3. Results and Discussion
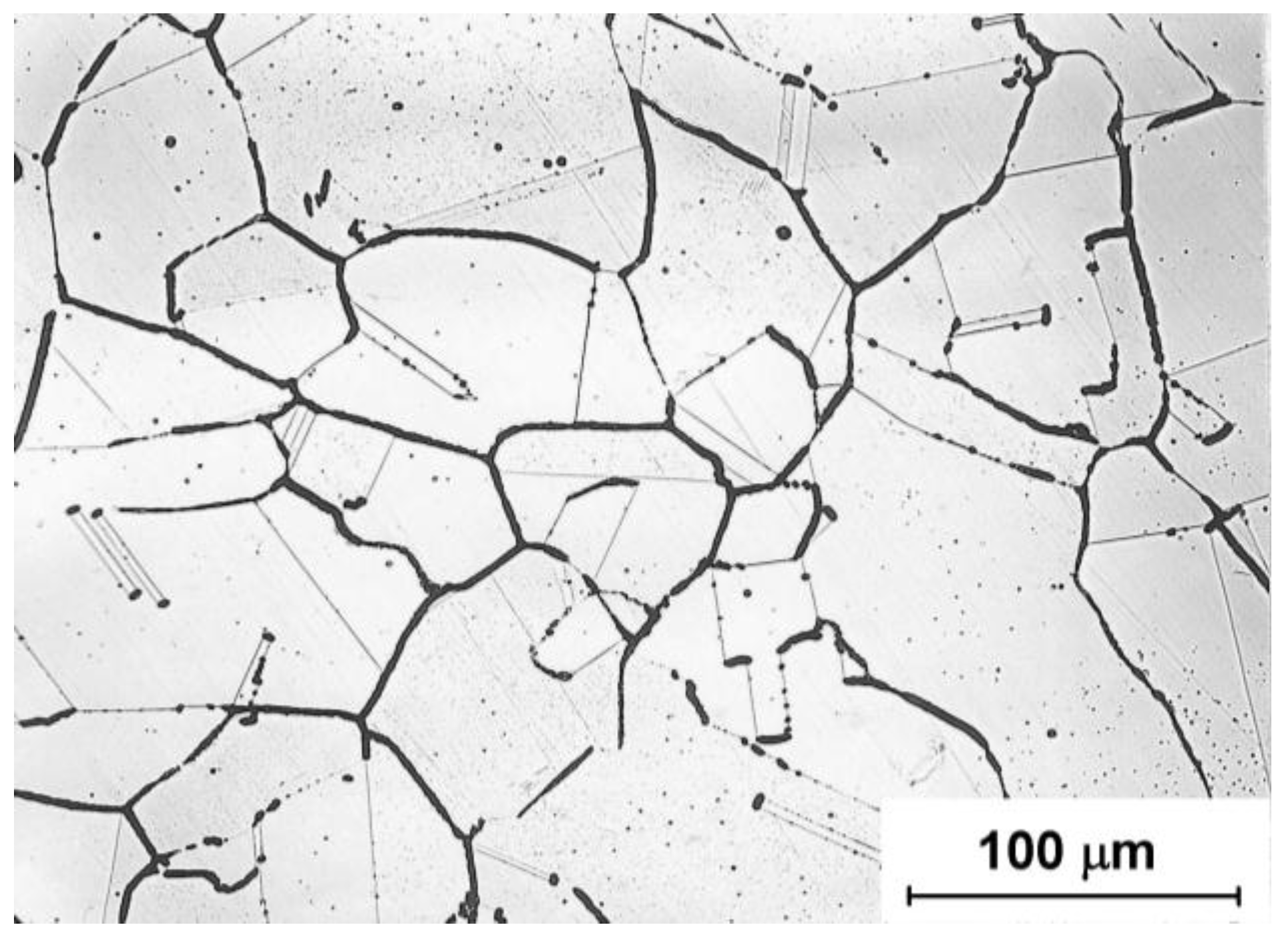
Figure 1,
Figure 2 and
Figure 3 show examples of the basic microstructure of 316L steel after all three variants of annealing. Microstructural analysis was performed using an electrolytic etching in 10% oxalic acid solution. The images suggest the possibility of depletion of chromium at grain boundaries, or annealing twins in the austenite matrix, and they provide basic information on potential susceptibility to the development of intergranular corrosion.
Figure 2 shows that the stabilization annealing is followed by discontinuous precipitation of M
23C
6 carbides, and thus the occurrence of localized areas depleted of chromium. The potential danger of intergranular corrosion in this case is negligible.
