Hydrogen Absorption in Metal Thin Films and Heterostructures Investigated in Situ with Neutron and X-ray Scattering
Abstract
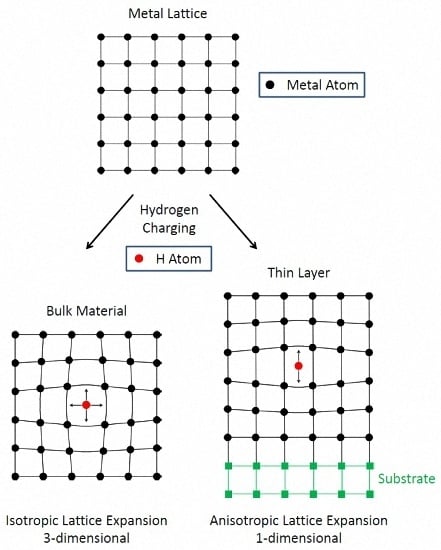
:1. Introduction: Hydrogen Absorption in Metals—Bulk versus Thin Films
2. Experimental Section
2.1. Materials and Applications
2.1.1. Hydrogen in Fe/Nb and W/Nb Films
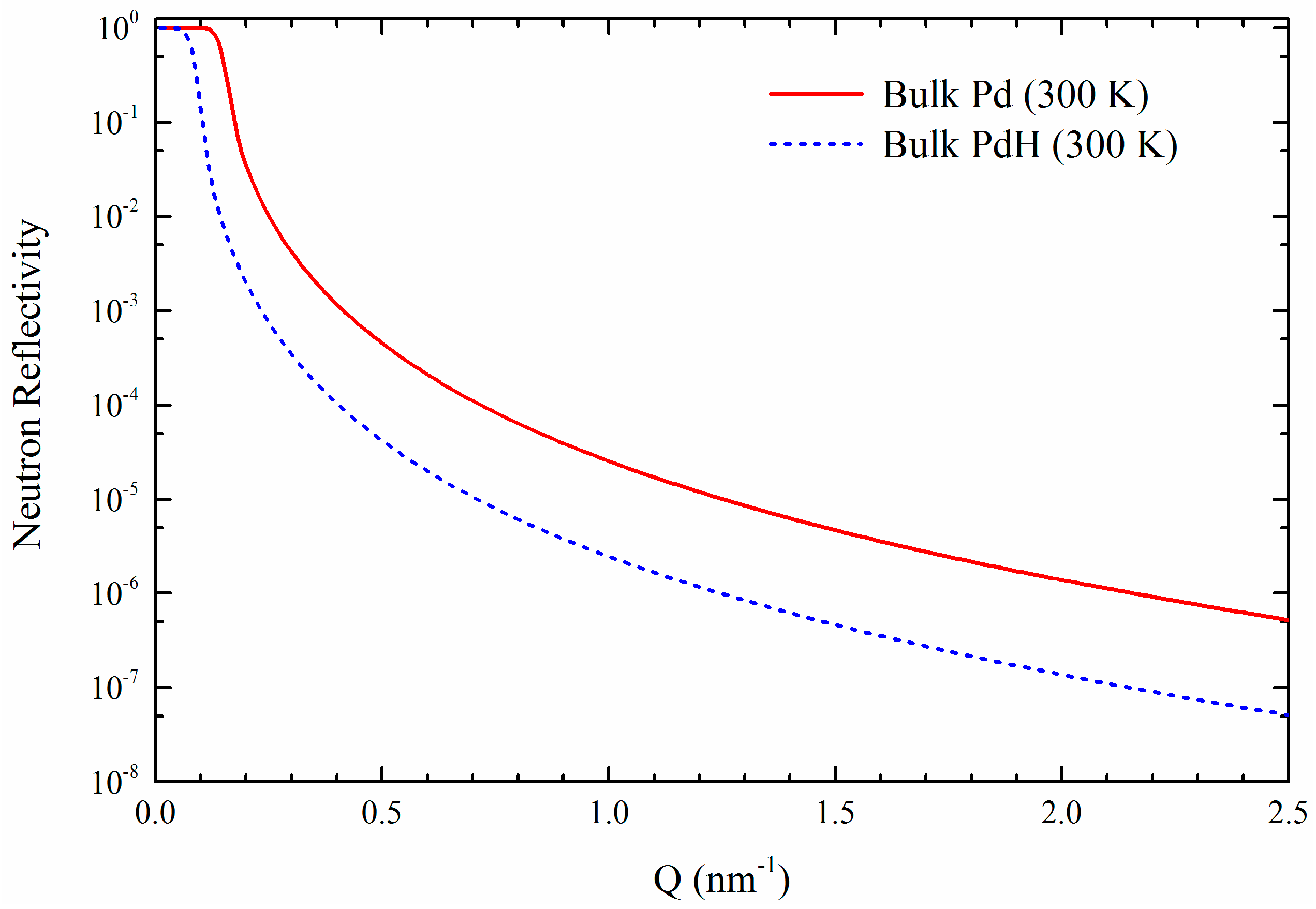
2.1.2. Hydrogen in Co/Pd Films
2.2. Experimental Methods
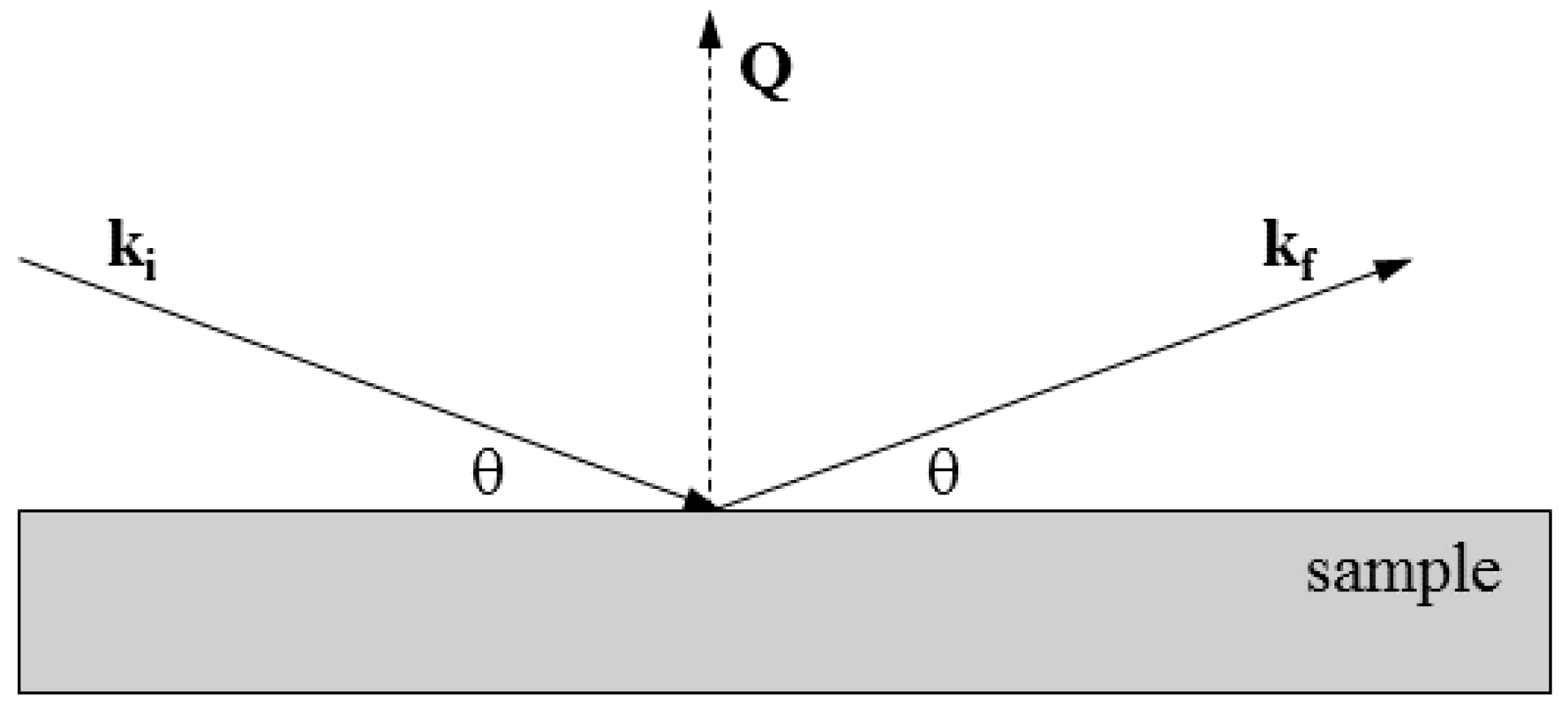
2.2.1. Neutron Reflectometry
2.2.2. Polarized Neutron Reflectometry (PNR)
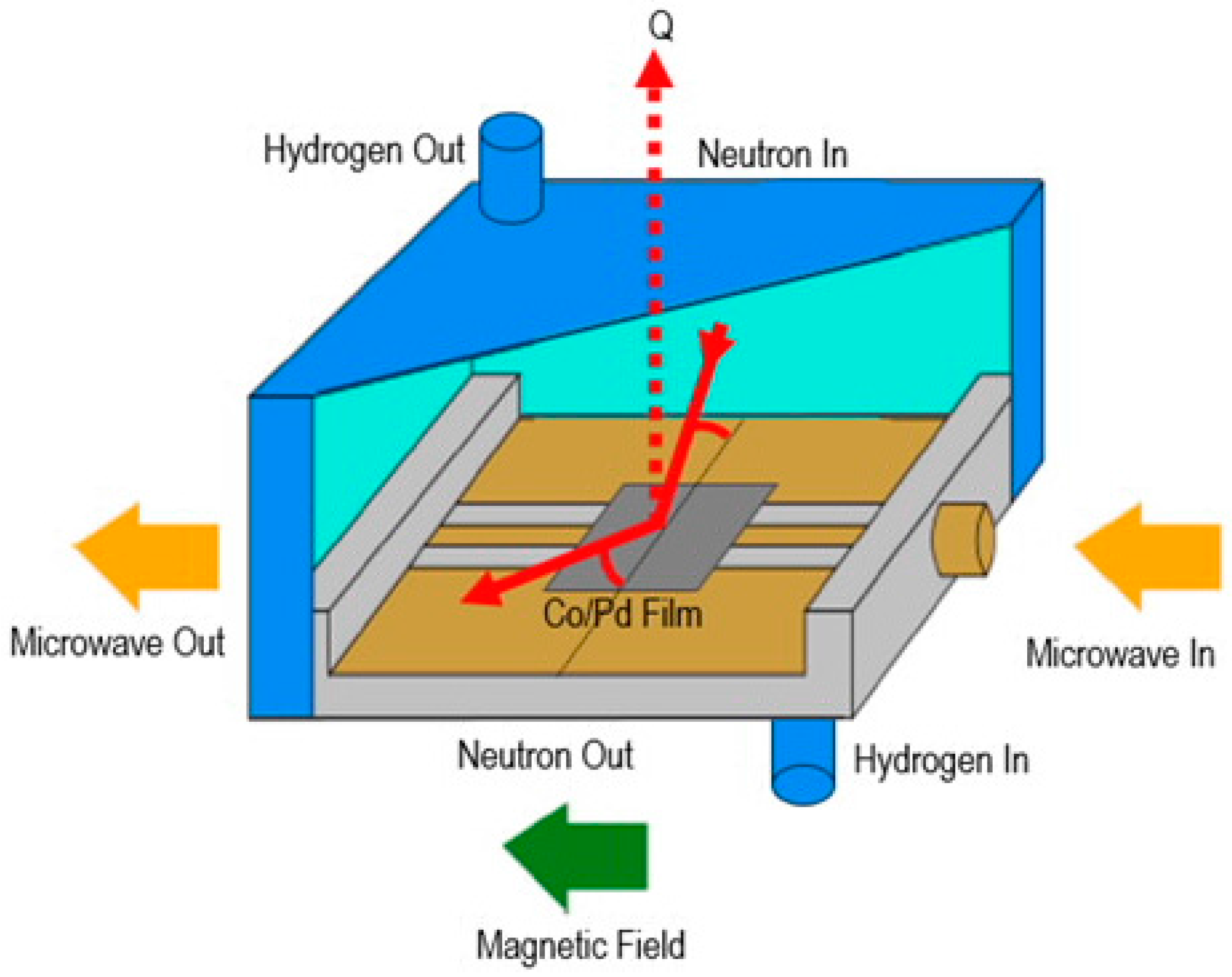
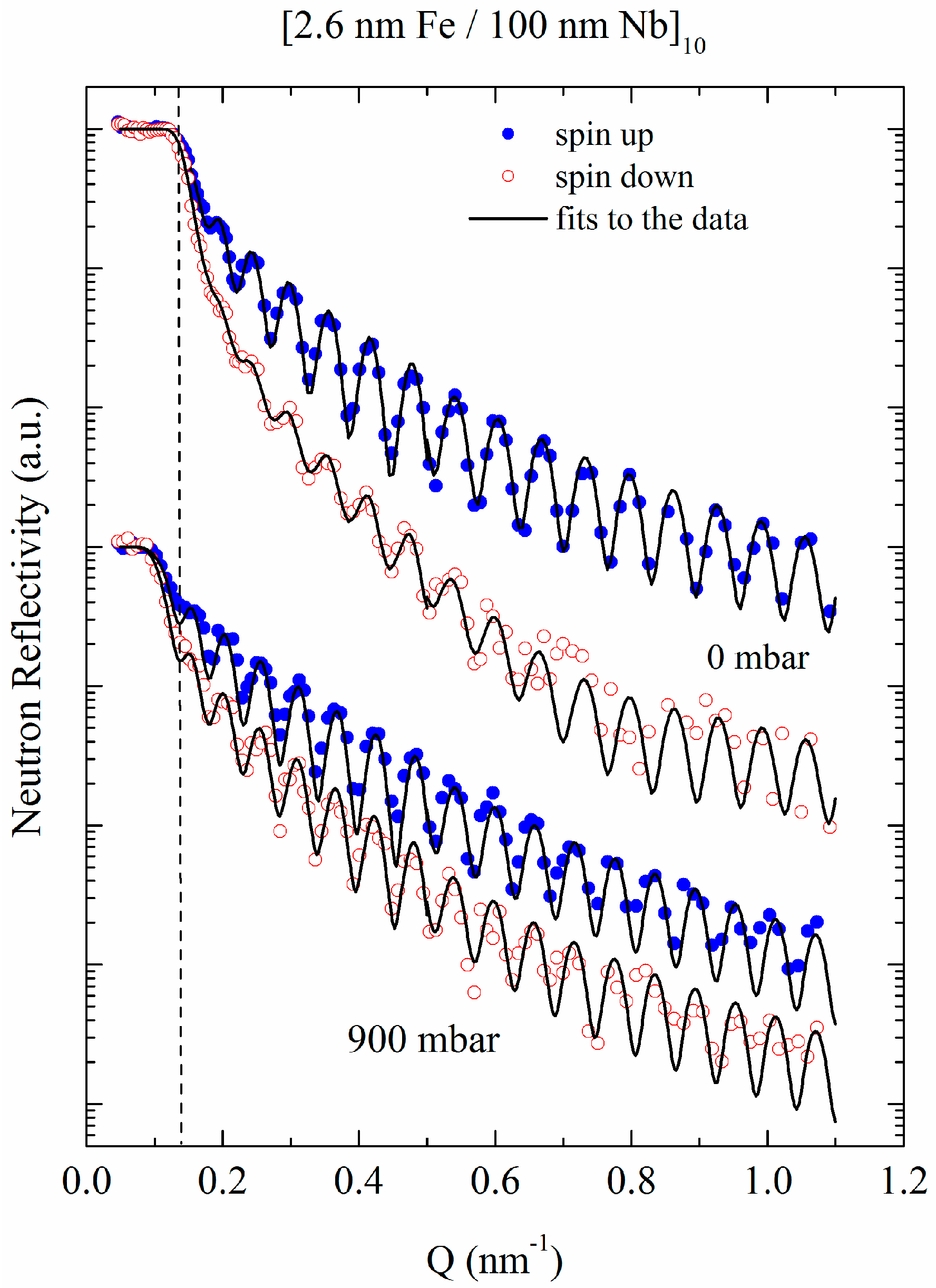
2.2.3. In Situ Ferromagnetic Resonance (FMR) Measurements and Polarized Neutron Reflectometry (PNR)
3. Results and Discussion
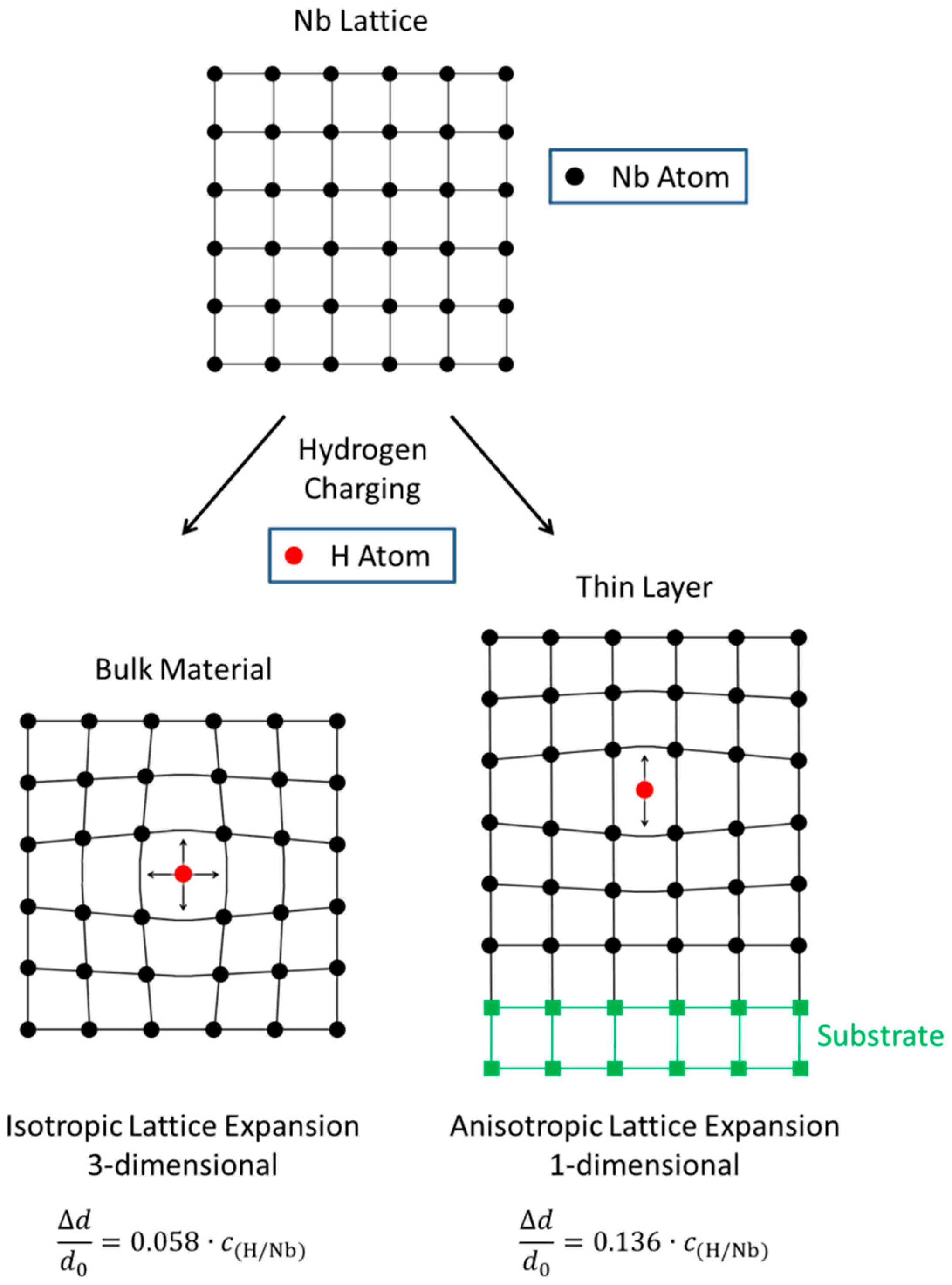
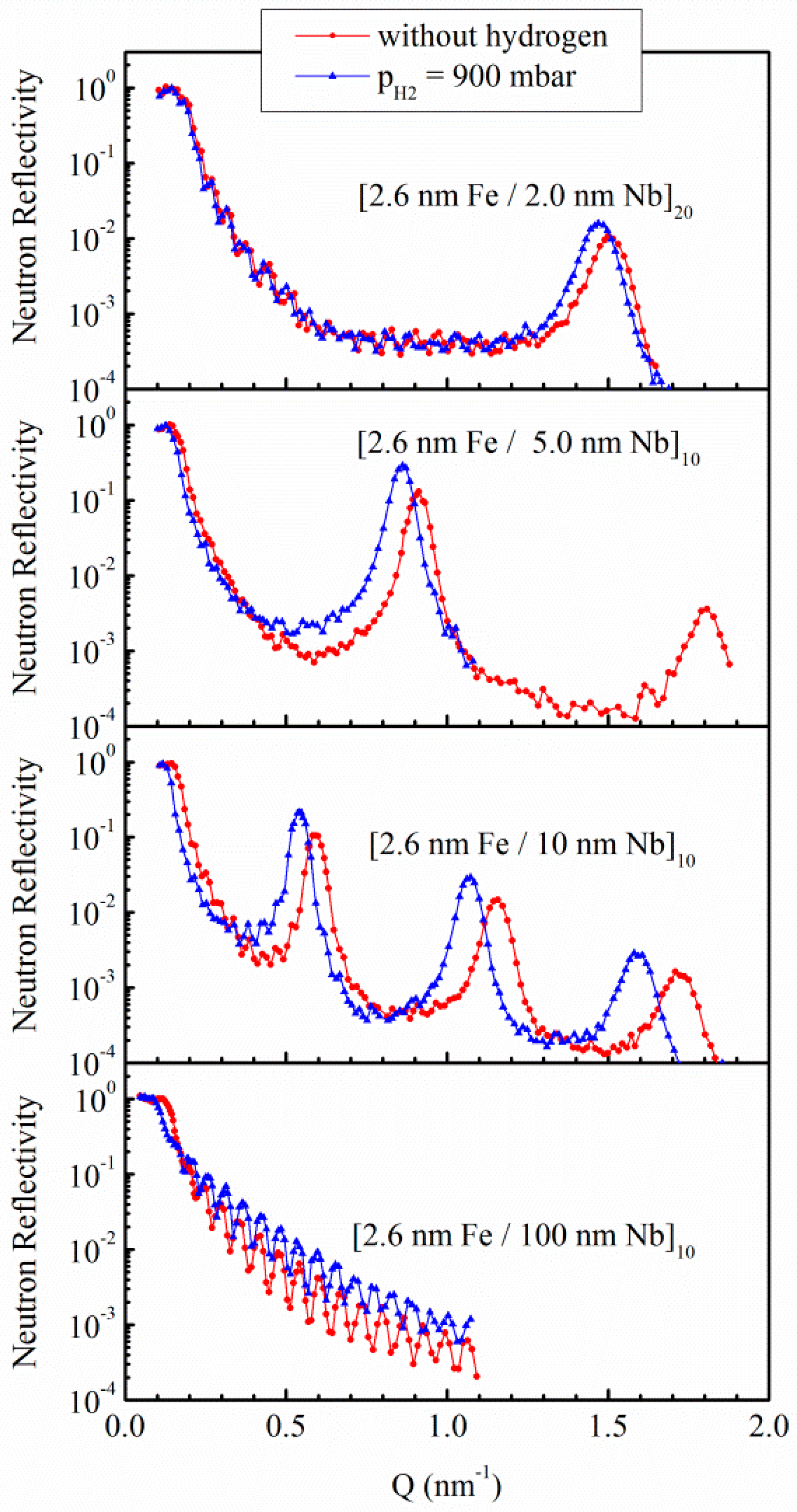
3.1. Nb Thin Films and Multilayers
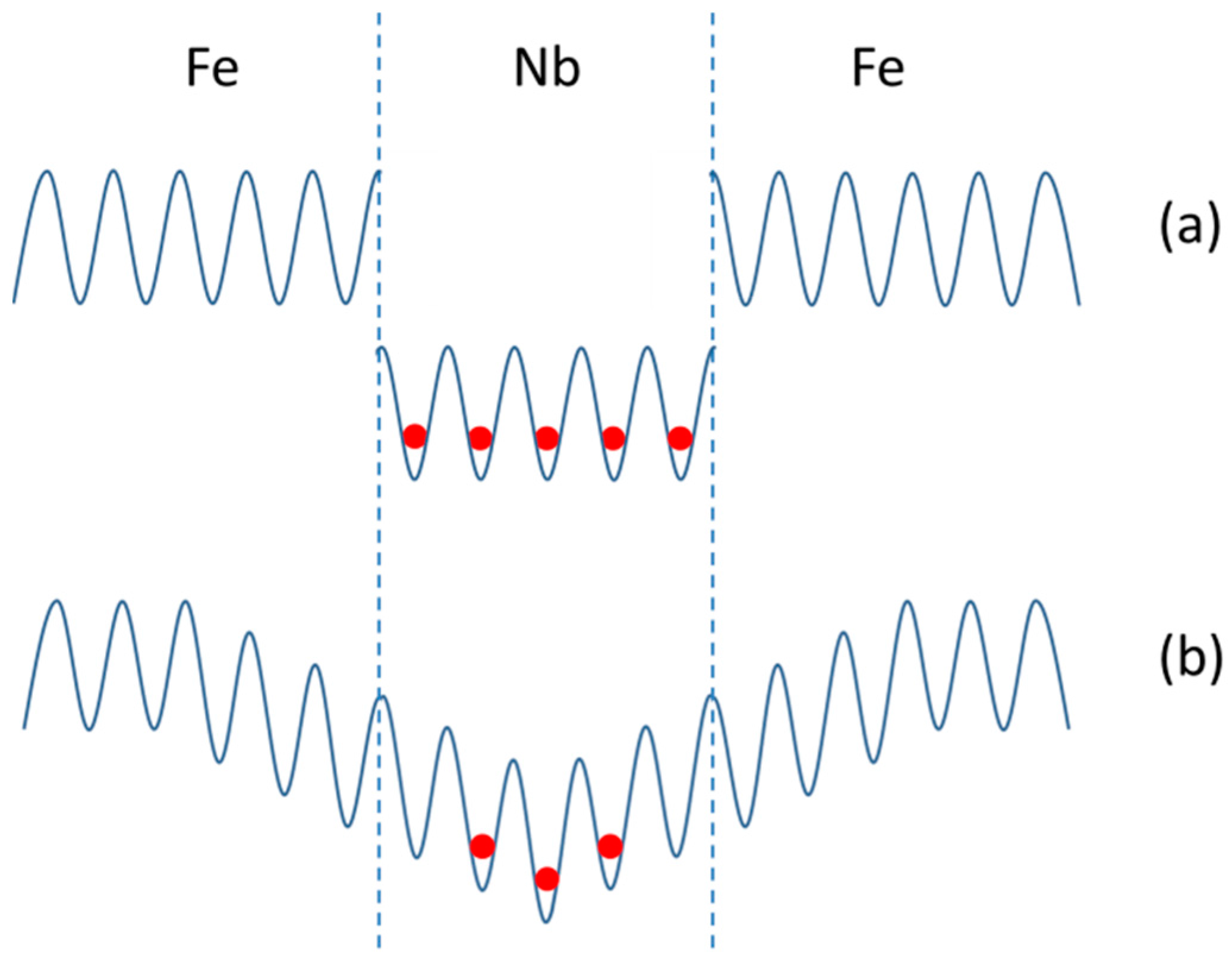
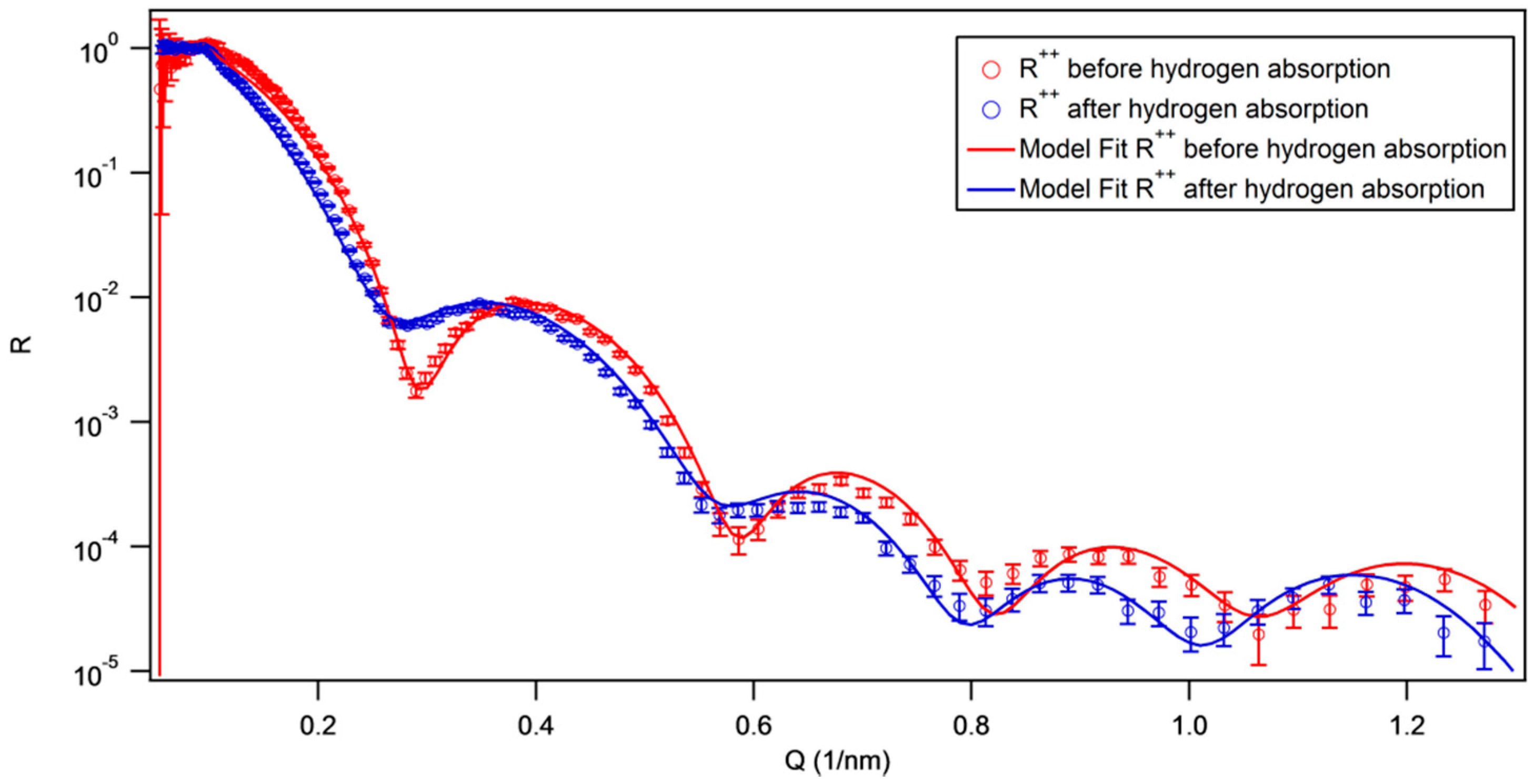
3.1.1. Fe/Nb Multilayers
3.1.2. W/Nb Multilayers
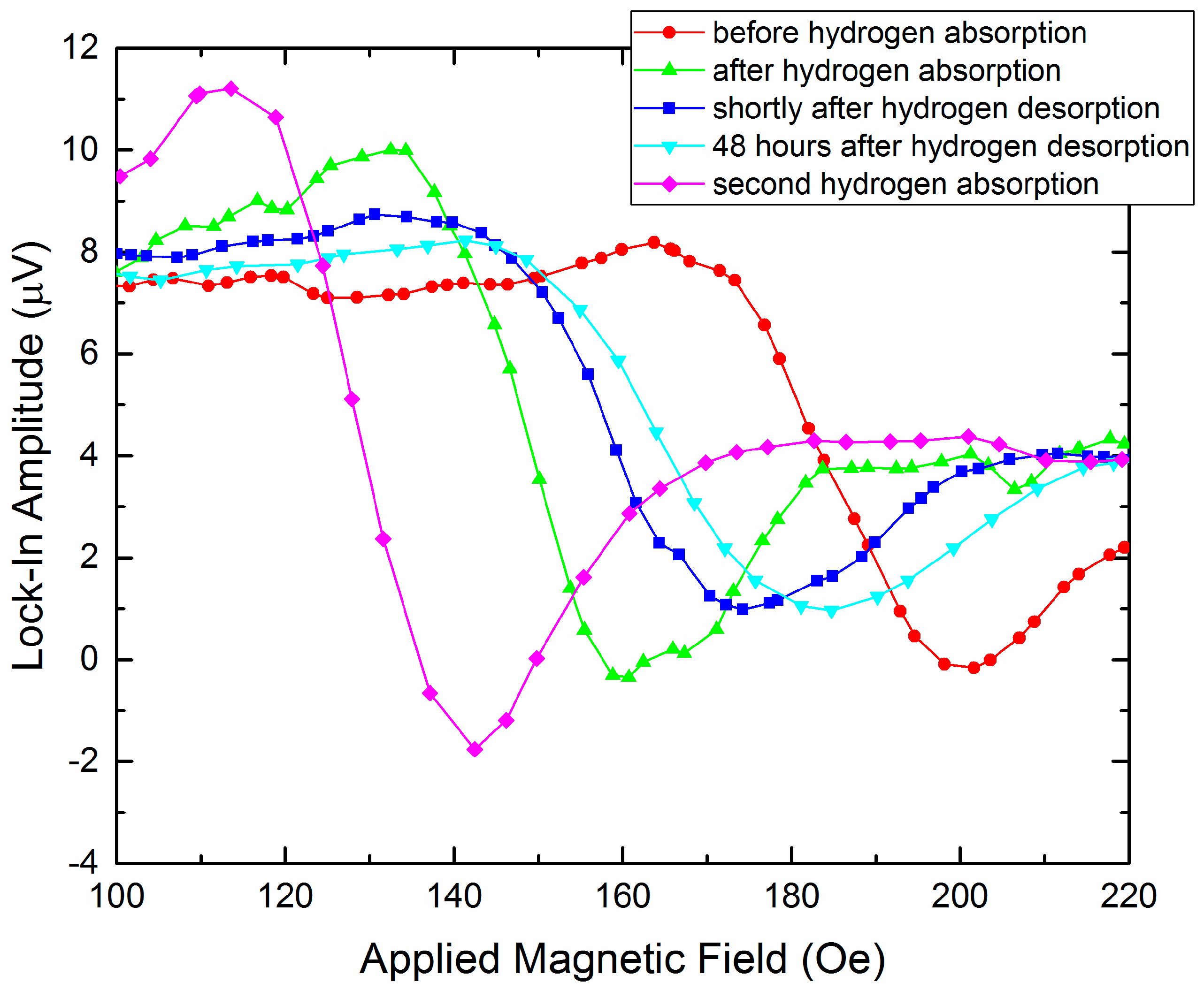
3.1.3. Co/Pd Bilayers
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrog. Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Van den Berg, A.W.C.; Areán, C.O. Materials for hydrogen storage: Current research trends and perspectives. Chem. Commun. 2008, 668–681. [Google Scholar] [CrossRef]
- Liu, C.; Li, F.; Ma, L.P.; Cheng, H.M. Advanced materials for energy storage. Adv. Mater. 2010, 22, E28–E62. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, C.; Grätzel, M.; Kostecki, R.; Mao, S.S. Nanomaterials for renewable energy production and storage. Chem. Soc. Rev. 2012, 41, 7909–7937. [Google Scholar] [CrossRef] [PubMed]
- Pukazhselvan, D.; Kumar, V.; Singh, S.K. High capacity hydrogen storage: Basic aspects, new developments and milestones. Nano Energy 2012, 1, 566–589. [Google Scholar] [CrossRef]
- Baldi, A.; Gonzalez-Silveira, M.; Palmisano, V.; Dam, B.; Griessen, R. Destabilization of the Mg–H System through Elastic Constraints. Phys. Rev. Lett. 2009, 102, 226102. [Google Scholar] [CrossRef] [PubMed]
