The Effects of Fe-Particles on the Tensile Properties of Al-Si-Cu Alloys
Abstract
:1. Introduction
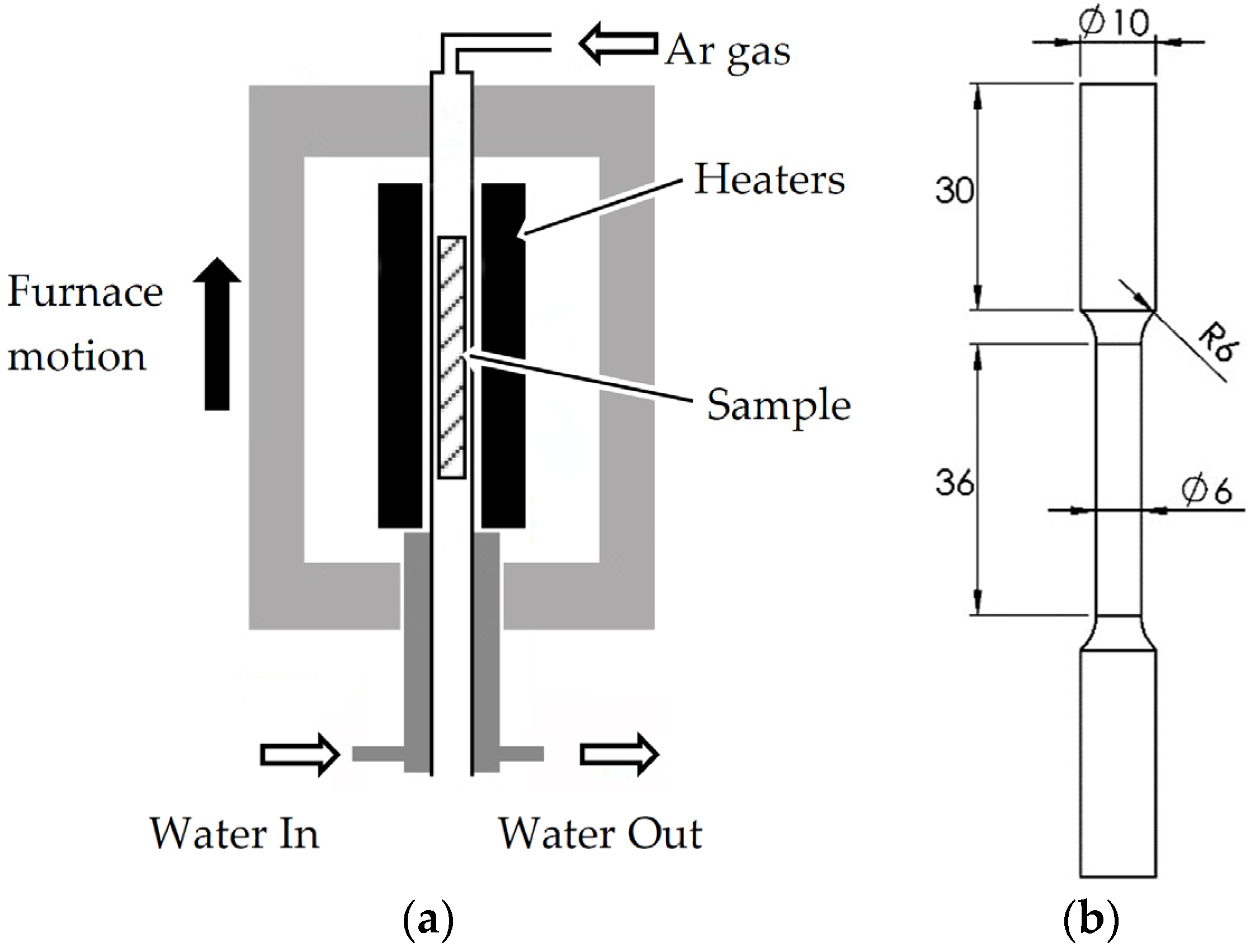
2. Materials and Methods
3. Results
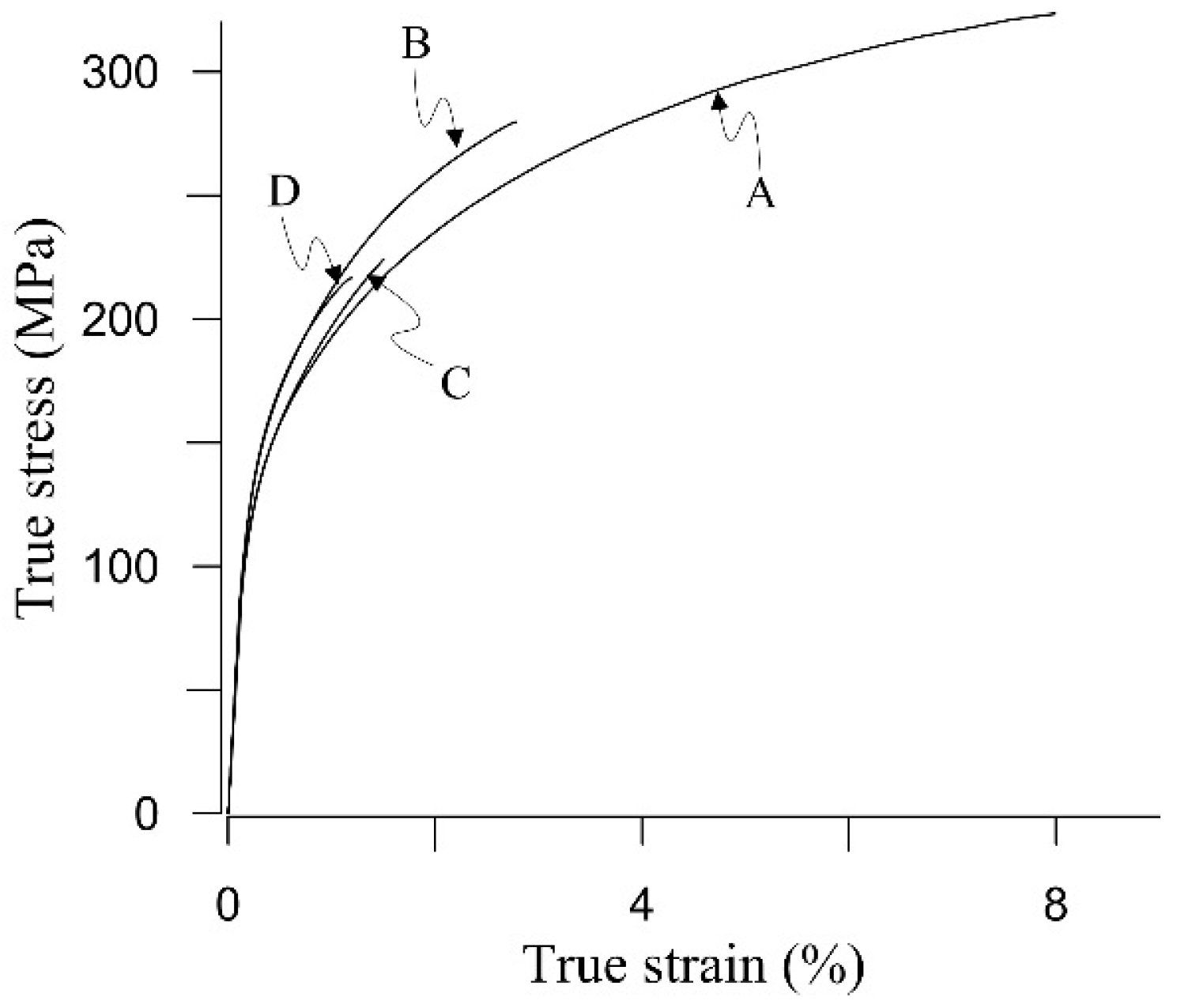
