Intermetallic Reactions during the Solid-Liquid Interdiffusion Bonding of Bi2Te2.55Se0.45 Thermoelectric Material with Cu Electrodes Using a Sn Interlayer
Abstract
:1. Introduction
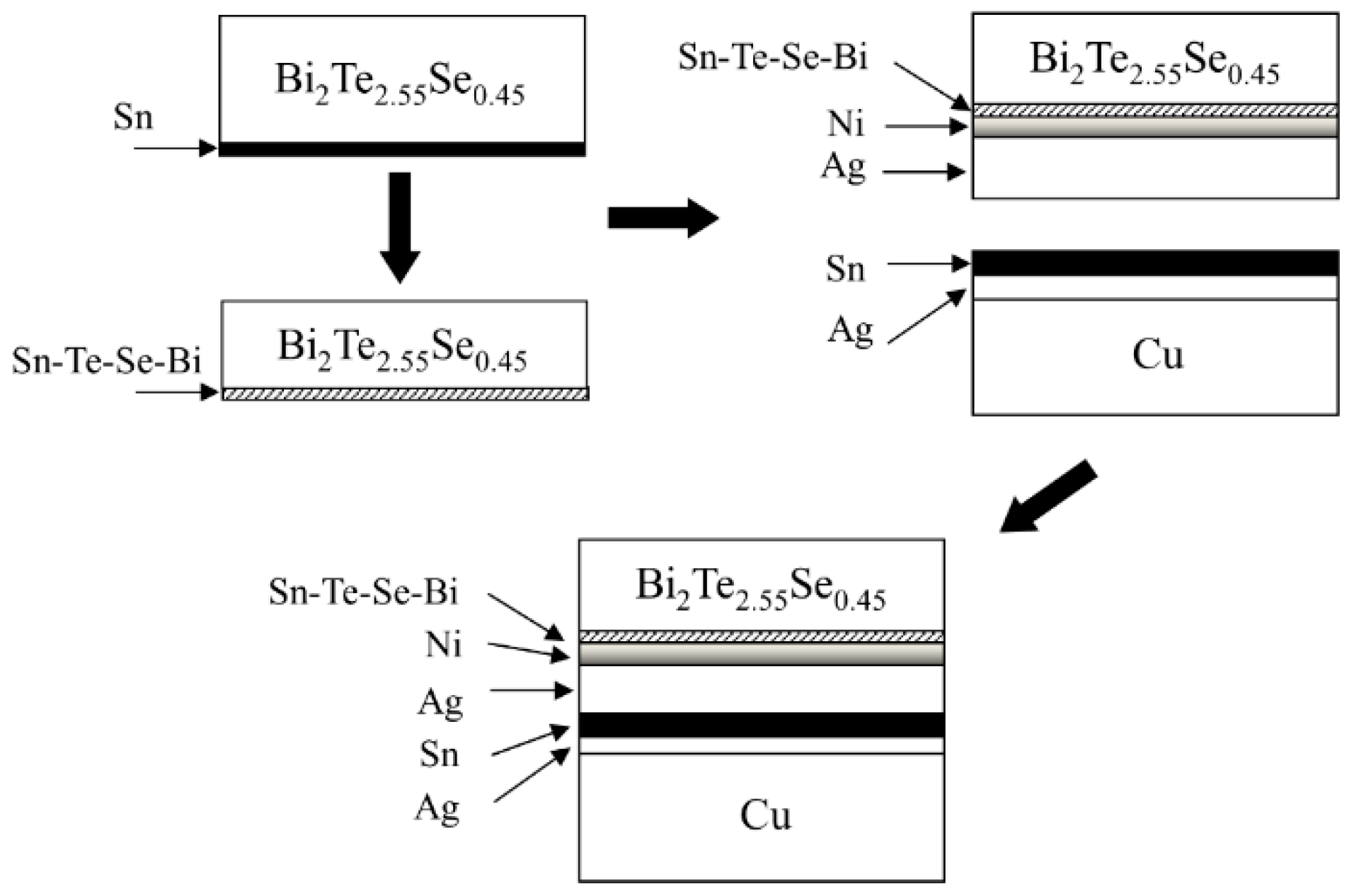
2. Experimental Section
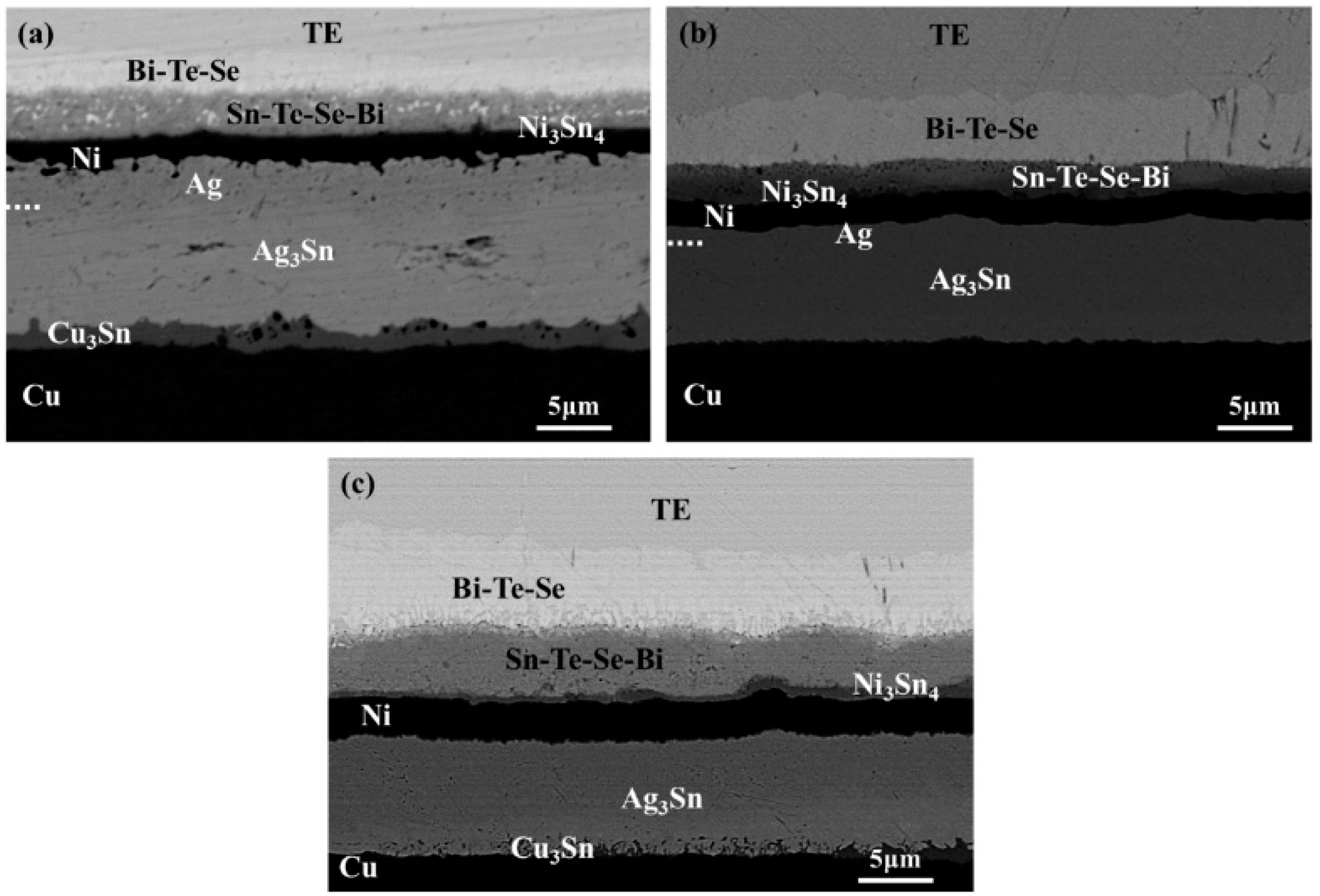
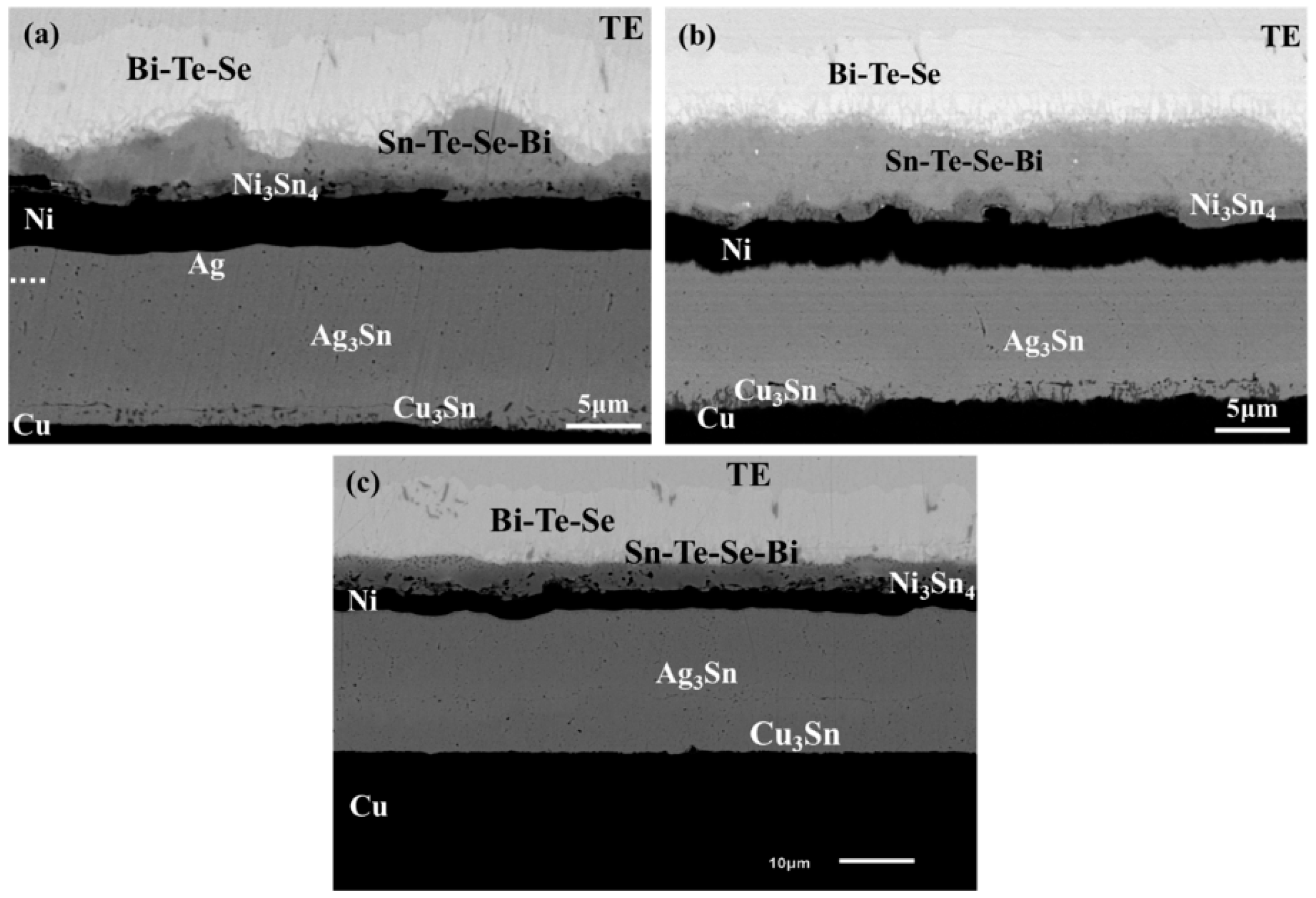
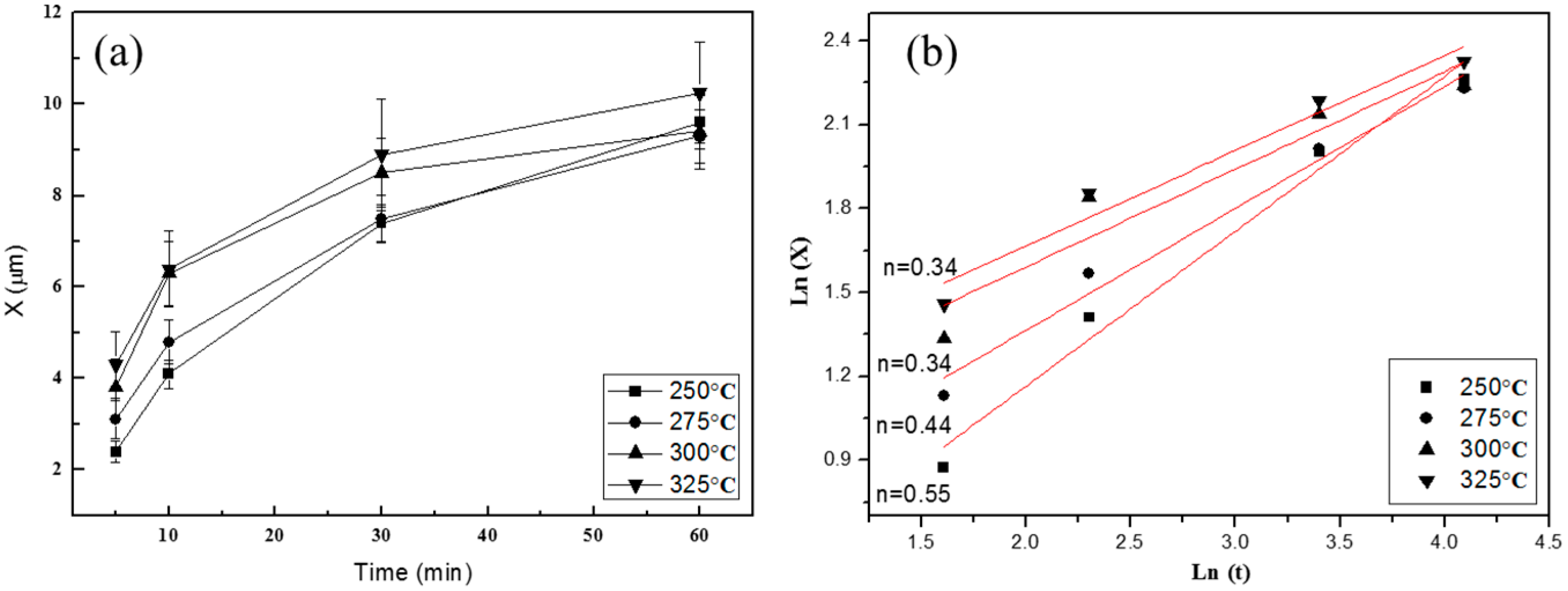
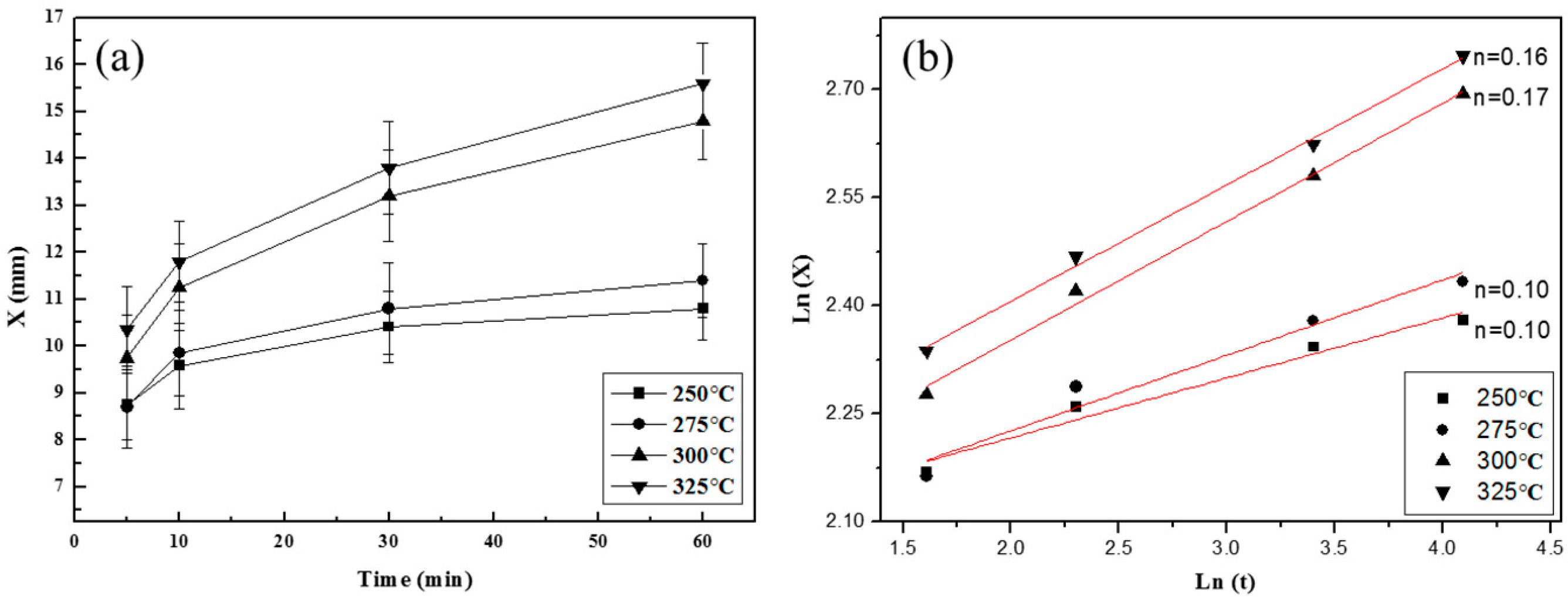
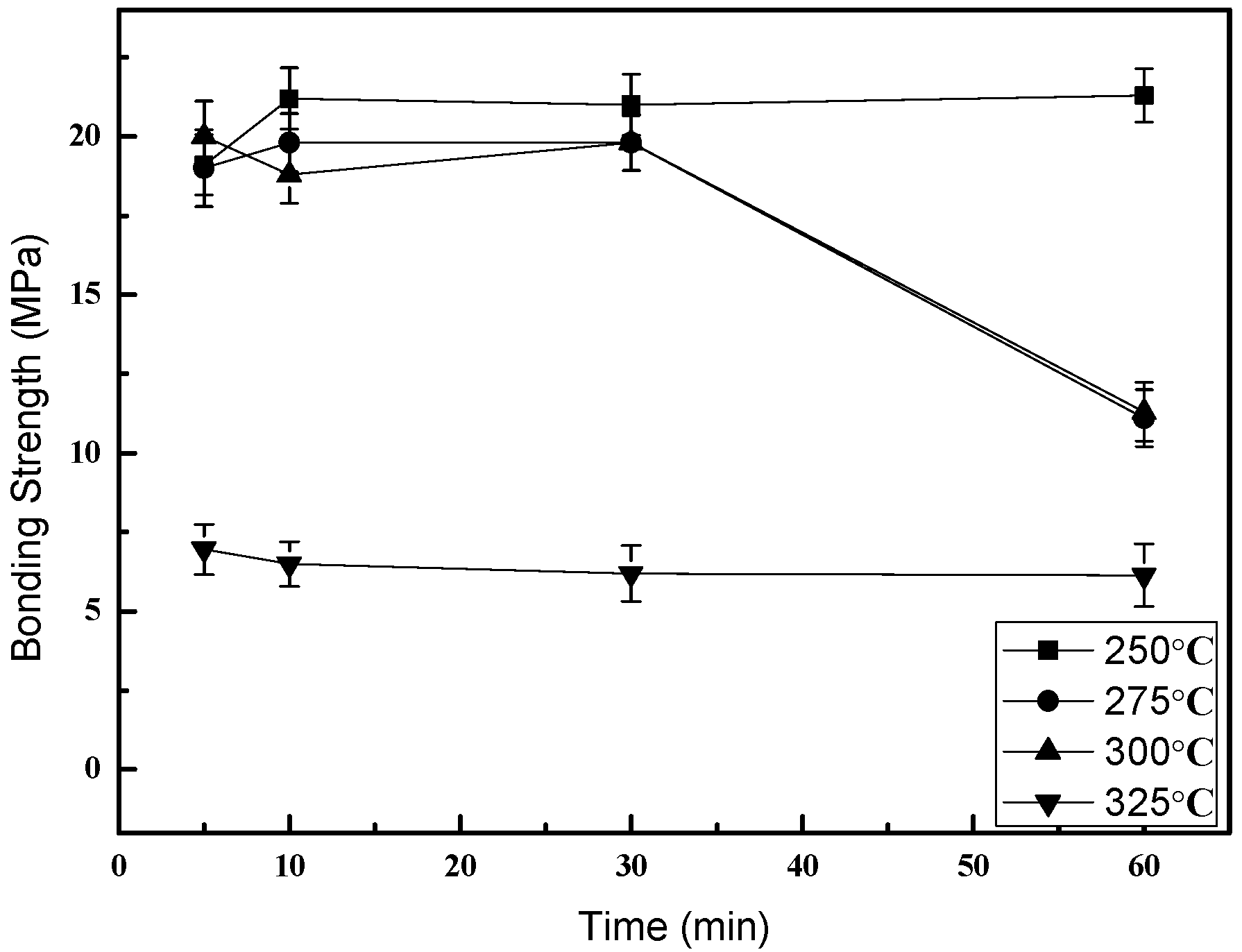
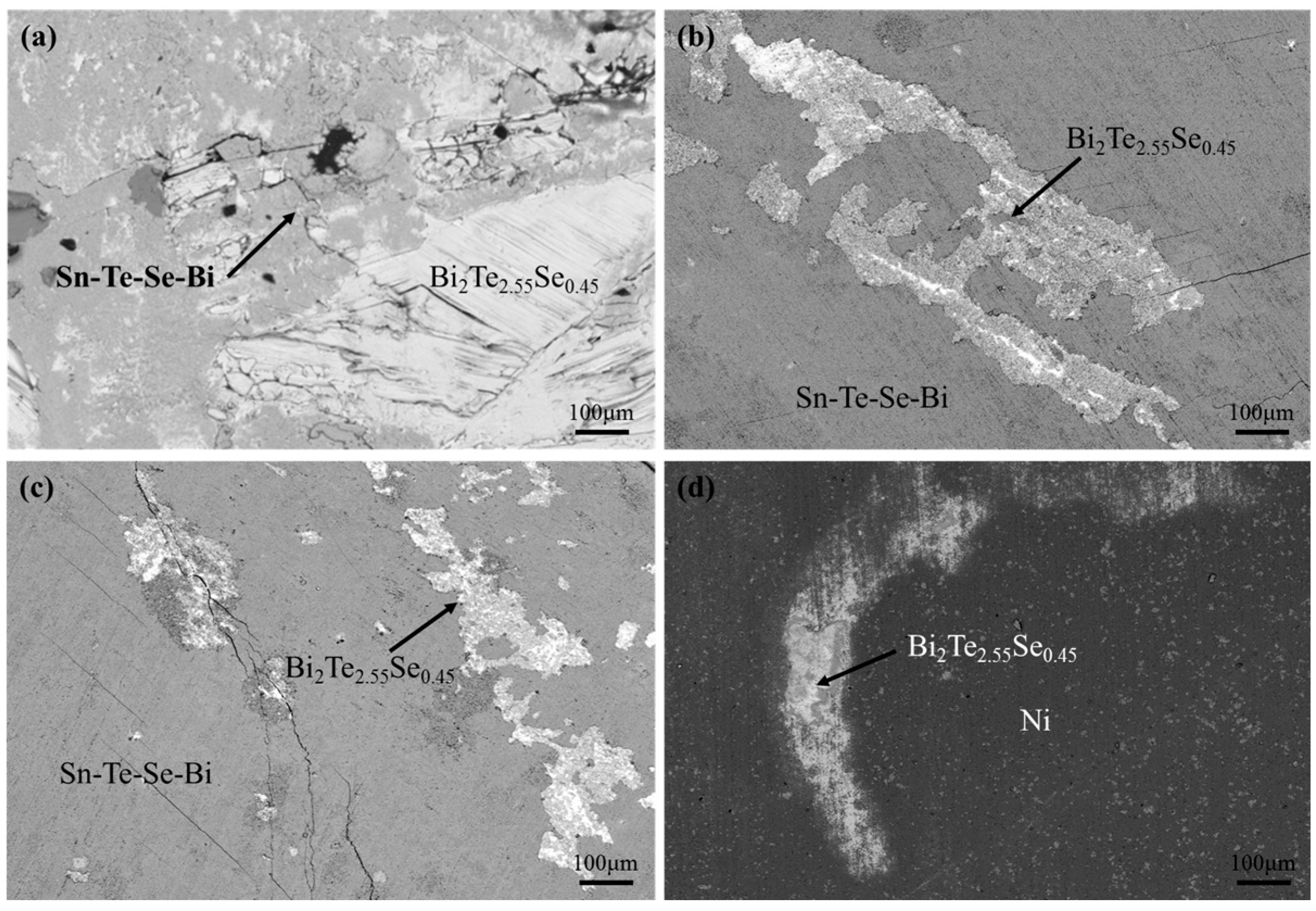
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, H.; Jing, H.Y.; Han, Y.D.; Xu, L.Y. Interfacial reaction between n-and p-type thermoelectric materials and SAC305 solders. J. Alloy. Compd. 2013, 576, 424–431. [Google Scholar] [CrossRef]