- Peisl, H. Lattice strains due to hydrogen in metals. In Hydrogen in Metals I; Springer Berlin Heidelberg: Berlin, Germany, 1978; pp. 53–74. [Google Scholar]
- Zabel, H. Thin films: Hydrogen. In Encyclopedia of Materials: Science and Technology, 2nd ed.; Buschow, K.H.J., Cahn, R.W., Flemings, M.C., Ilschner, B., Kramer, E.J., Mahajan, S., Veyssière, P., Eds.; Elsevier: Oxford, UK, 2001; pp. 9246–9250. [Google Scholar]
- Huiberts, J.N.; Griessen, R.; Rector, J.H.; Wijngaarden, R.J.; Dekker, J.P.; de Groot, D.G.; Koeman, N.J. Yttrium and lanthanum hydride films with switchable optical properties. Nature 1996, 380, 231–234. [Google Scholar] [CrossRef]
- Klose, F.; Rehm, C.; Nagengast, D.; Maletta, H.; Weidinger, A. Continuous and reversible change of the magnetic coupling in an Fe/Nb multilayer induced by hydrogen charging. Phys. Rev. Lett. 1997, 78, 1150–1153. [Google Scholar] [CrossRef]
- Hjörvarsson, B.; Dura, J.A.; Isberg, P.; Watanabe, T.; Udovic, T.J.; Andersson, G.; Majkrzak, C.F. Reversible tuning of the magnetic exchange coupling in Fe/V(001) superlattices using hydrogen. Phys. Rev. Lett. 1997, 79, 901–904. [Google Scholar] [CrossRef]
- Kravtsov, E.; Nefedov, A.; Nowak, G.; Zhernenkov, K.; Zabel, H.; Hjörvarsson, B.; Liebig, A.; Hoser, A.; McIntyre, G.J.; Paolasini, L.; et al. Fine-tuning of the spin-density-wave state in Cr/V heterostructures via hydrogen uptake. J. Phys. Condens. Matter 2009, 21, 336004. [Google Scholar] [CrossRef] [PubMed]
- Andersson, G.; Hjörvarsson, B.; Zabel, H. Hydrogen-induced lattice expansion of vanadium in a Fe/V(001) single-crystal superlattice. Phys. Rev. B 1997, 55, 15905. [Google Scholar] [CrossRef]
- Yang, Q.M.; Schmitz, G.; Fähler, S.; Krebs, H.U.; Kirchheim, R. Hydrogen in Pd/Nb multilayers. Phys. Rev. B 1996, 54, 9131–9140. [Google Scholar] [CrossRef]
- Chang, C.S.; Kostylev, M.; Ivanov, E. Metallic spintronic thin film as a hydrogen sensor. Appl. Phys. Lett. 2013, 102, 142405. [Google Scholar] [CrossRef]