3.1. Tensile Properties of As-Cast Samples
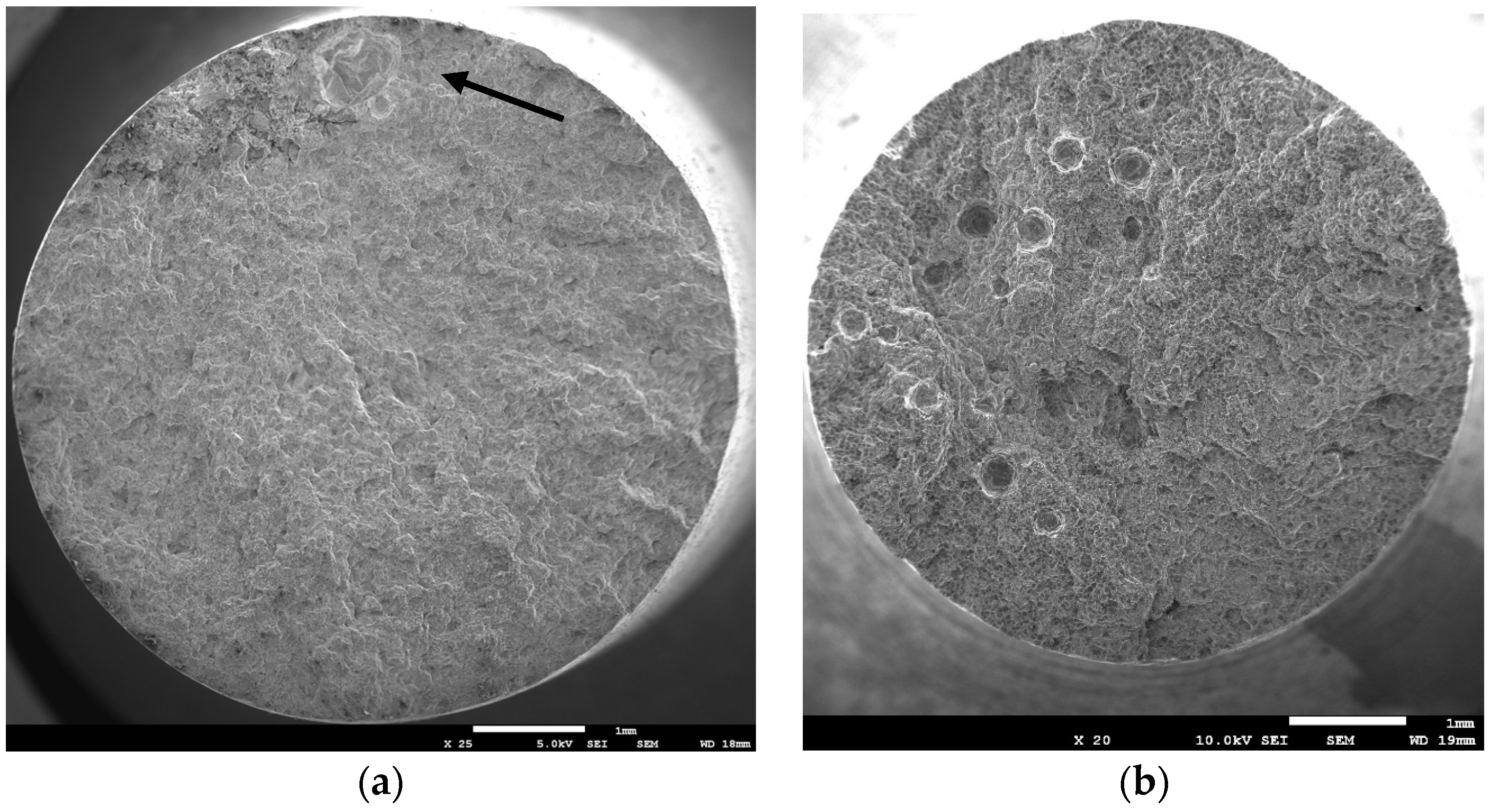
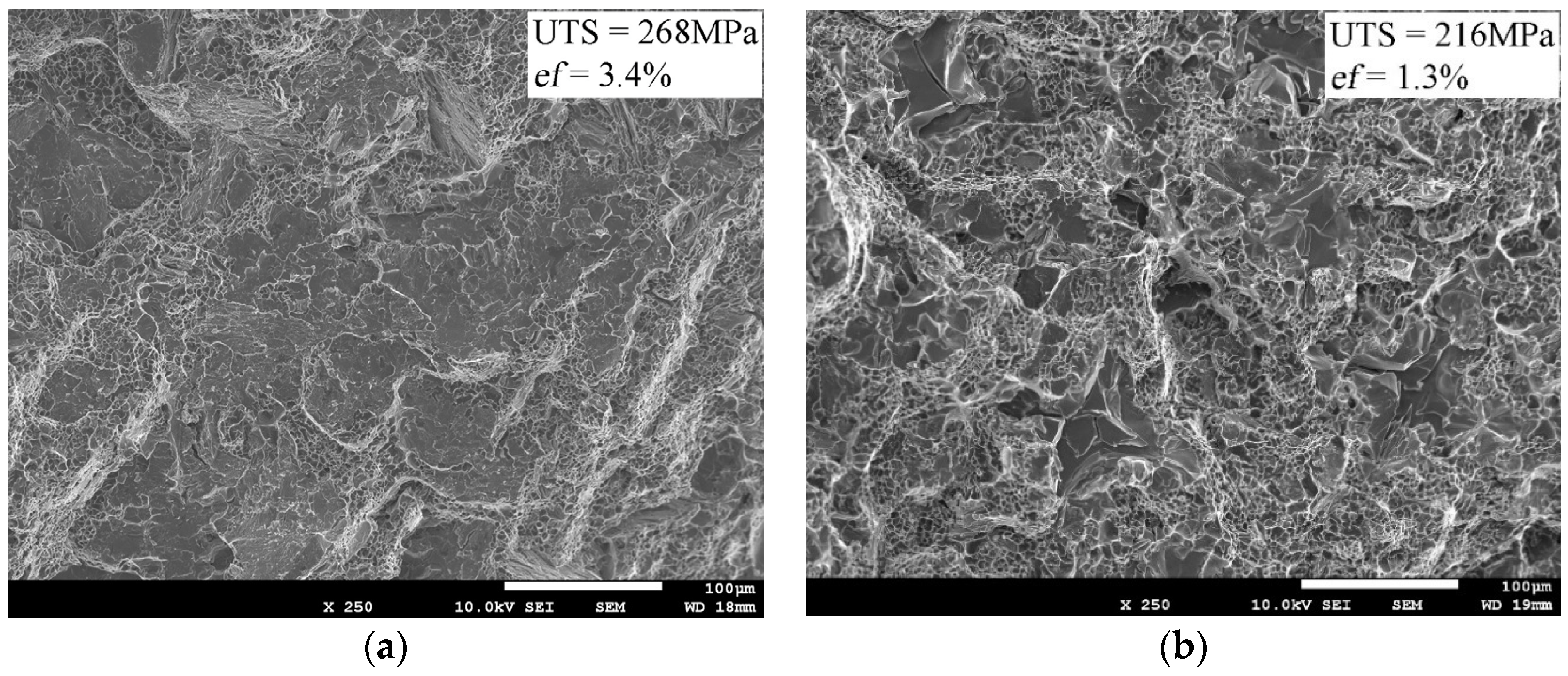