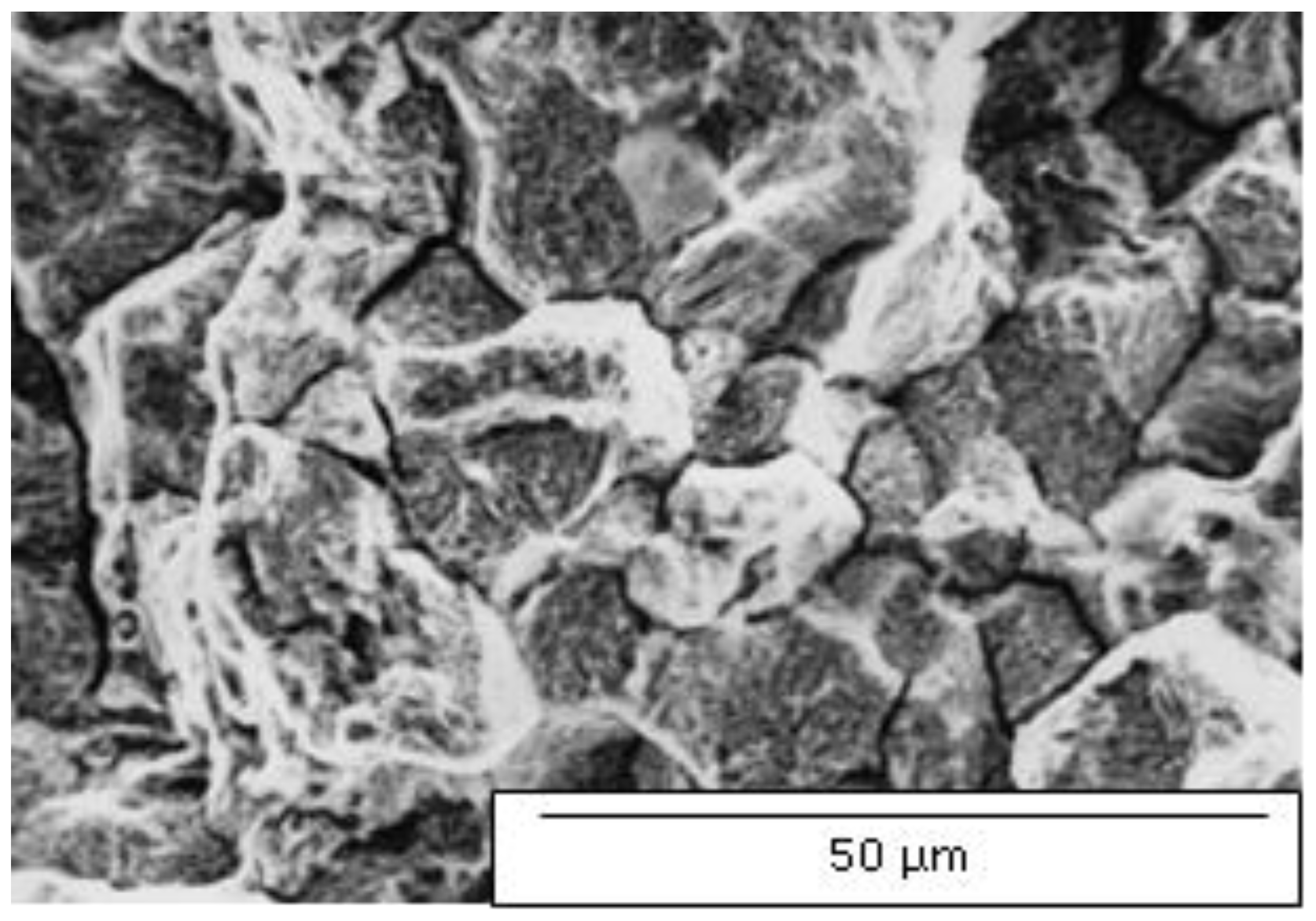
Figure 3 confirms intense continuous precipitation of M
23C
6 carbide in long-time sensitized samples, indicating susceptibility to intergranular corrosion. Identical characteristics were also found in 304L steel.
Fractographical analysis showed a higher incidence of brittle cleavage disruption on fracture surfaces of corrosion fatigue tests. In many cases, this type of disruption was accompanied by occurrence of intergranular areas, especially at lower levels of ∆
K and in the evaluation of corrosion fatigue under superposition environmental effects (see
Figure 4). As is known, the negative effect of harmful elements in steel (e.g., phosphorus, sulphur, and generally, other elements of subgroup IV.a to VI.A of the periodic table) lies in their ability to segregate on large-angle grain boundaries, which results in a reduction of the cohesive strength and the formation of low-energy intergranular fractures [
9]. Corrosion fatigue is precisely one of the degradation processes in which the formation of intergranular fractures through micro-segregation effect occurs [
10].