- Chien, P.Y.; Yeh, C.H.; Hsu, H.H.; Wu, A.T. Polarity Effect in a Sn3Ag0.5Cu/Bismuth Telluride Thermoelectric System. J. Electron. Mater. 2014, 43, 284–289. [Google Scholar] [CrossRef]
- Ritzer, T.M.; Lau, P.G.; Bogard, A.D. A Critical Evaluation of Today's Thermoelectric Modules. In Proceedings of the 16th International Conference on Thermoelectrics, Dresden, Germany, 26–29 August 1997; pp. 619–623.
- Zybala, R.; Wojciechowski, K.T.; Schmidt, M. Junctions and Diffusion Barriers for High Temperature Thermoelectric Modules. Mater. Ceram./Ceram. Mater. 2010, 62, 481–485. [Google Scholar]
- Jacobson, D.M.; Humpston, G. Diffusion Soldering. Solder. Surf. Mt. Technol. 1992, 10, 27–32. [Google Scholar] [CrossRef]
- Chuang, T.H.; Lin, H.J.; Tsao, C.W. Intermetallic compounds formed during diffusion soldering of Au/Cu/Al2O3 and Cu/Ti/Si with Sn/In interlayer. J. Electron. Mater. 2006, 35, 1566–1570. [Google Scholar] [CrossRef]
- Liang, M.W.; Hsieh, T.E.; Chang, S.Y.; Chuang, T.H. Thin-film reactions during diffusion soldering of Cu/Ti/Si and Au/Cu/Al2O3 with Sn interlayers. J. Electron. Mater. 2003, 32, 952–956. [Google Scholar] [CrossRef]
