Structural Origin of the Enhanced Glass-Forming Ability Induced by Microalloying Y in the ZrCuAl Alloy
Abstract
:1. Introduction
2. Experimental Section
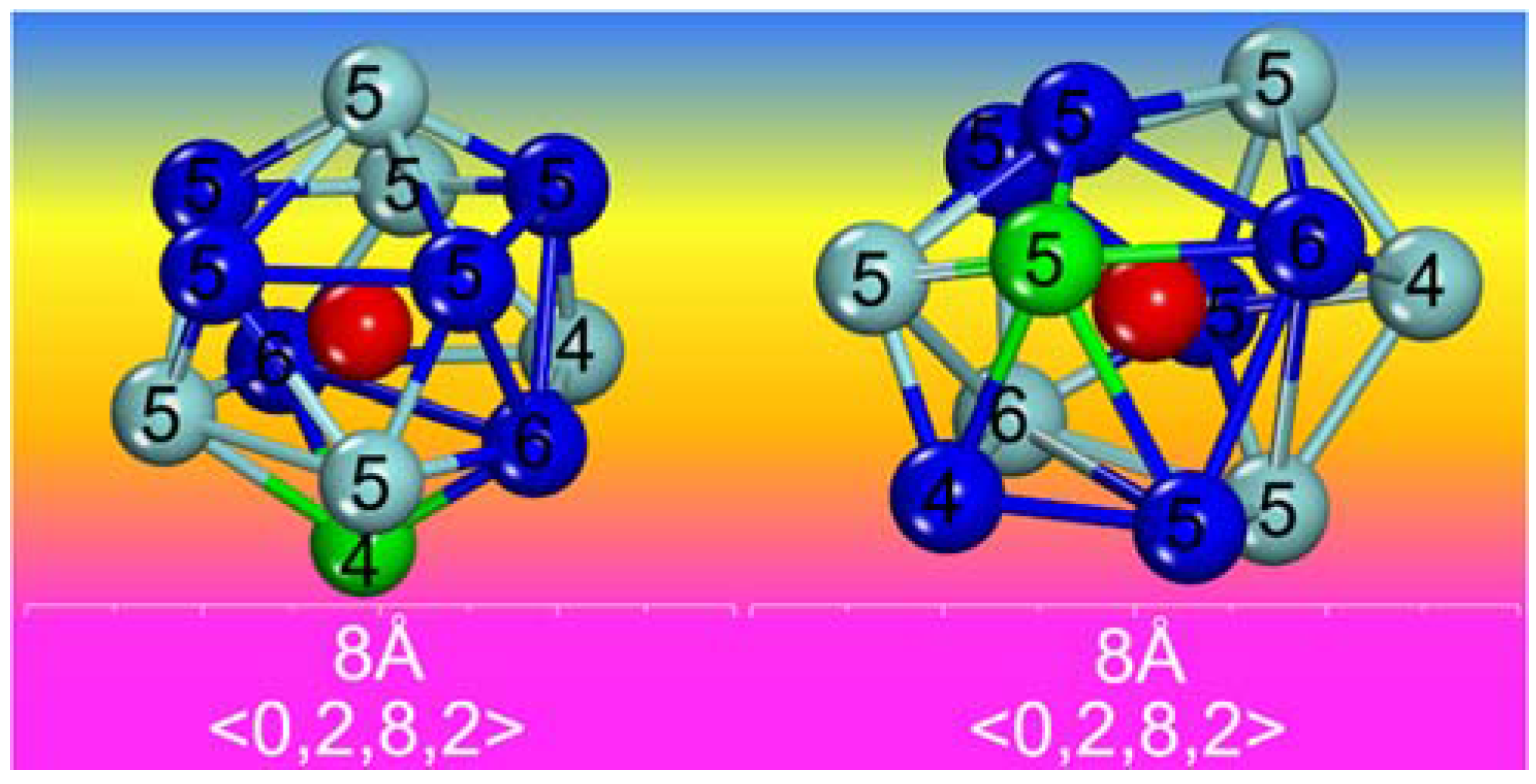
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Inoue, A. Stabilization of metallic supercooled liquid and bulk amorphous alloys. Acta Mater. 2000, 48, 279–306. [Google Scholar] [CrossRef]
- Turnbull, D. Under what conditions can a glass be formed? Contemp. Phys. 1969, 10, 473–488. [Google Scholar] [CrossRef]
- Greer, A.L. Confusion by Design. Nature 1993, 366, 303–304. [Google Scholar] [CrossRef]
- Johnson, W.L. Bulk glass-forming metallic alloys: Science and technology. MRS Bull. 1999, 24, 42–56. [Google Scholar] [CrossRef]
- Lu, Z.P.; Tan, H.; Li, Y.; Ng, S.C. The correlation between reduced glass transition temperature and glass forming ability of bulk metallic glasses. Scr. Mater. 2000, 42, 667–673. [Google Scholar] [CrossRef]
- Lu, Z.P.; Liu, C.T. Glass formation criterion for various glass-forming systems. Phys. Rev. Lett. 2003. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.H.; Dong, C.; Shek, C.H. Bulk metallic glasses. Mater. Sci. Eng. R 2004, 44, 45–89. [Google Scholar] [CrossRef]
- Schroers, J.; Johnson, W.L. Ductile bulk metallic glass. Phys. Rev. Lett. 2004. [Google Scholar] [CrossRef] [PubMed]
- Ashby, M.F.; Greer, A.L. Metallic glasses as structural materials. Scr. Mater. 2006, 54, 321–326. [Google Scholar] [CrossRef]
- Na, J.H.; Demetriou, M.D.; Floyd, M.; Hoff, A.; Garrett, G.R.; Johnson, W.L. Compositional landscape for glass formation in metal alloys. Proc. Natl. Acad. Sci. USA 2014, 111, 9031–9036. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Tan, H.; Wang, D.; Li, Y.; Ma, E. Strategy for pinpointing the best glass-forming alloys. Appl. Phys. Lett. 2005. [Google Scholar] [CrossRef]