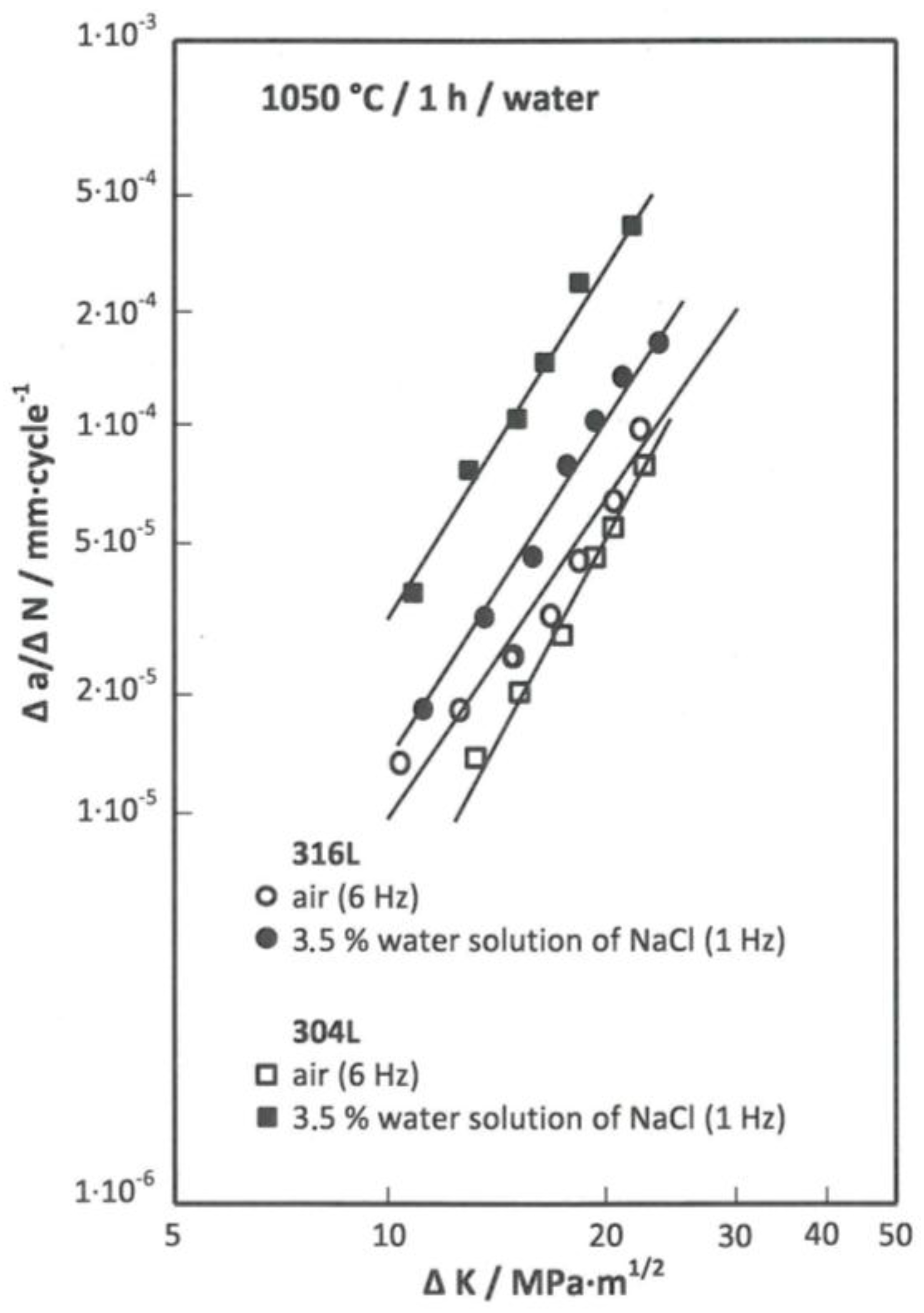
Figure 5 summarizes the plotted kinetic dependence of the development of fatigue cracks Δ
a/Δ
N at Δ
K of both studied steels after solution annealing.
For the 304L steel, after the application of solution annealing, the equation for evaluating corrosion fatigue in the air (1) and the equation in the specified corrosive environment (2) were determined.
For the 316L steel, the equation for the evaluation of corrosion fatigue in the air (3) and the equation for evaluation in the chosen environment (4) were determined after solution annealing.