- Welch, W.C.; Chae, J.; Najafi, K. Transfer of metal MEMS packages using a wafer-level solder transfer technique. IEEE Trans. Adv. Packag. 2005, 28, 643–649. [Google Scholar] [CrossRef]
- Made, R.I.; Gan, C.L.; Yan, L.L.; Yu, A.; Yoon, S.W.; Lau, J.H.; Lee, C.K. Study of Low-Temperature Thermocompression Bonding in Ag-In Solder for Packaging Applications. J. Electron. Mater. 2009, 38, 365–371. [Google Scholar] [CrossRef]
- Li, J.F.; Agyakwa, P.A.; Johnson, C.W. Kinetics of Ag3Sn growth in Ag-Sn-Ag system during transient liquid phase soldering process. Acta Mater. 2010, 58, 3429–3443. [Google Scholar] [CrossRef]
- Yan, L.L.; Lee, C.K.; Yu, D.Q.; Yu, A.B.; Choi, W.K.; Lau, J.H.; Yoon, S.U. A Hermetic Seal Using Composite Thin-Film In/Sn Solder as an Intermediate Layer and Its Interdiffusion Reaction with Cu. J. Electron. Mater. 2009, 38, 200–207. [Google Scholar] [CrossRef]
- Bader, S.; Gust, W.; Hieber, H. Rapid formation of intermetallic compounds interdiffusion in the Cu-Sn and Ni-Sn systems. Acta Metall. Mater. 1995, 43, 329–337. [Google Scholar]
- Yang, C.L.; Lai, H.J.; Hwang, J.D.; Chuang, T.H. Diffusion Soldering of Bi0.5Sb1.5Te3 Thermoelectric Material with Cu Electrode. J. Mater. Eng. Perform. 2013, 22, 2029–2037. [Google Scholar] [CrossRef]
- Yang, C.L.; Lai, H.J.; Hwang, J.D.; Chuang, T.H. Diffusion Soldering of Pb-Doped GeTe Thermoelectric Modules with Cu Electrodes Using a Thin-Film Sn Interlayer. J. Electron. Mater. 