Synchronous Upgrading Iron and Phosphorus Removal from High Phosphorus Oolitic Hematite Ore by High Temperature Flash Reduction
Abstract
:1. Introduction
2. Experimental
2.1. Raw Materials
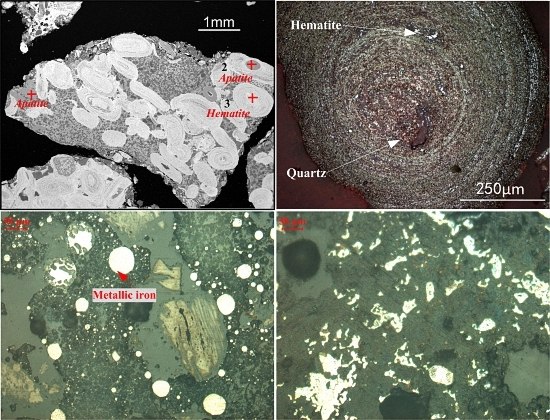
2.1.1. Oolitic Hematite Ore
2.1.2. Reducing Coal
2.1.3. Additive
2.1.4. Flux
2.2. Experimental Methods
2.2.1. High Temperature Flash Reduction
2.2.2. Magnetic Separation
2.2.3. Analytic Tests
2.3. Evaluation Indexes
3. Results and Discussion
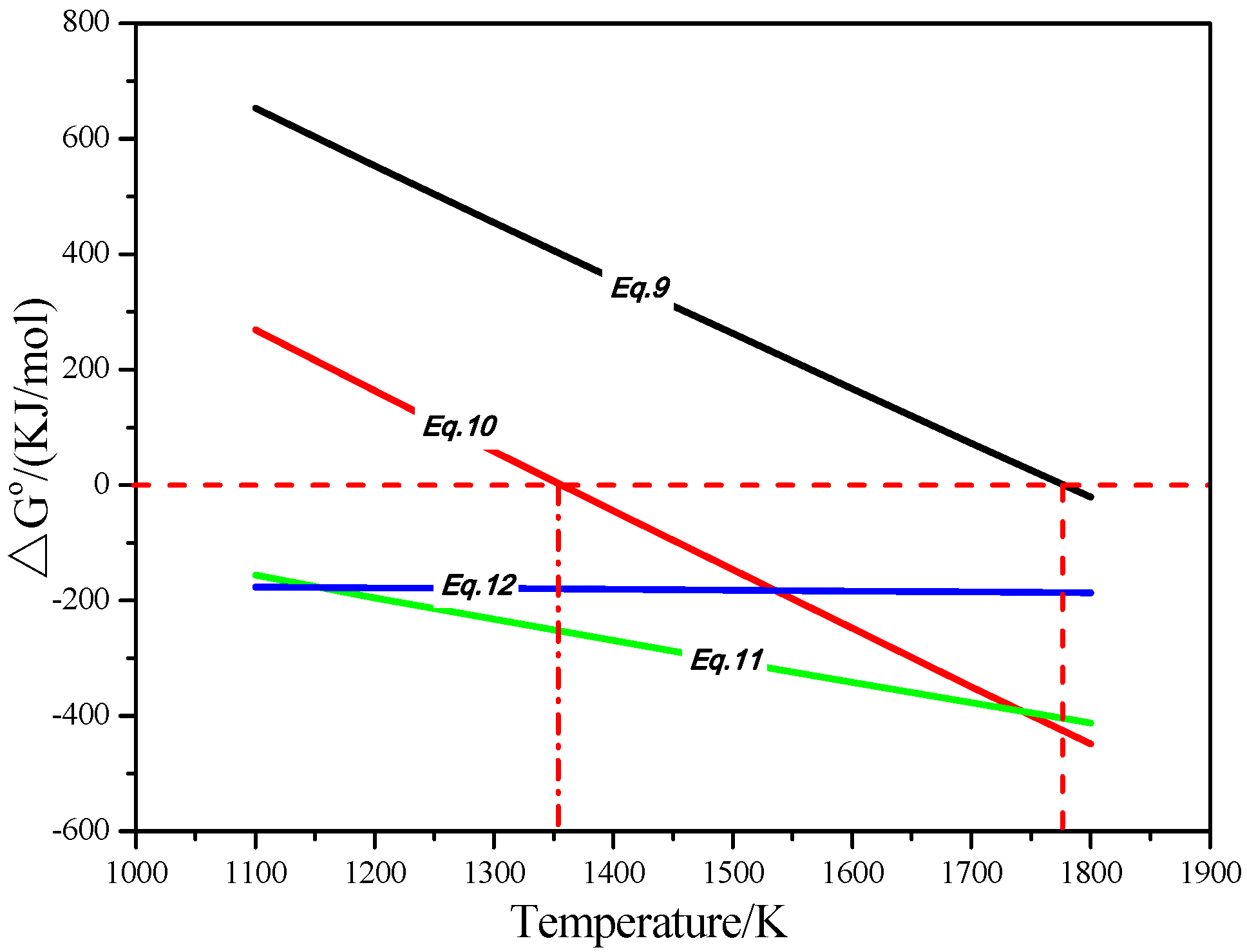
3.1. Thermodynamic Analysis
3.1.1. Thermodynamic Analysis of Iron Mineral Reactions in the Reduction