- Westerwaal, R.J.; Rooijmans, J.S.A.; Leclercq, L.; Gheorghe, D.G.; Radeva, T.; Mooij, L.; Mak, T.; Polak, L.; Slaman, M.; Dama, B.; et al. Nanostructured Pd-Au based fiber optic sensors for probing hydrogen concentrations in gas mixtures. Int. J. Hydrog. Energy 2013, 38, 4201–4212. [Google Scholar] [CrossRef]
- Ngene, P.; Radeva, T.; Slaman, M.; Westerwaal, R.J.; Schreuders, H.; Dam, B. Seeing hydrogen in colors: Low-cost and highly sensitive eye readable hydrogen detectors. Adv. Funct. Mater. 2014, 24, 2374–2382. [Google Scholar] [CrossRef]
- Hübert, T.; Boon-Brett, L.; Black, G.; Banach, U. Hydrogen sensors—A review. Sens. Actuators B 2011, 157, 329–352. [Google Scholar] [CrossRef]
- Ford, D.C.; Cooley, L.D.; Seidman, D.N. First principles calculations of niobium hydride formation in superconducting radio-frequency cavities. Supercond. Sci. Technol. 2013. [Google Scholar] [CrossRef]
- Fukai, Y. The Metal-Hydrogen System: Basic Bulk Properties; Springer: Berlin, Germany, 2005. [Google Scholar]
- Zabel, H.; Peisl, H. X-ray study of the phase diagram of hydrogen in niobium. Phys. Stat. Sol. A 1976, 37, K67–K70. [Google Scholar] [CrossRef]
- Walter, R.J.; Chandler, W.T. The columbium-hydrogen constitution diagram. Trans. Met. Soc. AIME 1965, 233, 762–765. [Google Scholar]
- Manchester, F.D.; San-Martin, A.; Pitre, J.M. The H-Pd (hydrogen-palladium) system. J. Phase Equilib. 1995, 15, 62–83. [Google Scholar] [CrossRef]
- Pundt, A.; Kirchheim, R. Hydrogen in Metals: Microstructural Aspects. Annu. Rev. Mater. Res. 2006, 36, 555–608. [Google Scholar] [CrossRef]
- Song, G.; Remhof, A.; Theis-Bröhl, K.; Zabel, H. Extraordinary adhesion of niobium on sapphire substrates. Phys. Rev. Lett. 1997, 79, 5062–5065. [Google Scholar] [CrossRef]
- Hamm, M.; Burlaka, V.; Wagner, S.; Pundt, A. Achieving reversibility of ultra-high mechanical stress by hydrogen loading of thin films. Appl. Phys. Lett. 2015. [Google Scholar] [CrossRef]
