 | 0.02 | 0.03 | 0.03 | 0.09 | 0.39 | 0.11 | 0.13 | 0.30 | 25.2 | 1.80 | 0.12 |
| CaO | Al2O3 | MgO | SiO2 |
|---|---|---|---|
| 95.7 | 1.1 | 2.4 | 0.60 |
| C | SiO2 | Al2O3 | CaO | MgO | Fe2O3 | S |
|---|---|---|---|---|---|---|
| 86.8 | 5.7 | 4.7 | 0.80 | 0.50 | 0.70 | 0.60 |
| Balance | Input, kg | Output, kg | ||||
|---|---|---|---|---|---|---|
| Pellets | Starting Slag | Slag | Metal | Dust | Gas | |
| Model | 85 | 15 | 24.8 | 33.8 | 13.3 | 28.1 |
| Experimental | 85 ± 0.5 | 15 ± 0.1 | 23.0 ± 0.5 | 31.1 ± 0.6 | 13.1 ± 0.3 | - |
| Element | Zn | Fe | Pb | Cd | Cl(Chlorides) | O(Oxides) | Others |
|---|---|---|---|---|---|---|---|
| wt. % | 71.1 | 0.4 | 8.5 | 0.5 | 1.1 | 18.1 | 0.3 |
| Element | Fe | C | Mn | Si | Cu | S | P | Ni | Cr | Sn | Zn | Others |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt. % | 90.3 | 2.7 | 5.4 | 0.75 | 0.30 | 0.10 | 0.12 | 0.05 | 0.03 | 0.06 | 0.03 | 0.16 |
| Phase | SiO2 | CaO | FeO | MgO | Al2O3 | MnO | Na2O | K2O | Cu | S | Others |
|---|---|---|---|---|---|---|---|---|---|---|---|
| wt. % | 27.8 | 36.5 | 11.6 | 10.1 | 9.4 | 2.3 | 0.3 | 0.8 | 0.1 | 0.2 | 0.9 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manojlović, V.; Kamberović, Ž.; Sokić, M.; Gavrilovski, M.; Korać, M. Designing of Synergistic Waste Mixtures for Multiphase Reactive Smelting. Metals 2016, 6, 138. https://doi.org/10.3390/met6060138
Manojlović V, Kamberović Ž, Sokić M, Gavrilovski M, Korać M. Designing of Synergistic Waste Mixtures for Multiphase Reactive Smelting. Metals. 2016; 6(6):138. https://doi.org/10.3390/met6060138
Chicago/Turabian StyleManojlović, Vaso, Željko Kamberović, Miroslav Sokić, Milorad Gavrilovski, and Marija Korać. 2016. "Designing of Synergistic Waste Mixtures for Multiphase Reactive Smelting" Metals 6, no. 6: 138. https://doi.org/10.3390/met6060138