3.1.2. Thermodynamic Analysis of Phosphorus Mineral Reactions in the Reduction
3.2. High Temperature Quick Reduction
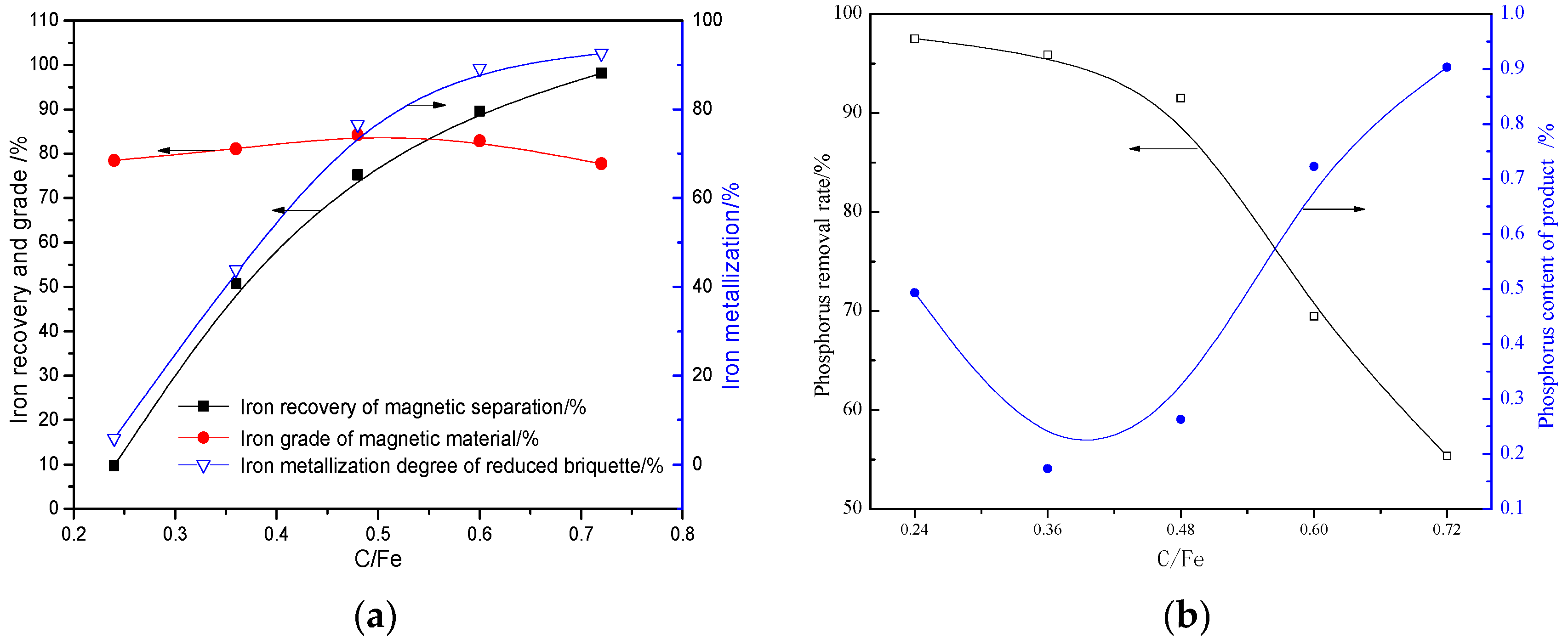
3.2.1. Effects of C/Fe (Mass Ratio)
3.2.2. Effects of Reduction Temperature
3.2.3. Effects of Reduction Duration
3.2.4. Effects of Basicity
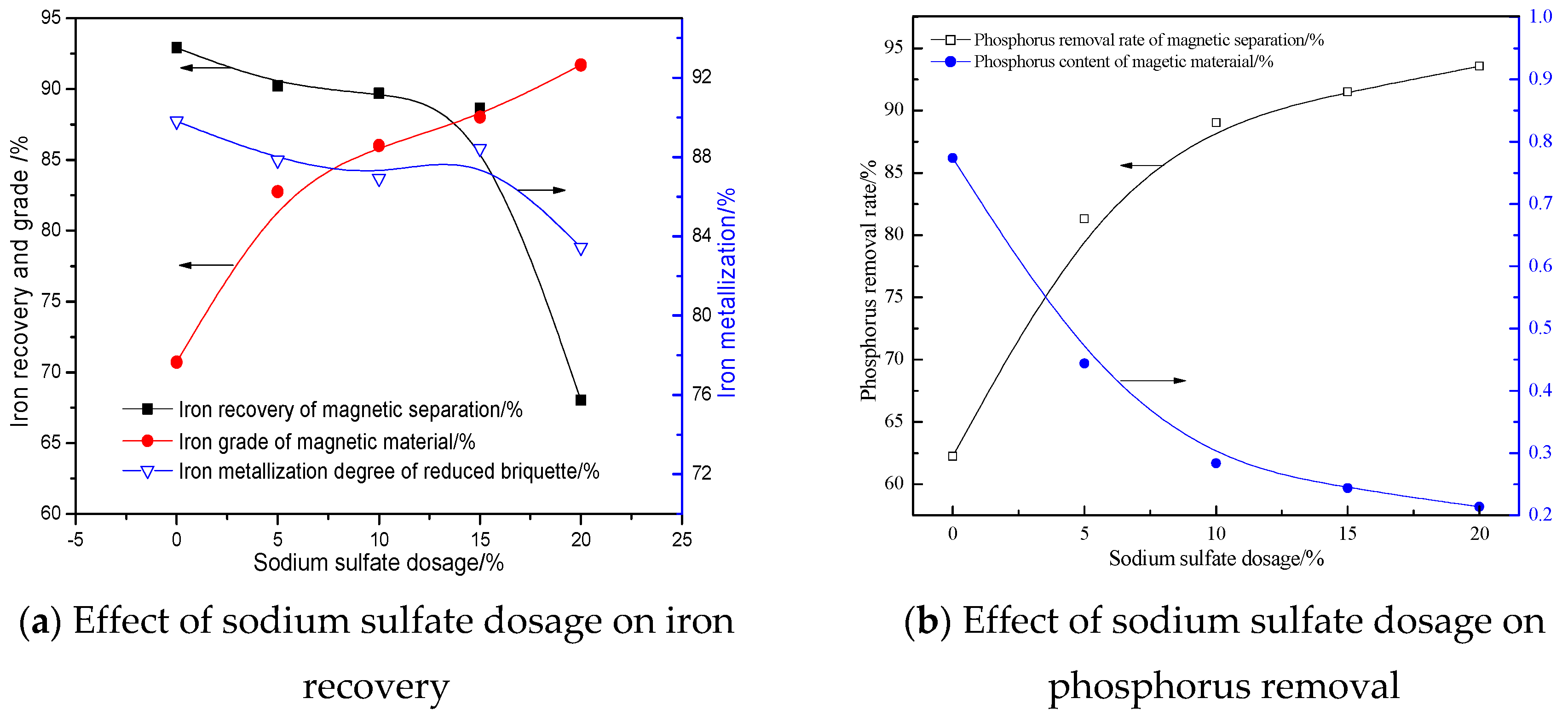
3.2.5. Effects of Sodium Sulfate Dosage
3.3. Magnetic Separation of Reduced Products
3.4. Analysis of Final Product
3.5. Mechanism of Removal of Phosphorus and Beneficiation of Iron
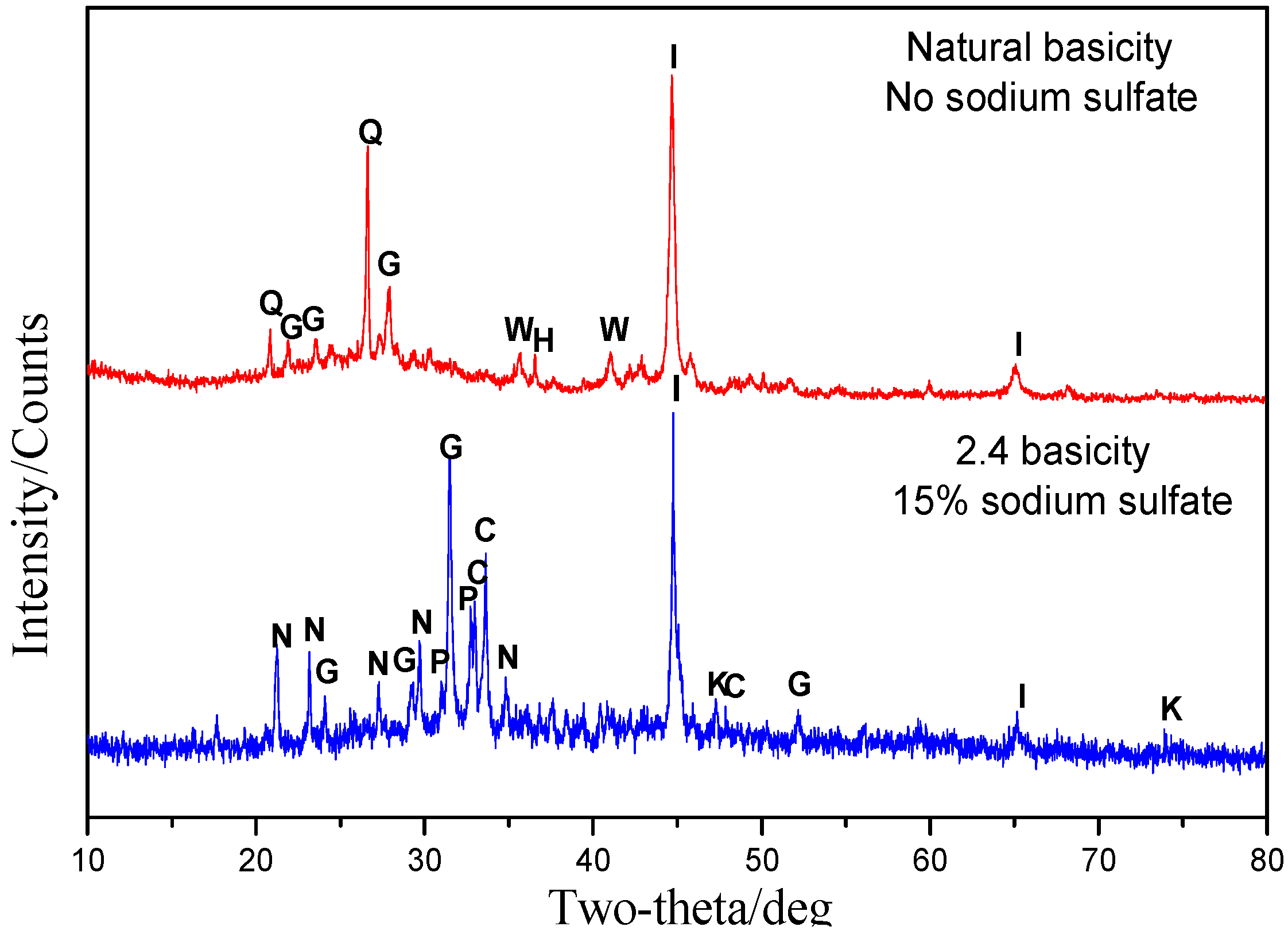
3.5.1. Phase Transformation of Oolitic Hematite Ore During Reduction Process