- Lu, Z.P.; Liu, C.T. Role of minor alloying additions in formation of bulk metallic glasses: A review. J. Mater. Sci. 2004, 39, 3965–3974. [Google Scholar] [CrossRef]
- Wang, W.H. Roles of minor additions in formation and properties of bulk metallic glasses. Prog. Mater. Sci. 2007, 52, 540–596. [Google Scholar] [CrossRef]
- Xu, D.H.; Duan, G.; Johnson, W.L. Unusual glass-forming ability of bulk amorphous alloys based on ordinary metal copper. Phys. Rev. Lett. 2004. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Tan, H.; Li, Y. Multiple maxima of GFA in three adjacent eutectics in Zr-Cu-Al alloy system-A metallographic way to pinpoint the best glass forming alloys. Acta Mater. 2005, 53, 2969–2979. [Google Scholar] [CrossRef]
- Zhang, Q.S.; Zhang, W.; Inoue, A. Preparation of Cu36Zr48Ag8Al8 bulk metallic glass with a diameter of 25 mm by copper mold casting. Mater. Trans. 2007, 48, 629–631. [Google Scholar] [CrossRef]
- Chen, L.Y.; Fu, Z.D.; Zhang, G.Q.; Hao, X.P.; Jiang, Q.K.; Wang, X.D.; Cao, Q.P.; Franz, H.; Liu, Y.G.; Xie, H.S.; et al. New class of plastic bulk metallic glass. Phys. Rev. Lett. 2008. [Google Scholar] [CrossRef] [PubMed]
- Egami, T.; Waseda, Y. Atomic size effect on the formability of metallic glasses. J. Non-Cryst. Solids 1984, 64, 113–134. [Google Scholar] [CrossRef]
- Hirata, A.; Guan, P.F.; Fujita, T.; Hirotsu, Y.; Inoue, A.; Yavari, A.R.; Sakurai, T.; Chen, M.W. Direct observation of local atomic order in a metallic glass. Nat. Mater. 2011, 10, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.W.; Li, M.Z.; Wang, W.H.; Liu, K.X. Hidden topological order and its correlation with glass-forming ability in metallic glasses. Nat. Commun. 2015. [Google Scholar] [CrossRef] [PubMed]