For the 316L steel, the kinetic equation for evaluating fatigue properties in the air (7) and the equation for evaluating the corrosion fatigue in the chosen environment (8) were determined after the application of stabilizing annealing.
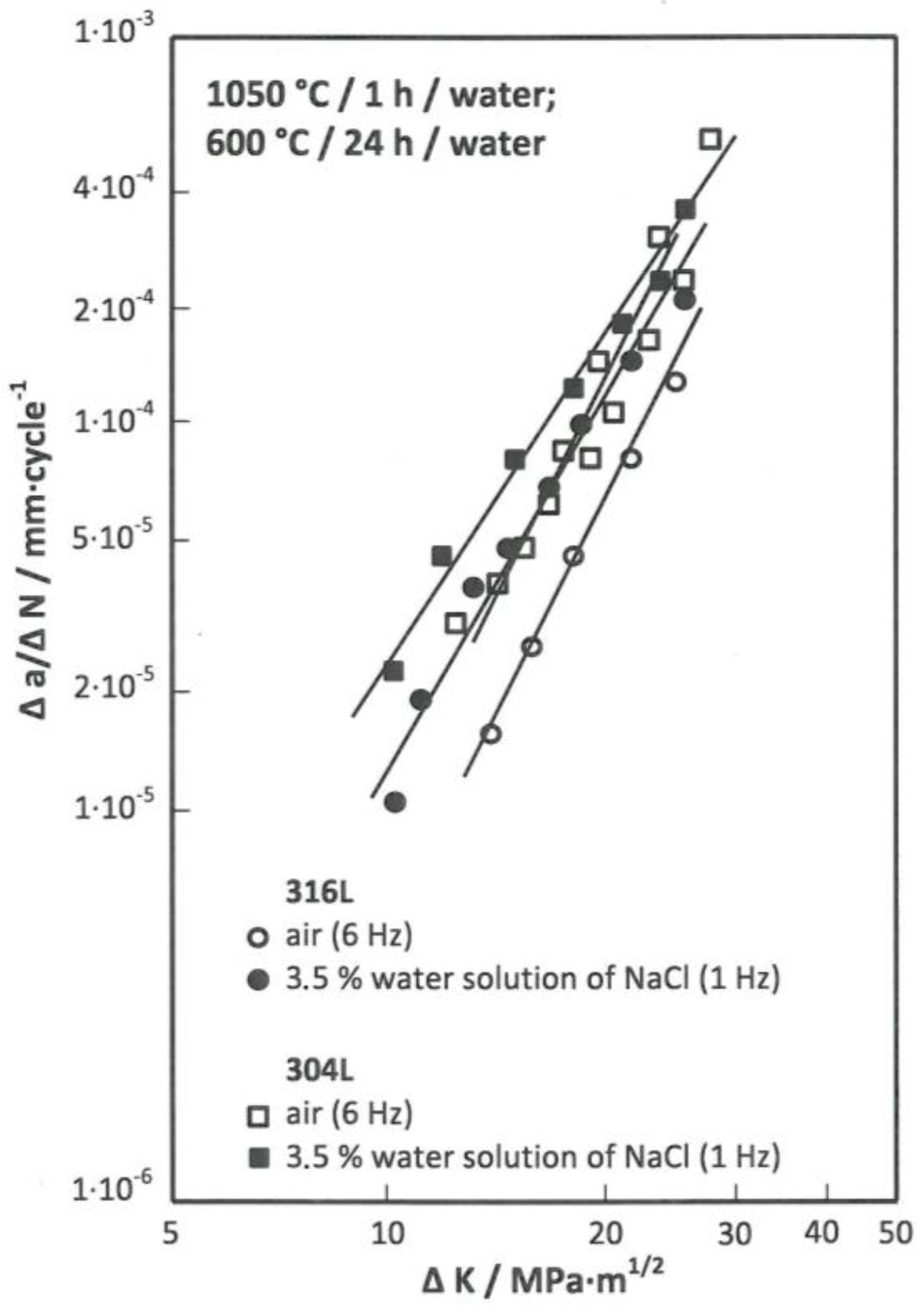
Figure 6 and Equations (5) and (7) show that the kinetic characteristics of both studied steels when evaluated in the air are identical.
Kinetics of the development of fatigue cracks in both austenitic steels after the application of sensitization annealing (
Figure 7) was also examined. For the 304L steel, kinetics of the fatigue crack growth for the evaluation of fatigue properties in the air after this treatment can be described by Equation (9), and in the chosen environment by Equation (10).