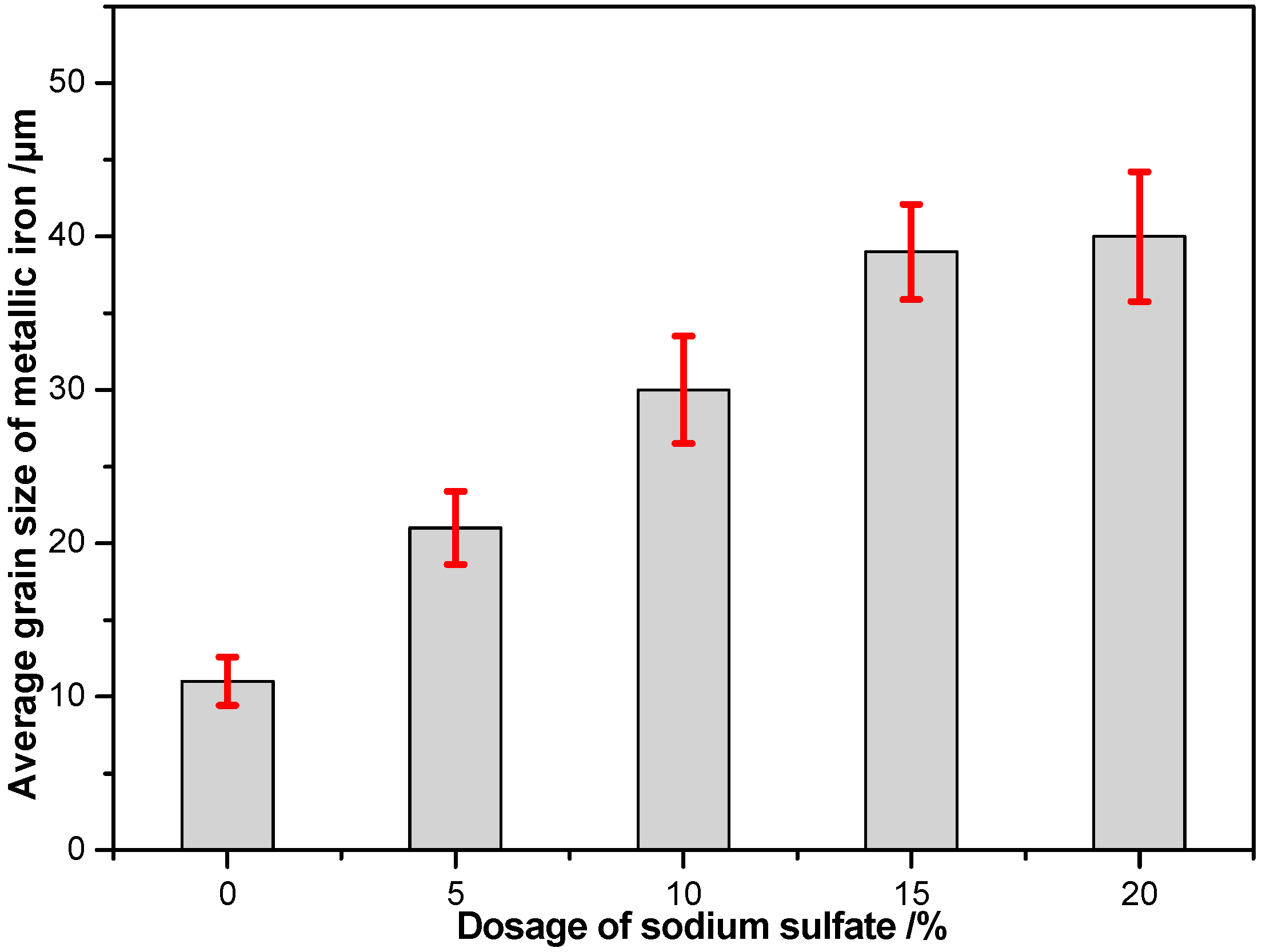
3.5.2. Growth of Metallic Iron Grains in Reduction
4. Conclusions
- Oolitic hematite ore, assaying 41.50% Fetotal, 1.24% P, 17.04%Al2O3 and 4.68% SiO2, was used as a raw material to produce metal iron powder. The characterization of the sample in mineralogy indicates that phosphorus appears mainly in calcium phosphate, and the main iron-bearing mineral, hematite, is superfinely disseminated and combined closely with other gangue minerals, resulting in poor beneficiation of iron and phosphorus removal by traditional dressing process.
- The quick high-temperature reduction process was conducted to improve the separation of Fe and P with the optimized conditions briquetting the ore mixture with 15% sodium sulphate and 2.4 basicity, and reducing at 1350 °C for 10 min with C/Fe of 0.48. The reduced briquettes obtained were then subjected to wet magnetic separation under the conditions of grinding at 95.72% over 0.074 mm, and magnetically separating the ground product in a Davi Tube at 0.10 T, and the final product (metallic iron powder), assaying 91.12% Fetotal and 0.25% P, was produced at overall iron recoveries of 90.08%, which can be used as the burden for steel-making.