- Bernal, J.D. A geometrical approach to the structure of liquids. Nature 1959, 183, 141–147. [Google Scholar]
- Gaskell, P.H. A new structural model for transition metal-metalloid glasses. Nature 1978, 276, 484–485. [Google Scholar] [CrossRef]
- Miracle, D.B. A structural model for metallic glasses. Nat. Mater. 2004, 3, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.W.; Luo, W.K.; Alamgir, F.M.; Bai, J.M.; Ma, E. Atomic packing and short-to-medium-range order in metallic glasses. Nature 2006, 439, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Guo, G.Q.; Chen, L.Y.; Huang, C.L.; Ge, T.; Chen, D.; Liaw, P.K.; Saksl, K.; Ren, Y.; Zeng, Q.S.; et al. Atomic-Scale Mechanisms of the Glass-Forming Ability in Metallic Glasses. Phys. Rev. Lett. 2012. [Google Scholar] [CrossRef] [PubMed]
- Hammersley, A.P.; Svensson, S.O.; Hanfland, M.; Fitch, A.N.; Häusermann, D. Two-dimensional detector software: From real detector to idealised image or two-theta scan. High Press. Res. 1996, 14, 235–248. [Google Scholar] [CrossRef]
- Qiu, X.; Thompson, J.W. PDFgetX2: A GUI-driven program to obtain the pair distribution function from X-ray powder diffraction data. J. Appl. Crystallogr. 2004, 37, 110–116. [Google Scholar] [CrossRef]
- Klementev, K.V. Extraction of the fine structure from X-ray absorption spectra. J. Phys. D 2001, 34, 209–217. [Google Scholar] [CrossRef]
- McGreevy, R.L. Reverse Monte Carlo modelling. J. Phys. Condens. Matt. 2001, 13, R877–R913. [Google Scholar] [CrossRef]
- Saksl, K.; Jovari, P.; Franz, H.; Zeng, Q.S.; Liu, J.F.; Jiang, J.Z. Atomic structure of Al89La6Ni5 metallic glass. J. Phys. Condens. Matter. 2006, 18, 7579–7592. [Google Scholar] [CrossRef] [PubMed]