2012, 42, 359–365. [Google Scholar] [CrossRef]
- Chuang, T.H.; Lin, H.J.; Chuang, C.H.; Yeh, W.T.; Hwang, J.D.; Chu, H.S. Solid Liquid Interdiffusion Bonding of (Pb, Sn)Te Thermoelectric Modules with Cu Electrodes Using a Thin-Film Sn Interlayer. J. Electron. Mater. 2014, 43, 4610–4618. [Google Scholar] [CrossRef]
- Chuang, T.H.; Yeh, W.T.; Chuang, C.H.; Hwang, J.D. Improvement of bonding strength of a (Pb, Sn)Te–Cu contact manufactured in a low temperature SLID-bonding process. J. Alloy. Compd. 2014, 613, 46–54. [Google Scholar] [CrossRef]
- Chiu, C.N.; Wang, C.H.; Chen, S.W. Interfacial Reactions in the Sn–Bi/Te Couples. J. Electron. Mater. 2007, 37, 40–44. [Google Scholar] [CrossRef]
- Chang, J.Y.; Chang, T.C.; Chuang, T.H.; Lee, C.Y. Dual-Phase Intermetallic Interconnections Structure and Method of Fabricating the Same. U.S. Patent 8,742,600 B2, 3 June 2014. [Google Scholar]
- Frear, D.R.; Burchett, S.N.; Morgan, H.S.; Lau, J.H. The Mechanics of Solder Alloy Interconnects; Van Nostrand Reinhold: New York, NY, USA, 1994; p. 60. [Google Scholar]



© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuang, C.-H.; Lin, Y.-C.; Lin, C.-W. Intermetallic Reactions during the Solid-Liquid Interdiffusion Bonding of Bi2Te2.55Se0.45 Thermoelectric Material with Cu Electrodes Using a Sn Interlayer. Metals 2016, 6, 92. https://doi.org/10.3390/met6040092
Chuang C-H, Lin Y-C, Lin C-W. Intermetallic Reactions during the Solid-Liquid Interdiffusion Bonding of Bi2Te2.55Se0.45 Thermoelectric Material with Cu Electrodes Using a Sn Interlayer. Metals. 2016; 6(4):92. https://doi.org/10.3390/met6040092
Chicago/Turabian StyleChuang, Chien-Hsun, Yan-Cheng Lin, and Che-Wei Lin. 2016. "Intermetallic Reactions during the Solid-Liquid Interdiffusion Bonding of Bi2Te2.55Se0.45 Thermoelectric Material with Cu Electrodes Using a Sn Interlayer" Metals 6, no. 4: 92. https://doi.org/10.3390/met6040092