- CaO and Na2SO4 can preferentially participate in the reactions with SiO2 and Al2O3, suppressing the reduction of calcium phosphate and intensifying the phosphorus removal. Meanwhile, sodium sulfate is suited to promoting the aggregation and growth of metallic iron grains, resulting in the elevation of the iron grade and recovery in the magnetic separation process.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cheng, C.Y.; Misra, V.N.; Clough, J.; Muni, R. Dephosphorisation of Western Australian iron ore by hydrometallurgical process. Miner. Eng. 1999, 12, 1083–1092. [Google Scholar] [CrossRef]
- Li, K.Q.; Ni, W.; Zhu, M.; Zheng, M.J.; Li, Y. Iron extraction from oolitic iron ore by a deep reduction process. J. Iron Steel Res. Int. 2011, 18, 9–13. [Google Scholar] [CrossRef]
- Forssberg, E.; Adolfsson, G. Dephosphorization of high phosphorus ores by acid leaching. J. Explor. Min. Metall. 1981, 34, 316–322. [Google Scholar]
- Jiang, T.; Jin, Y.S.; Li, Q.; Yang, Y.B.; Li, G.H.; Qiu, G. Dephosphorization technology of iron ores by Acidthiobacillus ferrooxidans. Chin. J. Nonferrous Met. 2007, 17, 1718–1722. [Google Scholar]
- Muhammed, M.; Zhang, Y. A hydrometallurgical process for the dephosphorization of iron ore. Hydrometallurgy 1989, 21, 277–292. [Google Scholar] [CrossRef]
- Delvasto, P.; Valverde, A.; Ballester, A.; Muñoz, J.; González, F.; Blázquez, M.; Igual, J.; García-Balboa, C. Diversity and activity of phosphate bioleaching bacteria from a high-phosphorus iron ore. Hydrometallurgy 2008, 92, 124–129. [Google Scholar] [CrossRef]
- Priha, O.; Sarlin, T.; Blomberg, P.; Wendling, L.; Mäkinen, J.; Arnold, M.; Kinnunen, P. Bioleaching phosphorus from fluorapatites with acidophilic bacteria. Hydrometallurgy 2014, 12, 269–275. [Google Scholar] [CrossRef]
- Tariq, M.B.; Wasim, Y. Bacterial solubilization of phosphorus from phosphate rock containing sulfur-mud. Hydrometallurgy 2014, 103, 54–59. [Google Scholar]