- Ravel, B.; Newville, M. Athena, Artemis, Hephaestus: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synch. Rad. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, N.N. The algorithm for three-dimensional Voronoi polyhedral. J. Comput. Phys. 1986, 67, 223–229. [Google Scholar] [CrossRef]
- Zeng, Q.S.; Sheng, H.W.; Ding, Y.; Wang, L.; Yang, W.G.; Jiang, J.Z.; Mao, W.L.; Mao, H.K. Long-range topological order in metallic glass. Science 2011, 332, 1404–1406. [Google Scholar] [CrossRef] [PubMed]
- Park, E.S.; Kim, D.H. Phase separation and enhancement of plasticity in Cu-Zr-Al-Y bulk metallic glasses. Acta Mater. 2006, 54, 2597–2604. [Google Scholar] [CrossRef]
- Yang, L.; Guo, G.Q.; Chen, L.Y.; Wei, S.H.; Jiang, J.Z.; Wang, X.D. Atomic structure in Al-doped multicomponent bulk metallic glass. Scr. Mater. 2010, 63, 879–882. [Google Scholar] [CrossRef]
- Wang, S.Y.; Kramer, M.J.; Xu, M.; Wu, S.; Hao, S.G.; Sordelet, D.J.; Ho, K.M.; Wang, C.Z. Experimental and ab initio molecular dynamics simulation studies of liquid Al60Cu40 alloy. Phys. Rev. B 2009. [Google Scholar] [CrossRef]
- Yang, L.; Guo, G.Q. Preferred clusters in metallic glasses. Chin. Phys. B 2010. [Google Scholar] [CrossRef]
- Fang, H.Z.; Hui, X.; Chen, G.L.; Liu, Z.K. Al-centered icosahedral ordering in Cu46Zr46Al8 bulk metallic glass. Appl. Phys. Lett. 2009. [Google Scholar] [CrossRef]
- Guo, G.Q.; Wu, S.Y.; Luo, S.; Yang, L. Detecting Structural Features in Metallic Glass via Synchrotron Radiation Experiments Combined with Simulations. Metals 2015, 5, 2093–2108. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Q.; Kalb, J.A.; Thompson, C.V. Matching glass-forming ability with the density of the amorphous phase. Science 2008, 322, 1816–1819. [Google Scholar]