- Hjörvarsson, B.; Andersson, G.; Karlsson, E. Metallic superlattices: Quasi two-dimensional playground for hydrogen. J. Alloy. Compd. 1997, 51, 253–254. [Google Scholar] [CrossRef]
- Čížek, J.; Procházka, I.; Bečvář, F.; Kužel, R.; Cieslar, M.; Brauer, G.; Anwand, W.; Kirchheim, R.; Pundt, A. Hydrogen-induced defects in bulk niobium. Phys. Rev. B 2004. [Google Scholar] [CrossRef]
- Romanenko, A.; Edwardson, C.J.; Coleman, P.G.; Simpson, P.J. The effect of vacancies on the microwave surface resistance of niobium revealed by positron annihilation spectroscopy. Appl. Phys. Lett. 2013. [Google Scholar] [CrossRef]
- Fritzsche, H.; Saoudi, M.; Haagsma, J.; Ophus, C.; Luber, E.; Harrower, C.T.; Mitlin, D. Neutron reflectometry study of hydrogen desorption in destabilized MgAl alloy thin films. Appl. Phys. Lett. B 2008. [Google Scholar] [CrossRef]
- Maxelon, M.; Pundt, A.; Pyckhout-Hintzen, W.; Barker, J.; Kirchheim, R. Interaction of hydrogen and deuterium with dislocations in palladium as observed by small angle neutron scattering. Acta Mater. 2001, 49, 2625–2634. [Google Scholar] [CrossRef]
- Munter, A.E.; Heuser, B.J. Deuterium phase behavior in thin-film Pd. Phys. Rev. B 1998, 58, 678–684. [Google Scholar] [CrossRef]
- Song, G.; Geitz, M.; Abromeit, A.; Zabel, H. Solubility isotherms of hydrogen in epitaxial Nb(110) films. Phys. Rev. B 1996, 54, 14093–14101. [Google Scholar] [CrossRef]
- Reimer, P.M.; Zabel, H.; Flynn, C.P.; Matheny, A.; Ritley, K. Elastic properties of hydrogen-loaded epitaxial films. Z. Phys. Chem. 1993, 181, 367–373. [Google Scholar] [CrossRef]
- Reimer, P.M.; Zabel, H.; Flynn, C.P.; Dura, J.A. Extraordinary alignment of Nb films with sapphire and the effects of added hydrogen. Phys. Rev. B 1992, 45, 11426–11429. [Google Scholar] [CrossRef]
- Laudahn, U.; Pundt, A.; Bicker, M.; Hülsen, U.; Geyer, U.; Wagner, T.; Kirchheim, R. Hydrogen-induced stress in Nb single layers. J. Alloy. Compd. 1999. [Google Scholar] [CrossRef]
- Hjörvarsson, B.; Rydén, J.; Karlsson, E.; Birch, J.; Sundgren, J.E. Interface effects of hydrogen uptake in Mo/V single-crystal superlattices. Phys. Rev. B 1991, 43, 6440–6445. [Google Scholar] [CrossRef]