- Matinde, E.; Hino, M. Dephosphorization treatment of high phosphorus iron ore by pre-reduction, air jet milling and screening methods. ISIJ Int. 2011, 51, 544–551. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.S.; Liu, Y.C. Flotation research on a high phosphorus-bearing oolitic hematite ore in Erxi. China Min. Mag. 2011, 11, 71–86. [Google Scholar]
- Yin, J.Q.; Lv, X.W.; Bai, C.G.; Qiu, G.B.; Ma, S.W.; Xie, B. Dephosphorization of iron ore bearing high phosphorous by carbothermic reduction assisted with microwave and magnetic separation. ISIJ Int. 2012, 52, 1579–1584. [Google Scholar] [CrossRef]
- Yu, W.; Sun, T.C.; Kou, J.; Wei, Y.X.; Xu, C.Y.; Liu, Z.Z. The Function of Ca(OH)2 and Na2CO3 as additive on the reduction of high-Phosphorus oolitic hematite-coal mixed pellets. ISIJ Int. 2013, 53, 427–433. [Google Scholar] [CrossRef]
- Bai, S.J.; Wen, S.M.; Liu, D.W.; Zhang, W.B.; Cao, Q.B. Beneficiation of high phosphorus limonite ore by sodium carbonate-added carbothermic reduction. ISIJ Int. 2012, 52, 1757–1763. [Google Scholar] [CrossRef]
- Li, G.H.; Zhang, S.H.; Rao, M.J.; Zhang, Y.B.; Jiang, T. Behavior of phosphorus during the carbothermic reduction of phosphorus-rich oolitic hematite ore in the presence of Na2SO4. Int. J. Miner. Process. 2015, 124, 26–34. [Google Scholar] [CrossRef]
- Li, Y.L.; Sun, T.C.; Xu, C.Y.; Liu, Z.H. New dephosphorizing agent for phosphorus removal from high-phosphorus oolitic hematite ore in direct reduction roasting. J. Cent. South Univ. Sci. Technol. 2012, 43, 827–834. [Google Scholar]
- Guo, Z.Q.; Zhu, D.Q.; Pan, J.; Wu, T.J.; Zhang, F. Improving beneficiation of copper and iron from copper slag by modifying the molten copper slag. Metals 2016. [Google Scholar] [CrossRef]
- Zhu, D.Q.; Chun, T.J.; Pan, J.; Zhang, J.L. Influence of basicity and MgO content on metallurgical performances of Brazilian specularite pellets. Int. J. Miner. Process. 2013, 125, 51–60. [Google Scholar] [CrossRef]
- Zhou, X.L.; Zhu, D.Q.; Jian, P.; Luo, Y.H.; Liu, X.Q. Upgrading of high-aluminum hematite-limonite ore by high temperature reduction-wet magnetic separation process. Metals 2016, 6, 57. [Google Scholar] [CrossRef]