- Greer, A.L.; Ma, E. Bulk metallic glasses: At the cutting edge of metals research. Mater. Res. Bull. 2007, 32, 611–615. [Google Scholar] [CrossRef]
- Fujita, T.; Konno, K.; Zhang, W.; Kumar, V.; Matsuura, M.; Inoue, A.; Sakurai, T.; Chen, M.W. Atomic-scale heterogeneity of a multicomponent bulk metallic glass with excellent glass forming ability. Phys. Rev. Lett. 2009. [Google Scholar] [CrossRef] [PubMed]
- Xi, X.K.; Li, L.L.; Zhang, B.; Wang, W.H.; Wu, Y. Correlation of atomic cluster symmetry and glass-forming ability of metallic glass. Phys. Rev. Lett. 2007. [Google Scholar] [CrossRef] [PubMed]
- Xi, X.K.; Sandor, M.T.; Liu, Y.H.; Wang, W.H.; Wu, Y. Structural changes induced by microalloying in Cu46Zr47−xAl7Gdx metallic glasses. Scr. Mater. 2009, 61, 967–969. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Chen, G.L.; Liu, X.J. Glass formation mechanism of minor yttrium addition in CuZrAl alloys. Appl. Phys. Lett. 2006. [Google Scholar] [CrossRef]
- Saida, J.; Matsushita, M.; Inoue, A. Direct observation of icosahedral cluster in Zr70Pd30 binary glassy alloy. Appl. Phys. Lett. 2001. [Google Scholar] [CrossRef]
- Saksl, K.; Franz, H.; Jovari, P.; Klementiev, K.; Welter, E.; Ehnes, A.; Saida, J.; Inoue, A.; Jiang, J.Z. Evidence of icosahedral short-range order in Zr70Cu30 and Zr70Cu29Pd1 metallic glasses. Appl. Phys. Lett. 2003. [Google Scholar] [CrossRef] [Green Version]
- Hentschel, H.G.E.; Moshe, M.; Procaccia, I.; Samwer, K.; Sharon, E. Microalloying and the mechanical properties of amorphous solids. 2015; arXiv:1510.03108. [Google Scholar]
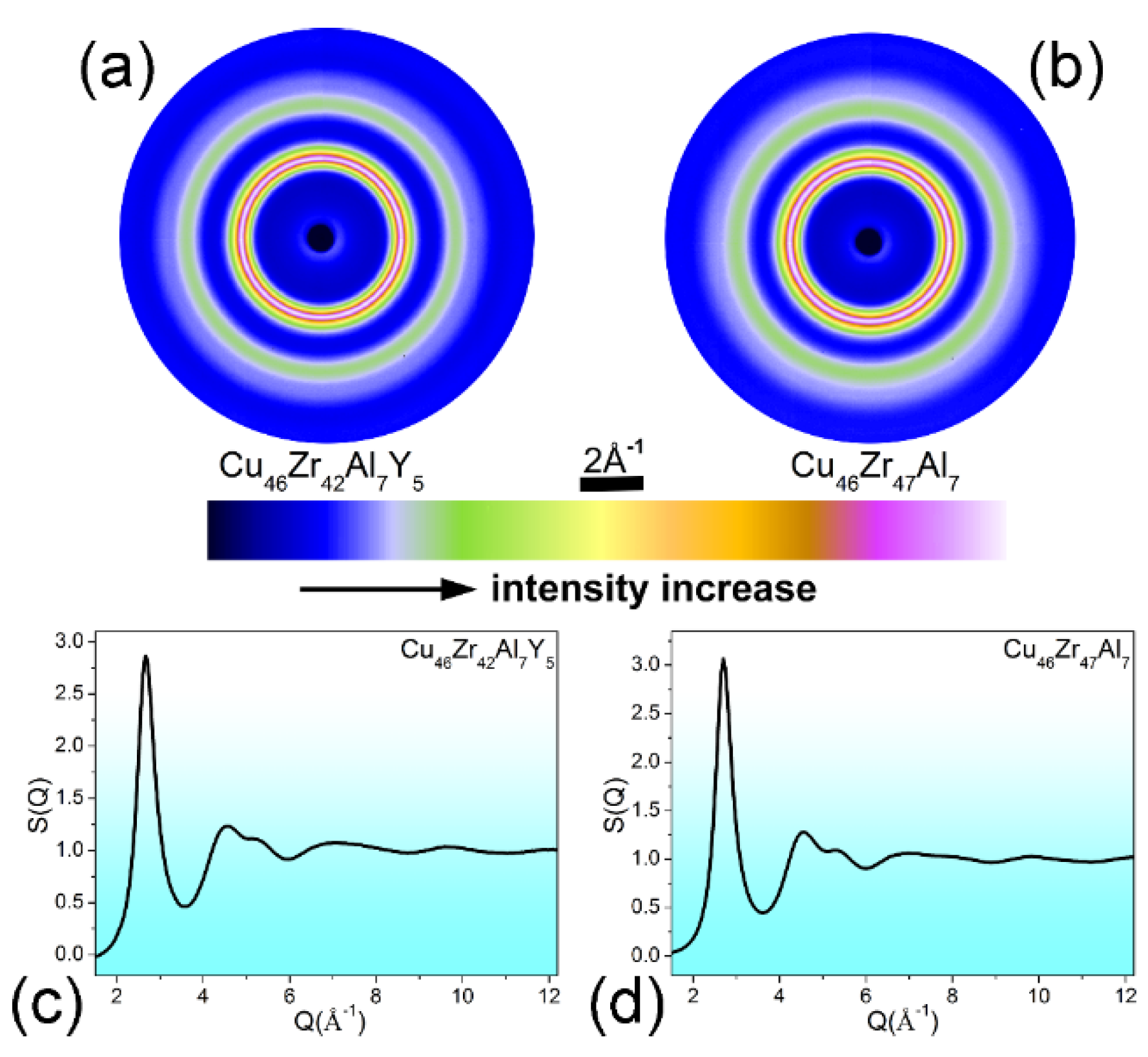
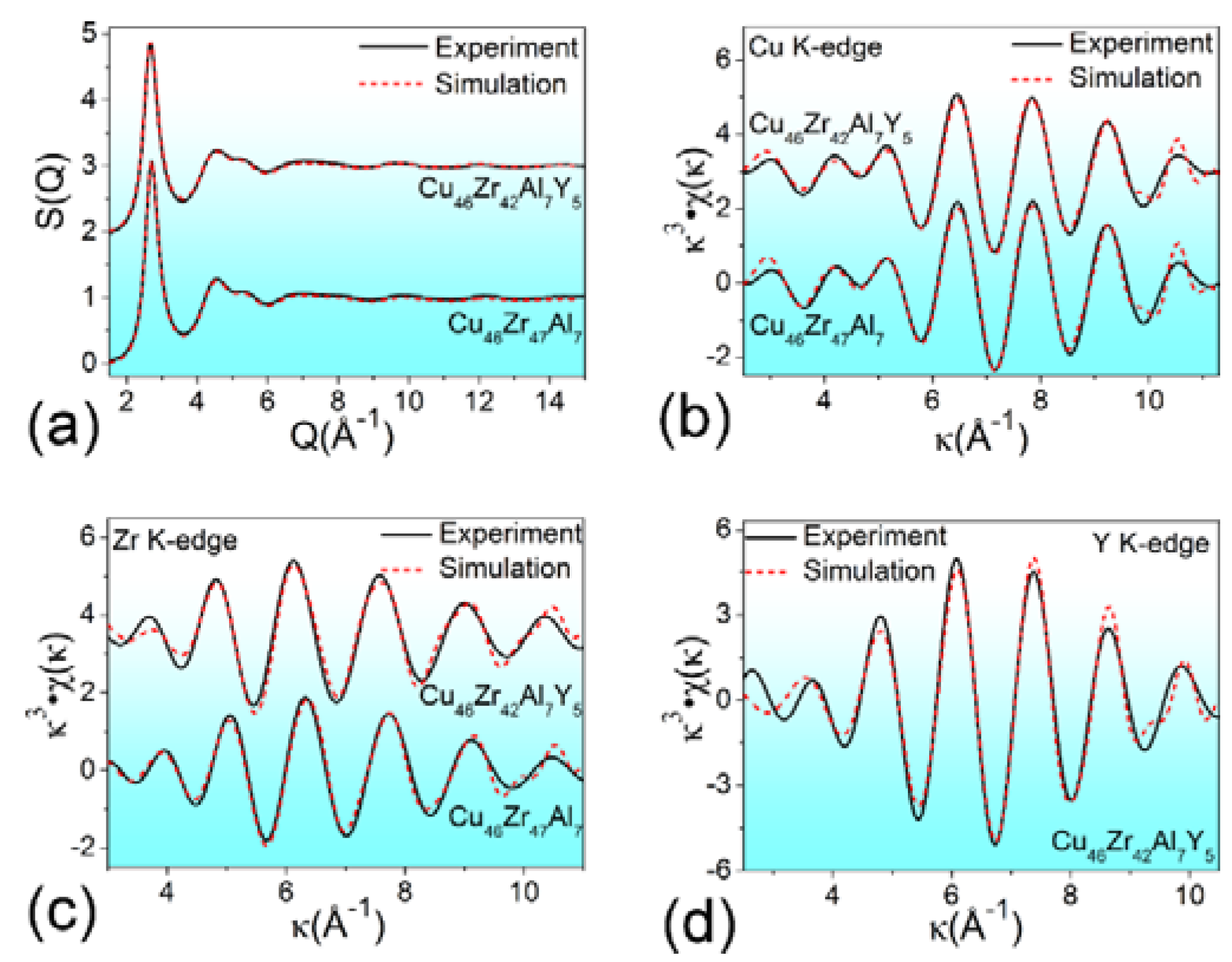


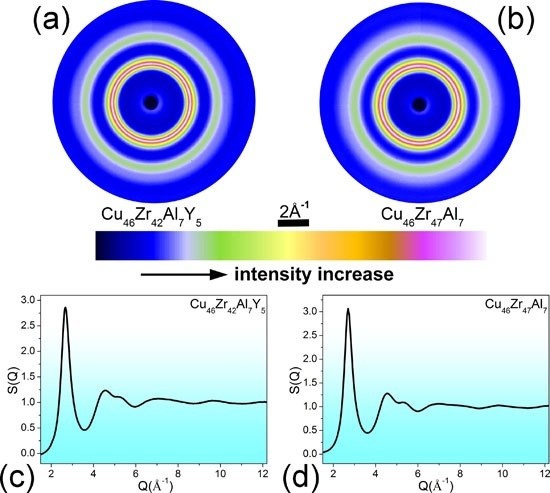