- Stillesjö, F.; Hjörvarsson, B.; Zabel, H. Hydrogen-induced lattice expansion in a (001)-oriented Mo/V superlattice. Phys. Rev. B 1996, 54, 3079–3083. [Google Scholar] [CrossRef]
- Munter, A.E.; Heuser, B.J.; Ruckman, M.W. In situ neutron reflectometry measurements of hydrogen and deuterium absorption in a Pd/Nb/Pd layered film. Phys. Rev. B 1997, 55, 14035–14038. [Google Scholar] [CrossRef]
- Gahr, S.; Grossbeck, M.L.; Birnbaum, H.K. Hydrogen embrittlement of Nb I-macroscopic behavior at low temperatures. Acta Met. 1977, 25, 125–134. [Google Scholar] [CrossRef]
- Rehm, C.; Fritzsche, H.; Maletta, H.; Klose, F. Hydrogen concentration and its relation to interplanar spacing and layer thickness of 1000-Å Nb(110) films during in situ hydrogen charging experiments. Phys. Rev. B 1999, 59, 3142–3152. [Google Scholar] [CrossRef]
- Klose, F.; Rehm, C.; Fieber-Erdmann, M.; Holub-Krappe, E.; Bleif, H.J.; Sowers, H.; Goyette, R.; Tröger, L.; Maletta, H. Hydrogen absorption in epitaxial W/Nb(001) and polycrystalline Fe/Nb(110) multilayers studied in situ by X-ray/neutron scattering techniques and X-ray absorption spectroscopy. Phys. B 2000, 283, 184–188. [Google Scholar] [CrossRef]
- Rehm, C.; Maletta, H.; Fieber-Erdmann, M.; Holub-Krappe, E.; Klose, F. Anomalous layer expansion in thin niobium films during hydrogen absorption. Phys. Rev. B 2002. [Google Scholar] [CrossRef]
- Buttner, W.J.; Post, M.B.; Burgess, R.; Rivkin, C. An overview of hydrogen safety sensors and requirements. Int. J. Hydrog. Energy 2011, 36, 2462–2470. [Google Scholar] [CrossRef]
- Lewis, F.A. The Palladium Hydrogen System; Academic Press: New York, NY, USA, 1967. [Google Scholar]
- Gupta, R.; Sagade, A.A.; Kulkarni, G.U. A low cost optical hydrogen sensing device using nanocrystalline Pd grating. Int. J. Hydrog. Energy 2012, 37, 9443–9449. [Google Scholar] [CrossRef]