- Cha, J.W.; Kim, D.Y.; Jung, S.M. Distribution behavior of phosphorus and metallization of Iron Oxide in carbothermic reduction of high-phosphorus iron ore. Metall. Mater. Trans. B 2015, 46, 2165–2171. [Google Scholar] [CrossRef]
- Han, H.L.; Duan, D.P.; Wang, X.; Chen, S. Innovative method for separating phosphorus and iron from high-phosphorus oolitic hematite by iron nugget process. Metall. Mater. Trans. B 2014, 45, 1634–1643. [Google Scholar] [CrossRef]
- Chun, T.J.; Zhu, D.Q.; Pan, J.; He, Z. Preparation of alumina from iron powder from red mud by sodium salt roasting and magnetic separation. Can. Metall. Q. 2014, 53, 183–189. [Google Scholar] [CrossRef]
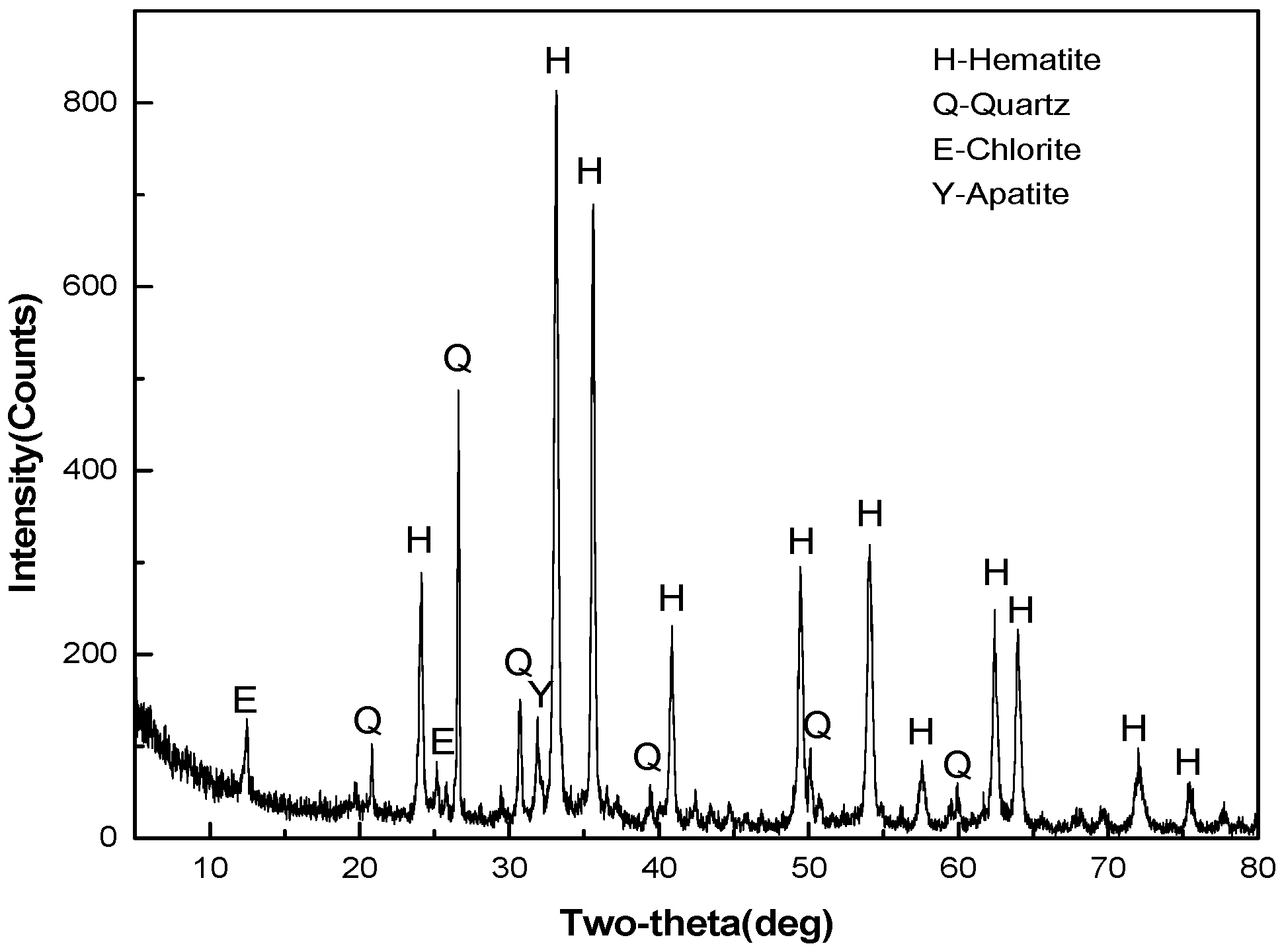
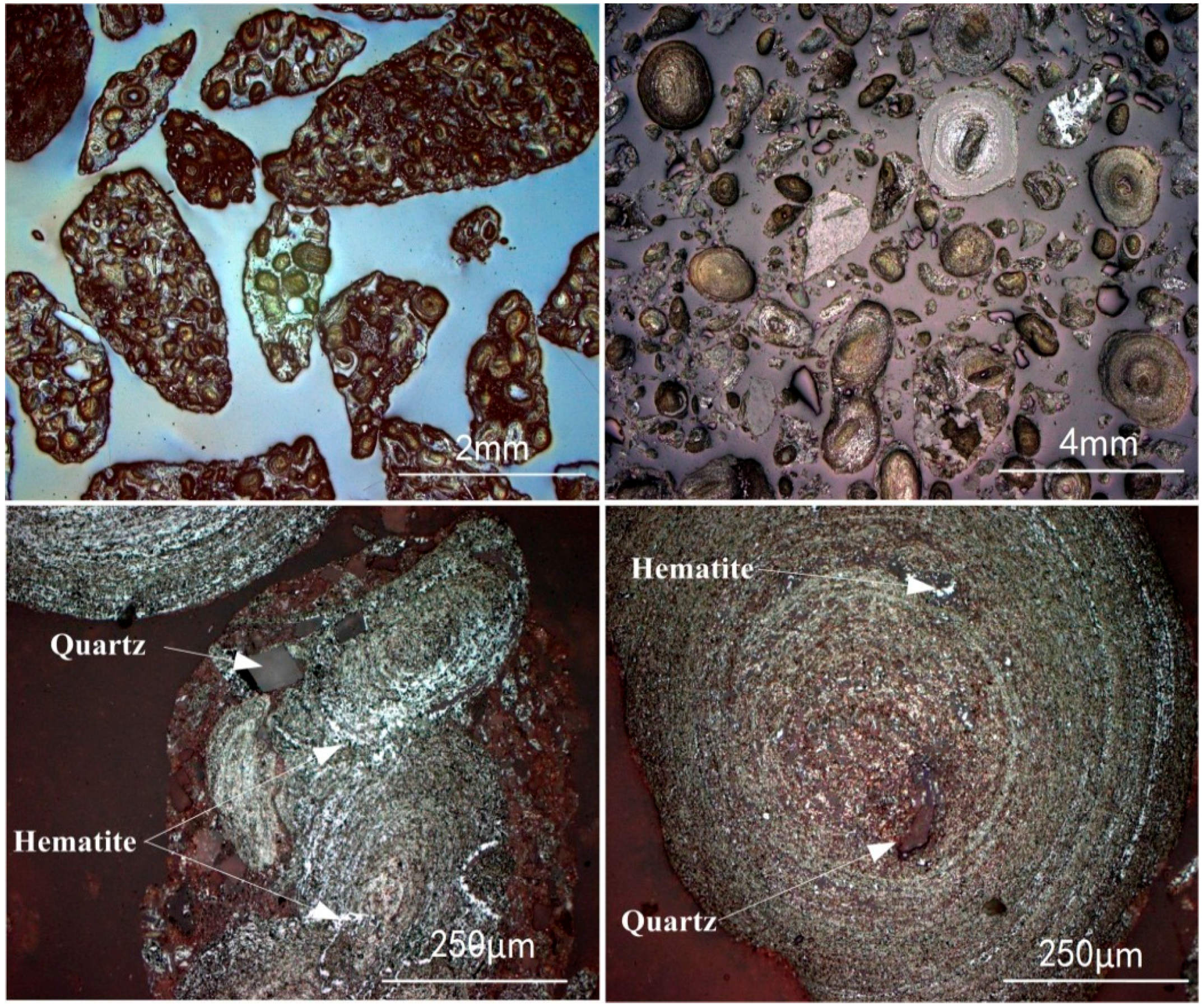






| TFe | P | SiO2 | Al2O3 | CaO | MgO | K2O | S | LOI * |
|---|---|---|---|---|---|---|---|---|
| 41.50 | 1.24 | 17.04 | 4.68 | 7.82 | 1.54 | 0.9 | 0.09 | 5.72 |
| Mineral | Apatite | Iron Phosphate | Others | Total Phosphorus |
|---|---|---|---|---|
| Content | 1.175 | 0.041 | 0.024 | 1.24 |
| Fraction | 94.76 | 3.31 | 1.94 | 100 |
| Fe2O3 | SiO2 | Al2O3 | CaO | MgO | P | S |
|---|---|---|---|---|---|---|
| 16.70 | 27.62 | 8.02 | 24.94 | 1.34 | 0.01 | 5.17 |
| Proximate Analysis/% | Ash Fusibility Analysis/°C | ||||||
|---|---|---|---|---|---|---|---|
| Mad | Aad | Vad | FCad | DT | ST | HT | FT |
| 12.98 | 4.49 | 30.41 | 52.12 | 1332 | 1376 | 1450 | 1469 |
| Reduction Duration/min | Metallization Degree/% | Magnetic Product | Iron Recovery/% | Phosphorus Removal Rate/% | |
|---|---|---|---|---|---|
| TFe/% | P/% | ||||
| 5 | 76.32 | 77.32 | 0.33 | 72.23 | 83.76 |
| 10 | 86.92 | 86.02 | 0.29 | 89.69 | 89.03 |
| 15 | 76.54 | 84.24 | 0.27 | 75.25 | 91.51 |
| 20 | 69.59 | 83.56 | 0.25 | 63.57 | 93.02 |
| TFe | SiO2 | Al2O3 | CaO | MgO | Na2O | P | S |
|---|---|---|---|---|---|---|---|
| 91.12 | 2.13 | 0.27 | 0.79 | 0.54 | 0.35 | 0.25 | 0.10 |