For the 316L steel, kinetic equations for evaluating the corrosion fatigue in the air (11) and for evaluating the fatigue characteristics in the selected corrosive environments (12) were determined after the application of sensitization annealing.
The comparison of the kinetic dependences shown in
Figure 5,
Figure 6 and
Figure 7 indicates that in the 304L steel, susceptibility to corrosion fatigue in the corrosive environment is significantly reflected. For example, after the application of annealing solution, the rate of fatigue crack growth increased at the level ∆
K = 15 MPa·m
1/2 due to the standard state by about 3/4 of the order, and at ∆
K = 20 MPa·m
1/2 from the value of 5 × 10
−5 mm/cycle corresponding to a standard condition to about 3.5 × 10
−4 mm/cycle. In the other two variants of heat treatment, the stated increase in speed decreased with respect to the standard condition. The decreased difference between the compared levels of fatigue crack growth rate is related to the fact that for the standard evaluation conditions after application of solution annealing, very low rate of fatigue crack growth was achieved, while for standard conditions, after application of stabilizing annealing or sensitization annealing, the rate of fatigue crack growth was higher.
Figure 5,
Figure 6 and
Figure 7 indicate favourable characteristics of corrosion fatigue in the 316L steel. After the application of solution annealing, the increase in growth rate for this steel compared to 304L steel at a comparable level of ∆
K is roughly one half. Similar is true after the application of stabilizing annealing (
Figure 3). After sensitization annealing, as compared to stabilizing annealing, the increase the rate of crack growth during corrosion fatigue was slightly higher (
Figure 4).
Results of the fatigue crack growth rate are in accordance with the general conclusions [
11] that in the field of high rate, the sudden final fracture is not strongly affected by corrosive environment. In the field of low and medium fatigue crack growth rate, an increase in rate and lower threshold values can be observed due to the presence of a corrosive environment.
Acknowledgments
This paper was created with the contribution of the projects Student Grant Competition SP 2016/103 Specific research in metallurgy, materials and process engineering, and No.LO1203 “Regional Materials and Technology Centre—Feasibility Programme”.
Author Contributions
P.J. and Z.J. conceived and designed the experiments; I.V. performed the experiments; P.V. and T.K. analyzed the data; I.V. contributed reagents/materials/analysis tools; P.J. and Z.J. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sakata, K. Technology for production of austenite type clean stainless steel. ISIJ Int. 2016, 46, 1795–1799. [Google Scholar] [CrossRef]
- Bursak, M.; Bokuvka, O. Fatigue properties of steel with increased atmospheric corrosion resistance. Commun. Sci. Lett. Univ. Zilina 2009, 11, 27–30. [Google Scholar]
- Bursak, M.; Bokuvka, O. Influence of technological factors on fatigue properties of steel sheets. Commun. Sci. Lett. Univ. Zilina 2006, 8, 34–37. [Google Scholar]
- Matocha, K. Corrosion fatigue and stress corrosion cracking of structural steels in water environments at temperatures 20–300 °C. In Habilitation Work; VSB-TU Ostrava: Ostrava, Czech Republic, 2006; p. 93. [Google Scholar]
- Vehovar, L.; Vehovar, A.; Tandler, M. The corrosion resistance of austenitic stainless steel, alloyed with nitrogen. Metalurgija 2001, 40, 4. [Google Scholar]
- Paris, P.C.; Erdogan, J. A critical analysis of crack propagation laws. J. Basic Eng. Trans. 1963, 85, 528–534. [Google Scholar] [CrossRef]
- Wei, P.R. A perspective on environmetally assisted crack growth in steels. In Proceedings of the International Conference on Environmental Degradation of Engineering Materials, Gdansk-Jurata, Poland, 19–23 September 1999; pp. 19–23.
- Cihal, V. Stainless Steels and Alloys; Academia: Prague, Czech Republic, 1999; p. 437. [Google Scholar]
- Tvrdy, M. Mechanical and metallurgical characteristics of steels for pressure systems. Metall. Innov. 1987, 8, 53. [Google Scholar]
- Lea, C.; Seah, M.P.; Hondros, E.D. The intergranular fragility index—An engineering materials parameter. Mater. Sci. Eng. 1980, 42, 233. [Google Scholar] [CrossRef]
- Klesnil, M.; Lukáš, P. Fatigue of Metallic Materials under Mechanical Stress; Academia Prague: New York, NY, USA, 1975; p. 222. [Google Scholar]
- American Society of Mechanical Engineers. ASME Boiler and Pressure Vessel Code, Section XI, Rules for In-Service Inspection of Nuclear Power Plant Components; ASME: New York, NY, USA, 1980. [Google Scholar]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).