- Engel, B.N.; England, C.D.; van Leeuwen, R.A.; Wiedmann, M.H.; Falco, C.M. Interface magnetic anisotropy in epitaxial superlattices. Phys. Rev. Lett. 1991, 67, 1910–1913. [Google Scholar] [CrossRef] [PubMed]
- Neutron Activation Calculator. Available online: https://www.ncnr.nist.gov/resources/activation/ (accessed on 12 April 2016).
- Mezei, F.; Golub, R.; Klose, F.; Toews, H. Focussed beam reflectometer for solid and liquid surfaces. Phys. B 1995. [Google Scholar] [CrossRef]
- Paul, A.; Krist, T.; Teichert, A.; Steitz, R. Specular and off-specular scattering with polarization and polarization analysis on reflectometer V6 at BER II, HZB. Physica B 2011, 406, 1598–1606. [Google Scholar] [CrossRef]
- Crawford, R.K.; Felcher, G.P.; Kleb, R.; Epperson, J.E.; Thiyagarajan, P. New Instruments at IPNS: POSY II and SAD II; Hyer, D.K., Ed.; IOP Publishing Ltd.: Bristol, UK, 1989. [Google Scholar]
- Gurevich, A.G.; Melkov, G.A. Magnetization Oscillation and Waves; C.R.C. Press: New York, NY, USA, 1996. [Google Scholar]
- James, M.; Nelson, A.; Brule, A.; Schulz, J.C. Platypus: A time-of-flight neutron reflectometer at Australia’s new research reactor. J. Neutron Res. 2006, 14, 91–108. [Google Scholar] [CrossRef]
- James, M.; Nelson, A.; Holt, S.A.; Saerbeck, T.; Hamilton, W.A.; Klose, F. The multipurpose time-of-flight neutron reflectometer “Platypus” at Australia’s OPAL reactor. Nucl. Instrum. Methods Phys. Res. Sec. A 2011, 632, 112–123. [Google Scholar] [CrossRef]
- Saerbeck, T.; Klose, F.; LeBrun, A.P.; Füzi, J.; Brule, A.; Nelson, A.; Holt, S.A.; James, M. Polarization “Down Under”: The polarized time-of-flight neutron reflectometer PLATYPUS. Rev. Sci. Instrum. 2012. [Google Scholar] [CrossRef] [PubMed]
- Maletta, H.; Rehm, C.; Klose, F.; Fieber-Erdmann, M.; Holub-Krappe, E. Anomalous effects of hydrogen absorption in Nb films. J. Magn. Magn. Mater. 2002, 240, 475–477. [Google Scholar] [CrossRef]
- Hydrogen in Metals I; Alefeld, G.; Völkl, J. (Eds.) Springer: Berlin, Germany, 1978; Volume 28.
- Hydrogen in Metals III; Wipf, H. (Ed.) Springer: Berlin, Germany, 1997; Volume 73.