| Grinding Fineness (–0.074 mm/%) | Magnetic Field Intensity/T | Magnetic Product | Iron Recovery/% | Phosphorus Removal Rate/% | |
|---|---|---|---|---|---|
| TFe/% | P/% | ||||
| 88.49 | 0.06 | 89.45 | 0.26 | 82.30 | 91.91 |
| 0.08 | 83.75 | 0.32 | 86.29 | 88.85 | |
| 0.10 | 85.70 | 0.29 | 83.51 | 90.44 | |
| 95.72 | 0.06 | 89.36 | 0.23 | 89.20 | 93.11 |
| 0.08 | 88.78 | 0.24 | 88.26 | 92.48 | |
| 0.10 | 91.12 | 0.25 | 90.08 | 91.79 | |
| 97.86 | 0.06 | 89.37 | 0.27 | 79.30 | 91.90 |
| 0.08 | 90.16 | 0.23 | 77.40 | 93.32 | |
| 0.10 | 88.01 | 0.25 | 88.63 | 91.49 | |
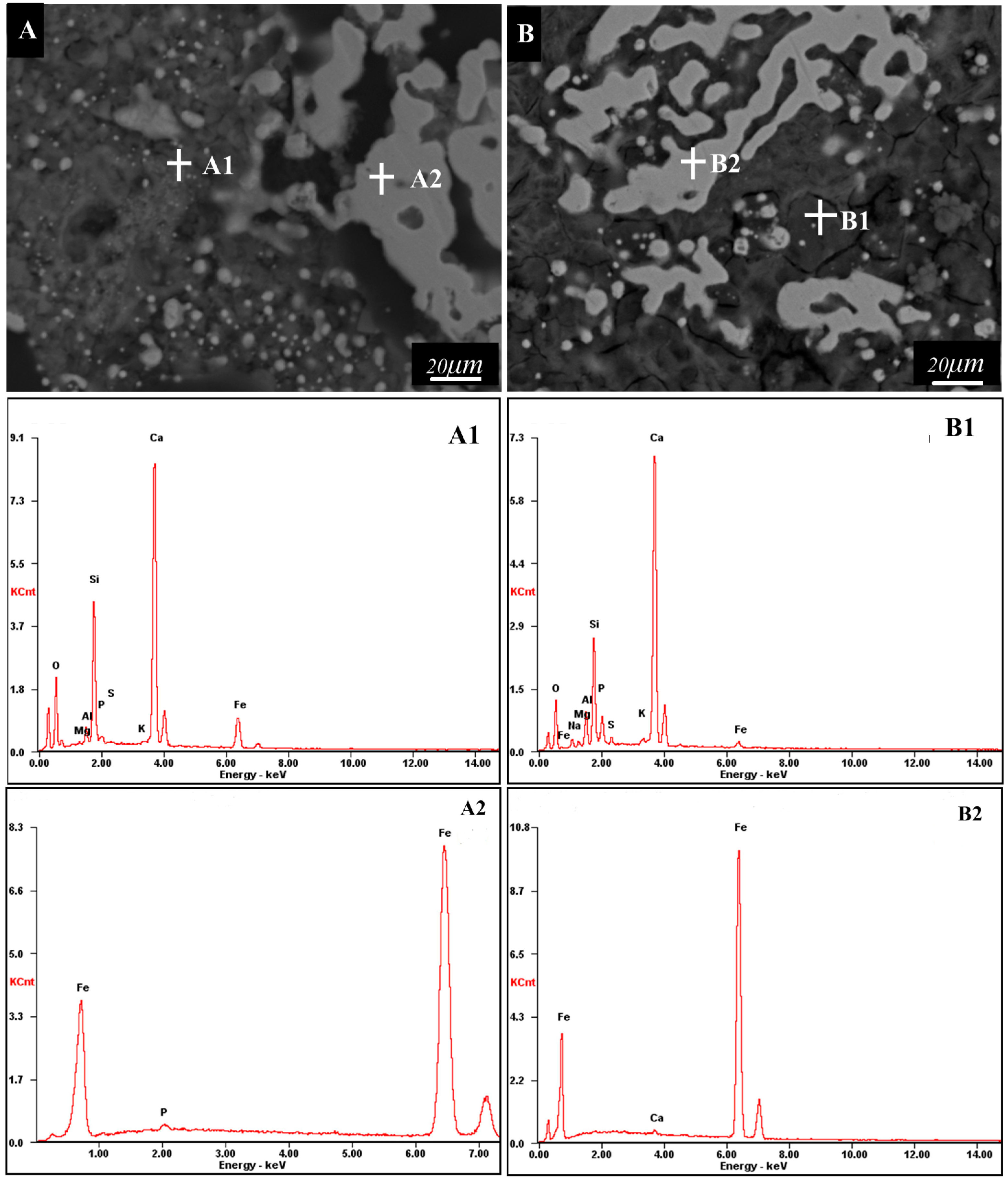
| Element | OK | MgK | AlK | SiK | PK | SK | KK | CaK | FeK | NaK | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | Wt% | 28.72 | 0.28 | 1.63 | 13.60 | 0.88 | 0.18 | 0.53 | 40.94 | 13.25 | / |
| At% | 49.08 | 0.31 | 1.65 | 13.24 | 0.78 | 0.15 | 0.37 | 27.93 | 6.49 | / | |
| B1 | Wt% | 24.83 | 0.93 | 2.44 | 10.80 | 5.21 | 3.93 | 1.15 | 44.67 | 1.81 | 4.23 |
| At% | 41.76 | 1.03 | 2.43 | 10.35 | 4.53 | 3.30 | 0.79 | 29.99 | 0.87 | 4.96 | |
| A2 | Wt% | / | / | / | / | 0.78 | / | / | / | 99.22 | / |
| At% | / | / | / | / | 1.39 | / | / | / | 98.61 | / | |
| B2 | / | / | / | / | / | / | / | / | 0.45 | 99.55 | / |
| / | / | / | / | / | / | / | / | 0.63 | 99.37 | / | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, D.; Guo, Z.; Pan, J.; Zhang, F. Synchronous Upgrading Iron and Phosphorus Removal from High Phosphorus Oolitic Hematite Ore by High Temperature Flash Reduction. Metals 2016, 6, 123. https://doi.org/10.3390/met6060123
Zhu D, Guo Z, Pan J, Zhang F. Synchronous Upgrading Iron and Phosphorus Removal from High Phosphorus Oolitic Hematite Ore by High Temperature Flash Reduction. Metals. 2016; 6(6):123. https://doi.org/10.3390/met6060123
Chicago/Turabian StyleZhu, Deqing, Zhengqi Guo, Jian Pan, and Feng Zhang. 2016. "Synchronous Upgrading Iron and Phosphorus Removal from High Phosphorus Oolitic Hematite Ore by High Temperature Flash Reduction" Metals 6, no. 6: 123. https://doi.org/10.3390/met6060123