| Atomic Pairs | Cu46Zr47Al7 | Cu46Zr42Al7Y5 | ||||
|---|---|---|---|---|---|---|
| R(Å) ± 0.01 | CNs | Uncertainty of CNs | R(Å) ± 0.01 | CN | Uncertainty of CNs | |
| Cu-Cu | 2.59 | 5.0 | ±0.2 | 2.60 | 5.3 | ±0.3 |
| Cu-Zr | 2.88 | 5.4 | ±0.3 | 2.89 | 4.2 | ±0.3 |
| Cu-Al | 2.61 | 0.7 | +0.3,−0.1 | 2.60 | 0.7 | +0.2, −0.1 |
| Cu-Y | - | - | - | 2.98 | 0.9 | - |
| Cu-M | - | 11.1 | - | - | 11.1 | - |
| Zr-Cu | 2.88 | 5.4 | ±0.2 | 2.89 | 5.5 | ±0.2 |
| Zr-Zr | 3.20 | 5.9 | ±0.2 | 3.20 | 5.3 | ±0.3 |
| Zr-Al | 2.82 | 0.8 | +0.2,−0.1 | 2.79 | 0.7 | +0.3,−0.1 |
| Zr-Y | - | - | - | 3.54 | 0.5 | +0.1 |
| Zr-M | - | 12.1 | - | - | 12.0 | - |
| Al-Cu | 2.61 | 4.9 | ±0.2 | 2.60 | 5.5 | ±0.3 |
| Al-Zr | 2.82 | 5.7 | ±0.3 | 2.79 | 4.9 | ±0.2 |
| Al-Al | 2.70 | 0.1 | +0.1 | 2.70 | 0.1 | +0.1 |
| Al-Y | - | - | - | 3.29 | 0.2 | +0.2 |
| Al-M | - | 10.7 | - | - | 10.7 | - |
| Y-Cu | - | - | - | 2.98 | 7.7 | ±0.4 |
| Y-Zr | - | - | - | 3.54 | 4.5 | ±0.3 |
| Y-Al | - | - | - | 3.29 | 0.2 | +0.2 |
| Y-Y | - | - | - | 3.76 | 0.3 | +0.1 |
| Y-M | - | - | - | - | 12.7 | - |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, G.-Q.; Wu, S.-Y.; Yang, L. Structural Origin of the Enhanced Glass-Forming Ability Induced by Microalloying Y in the ZrCuAl Alloy. Metals 2016, 6, 67. https://doi.org/10.3390/met6040067
Guo G-Q, Wu S-Y, Yang L. Structural Origin of the Enhanced Glass-Forming Ability Induced by Microalloying Y in the ZrCuAl Alloy. Metals. 2016; 6(4):67. https://doi.org/10.3390/met6040067
Chicago/Turabian StyleGuo, Gu-Qing, Shi-Yang Wu, and Liang Yang. 2016. "Structural Origin of the Enhanced Glass-Forming Ability Induced by Microalloying Y in the ZrCuAl Alloy" Metals 6, no. 4: 67. https://doi.org/10.3390/met6040067