- Remhof, A.; Song, G.; Sutter, C.; Schreyer, A.; Siebrecht, R.; Zabel, H.; Güthoff, F.; Windgasse, J. Hydrogen and deuterium in epitaxial Y(0001) films: Structural properties and isotope exchange. Phys. Rev. B 1999, 59, 6689–6699. [Google Scholar] [CrossRef]
- Pivak, Y.; Gremaud, R.; Gross, K.; Gonzalez-Silveira, M.; Walton, A.; Book, D.; Schreuders, H.; Dam, B.; Griessen, R. Effect of the substrate on the thermodynamic properties of PdHx films studied by hydrogenography. Scr. Mater. 2009, 60, 348–351. [Google Scholar] [CrossRef]
- Gremaud, R.; Gonzalez-Silveira, M.; Pivak, Y.; de Mana, S.; Slaman, M.; Schreuders, H.; Dam, B.; Griessen, R. Hydrogenography of PdHx thin films: Influence of H-induced stress relaxation processes. Acta Mater. 2009, 57, 1209–1219. [Google Scholar] [CrossRef]
- Maksymov, I.S.; Kostylev, M. Broadband stripline ferromagnetic resonance spectroscopy of ferromagnetic films, multilayers and nanostructures. Phys. E 2015, 69, 253–293. [Google Scholar] [CrossRef]
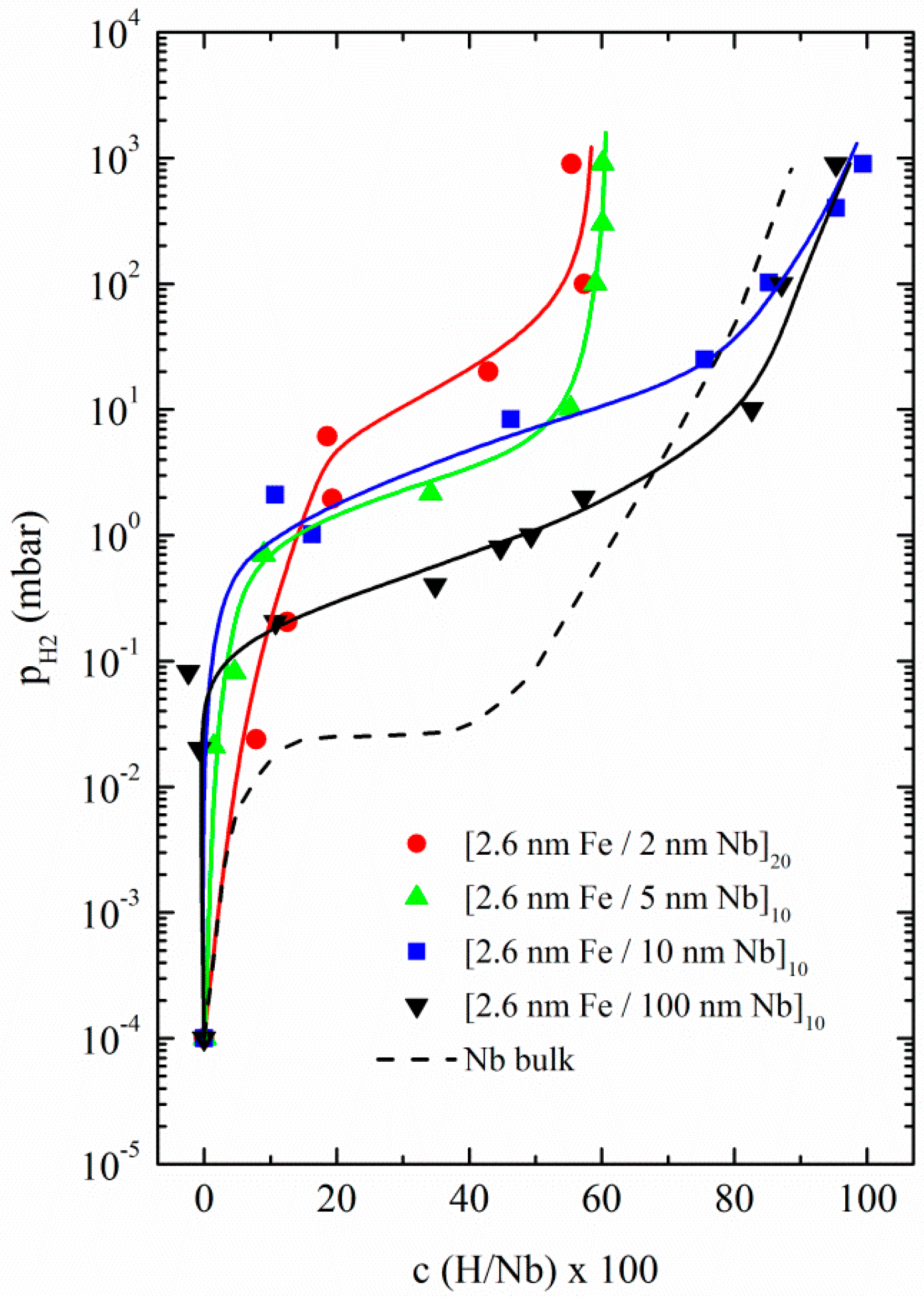

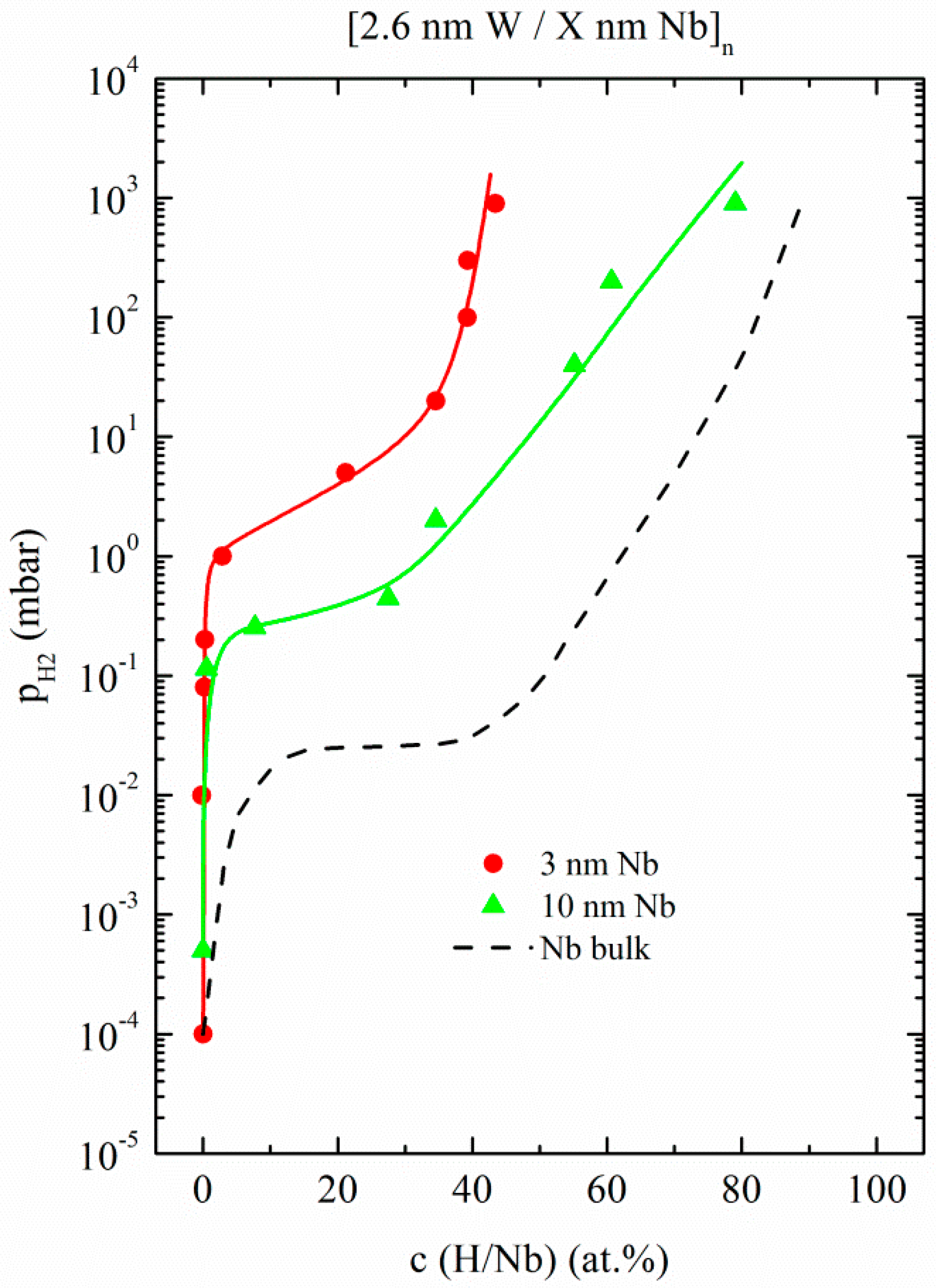
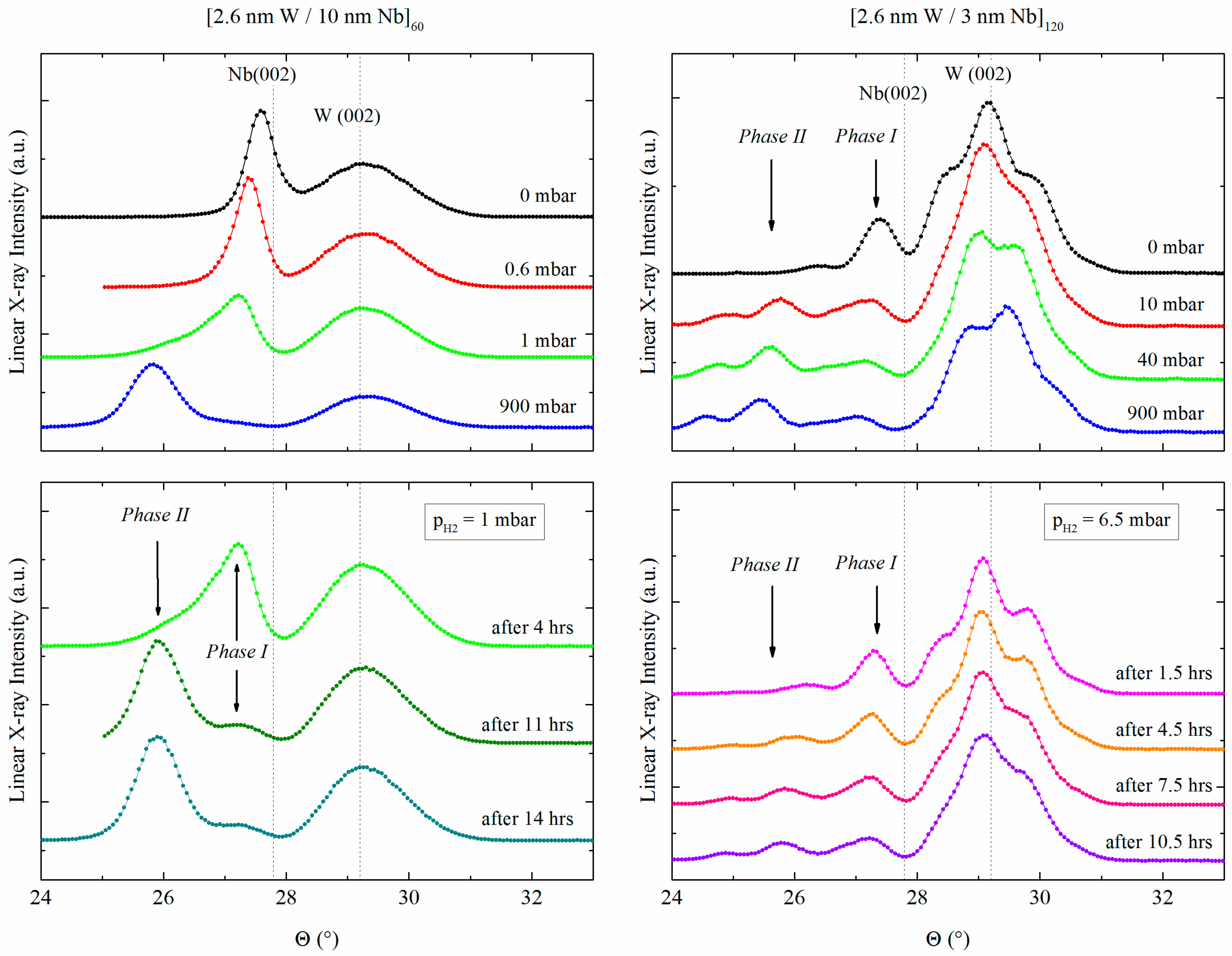
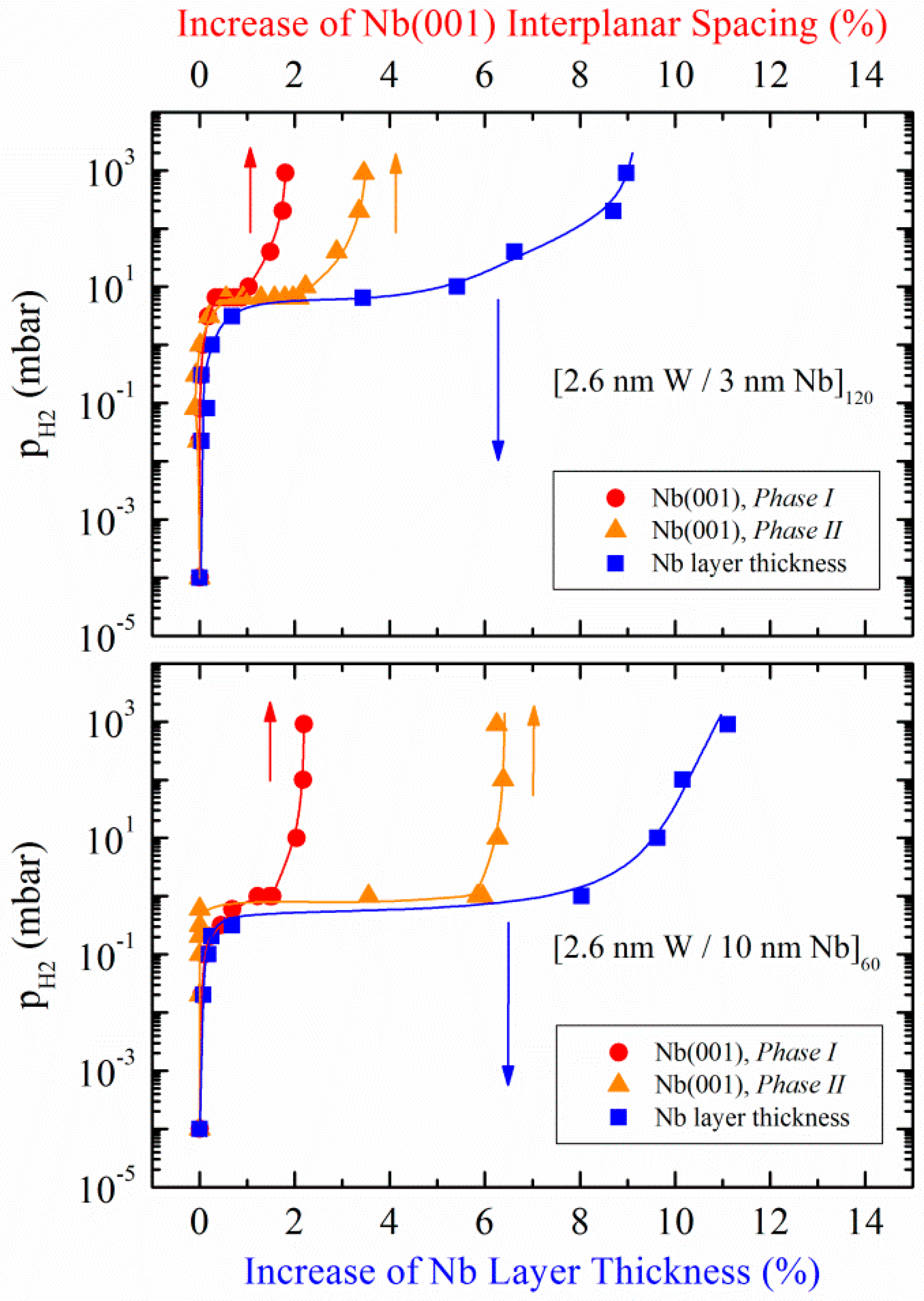
| Fe/Nb(110)—Polycrystalline | W/Nb(110)—Single Crystal |
|---|---|
| [2.6 nm Fe/2 nm Nb]20 | [2.6 nm W/3 nm Nb]120 |
| [2.6 nm Fe/5 nm Nb]10 | [2.6 nm W/10 nm Nb]100 |
| [2.6 nm Fe/10 nm Nb]10 | - |
| [2.6 nm Fe/100 nm Nb]10 | - |
| Layer Material | SLD at 0 mbar (μm−2) | SLD at 900 mbar (μm−2) | Bulk SLD (μm−2) | d at 0 mbar (nm) | d at 900 mbar (nm) |
|---|---|---|---|---|---|
| Pd | 402 | 402 | 402 | 5 | 5 |
| Nb | 380 | 170 | 392 | 93.6 | 103.5 |
| Fe (R+) | 1010 | 1030 | 1300 | 2.6 | 2.6 |
| Fe (R−) | 490 | 570 | 303 | 2.6 | 2.6 |
| Cr | 303 | 303 | 303 | 5 | 5 |
| Si | 207 | 207 | 207 | - | - |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Callori, S.J.; Rehm, C.; Causer, G.L.; Kostylev, M.; Klose, F. Hydrogen Absorption in Metal Thin Films and Heterostructures Investigated in Situ with Neutron and X-ray Scattering. Metals 2016, 6, 125. https://doi.org/10.3390/met6060125
Callori SJ, Rehm C, Causer GL, Kostylev M, Klose F. Hydrogen Absorption in Metal Thin Films and Heterostructures Investigated in Situ with Neutron and X-ray Scattering. Metals. 2016; 6(6):125. https://doi.org/10.3390/met6060125
Chicago/Turabian StyleCallori, Sara J., Christine Rehm, Grace L. Causer, Mikhail Kostylev, and Frank Klose. 2016. "Hydrogen Absorption in Metal Thin Films and Heterostructures Investigated in Situ with Neutron and X-ray Scattering" Metals 6, no. 6: 125. https://doi.org/10.3390/met6060125