3.2. Macroscopic Fracture Surface Investigation, As-Cast Samples
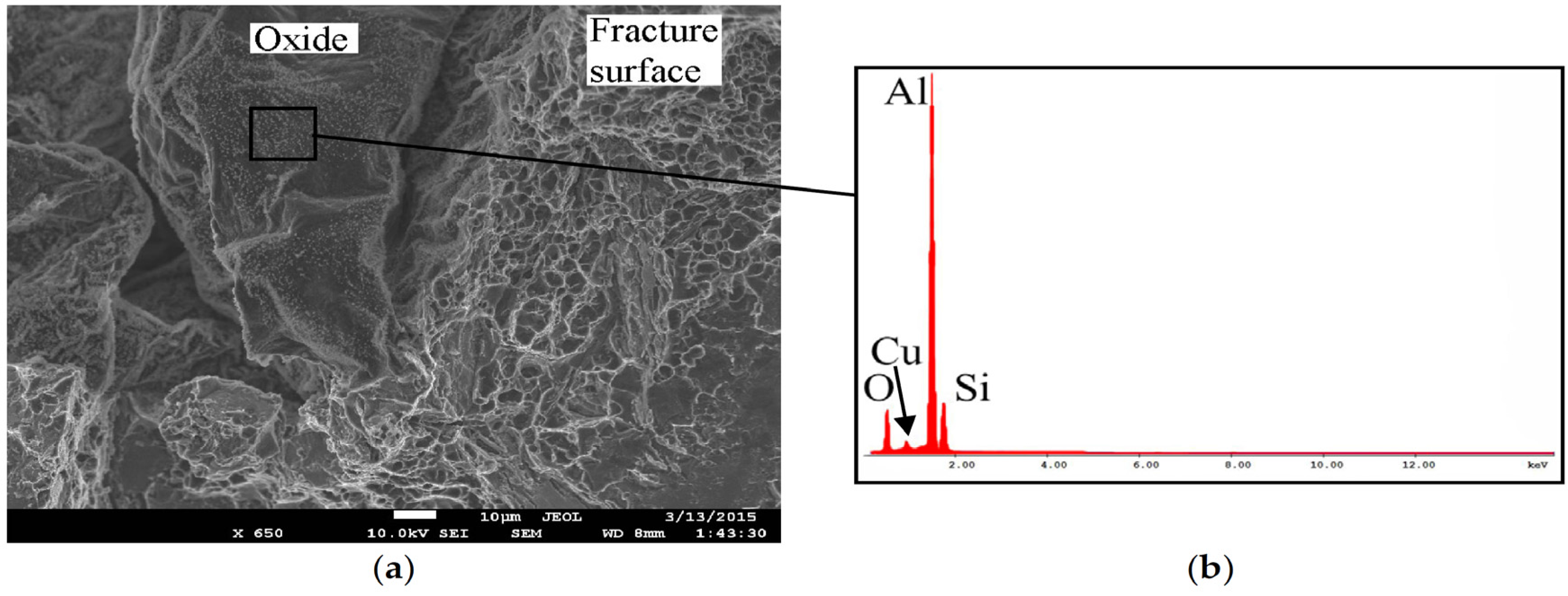
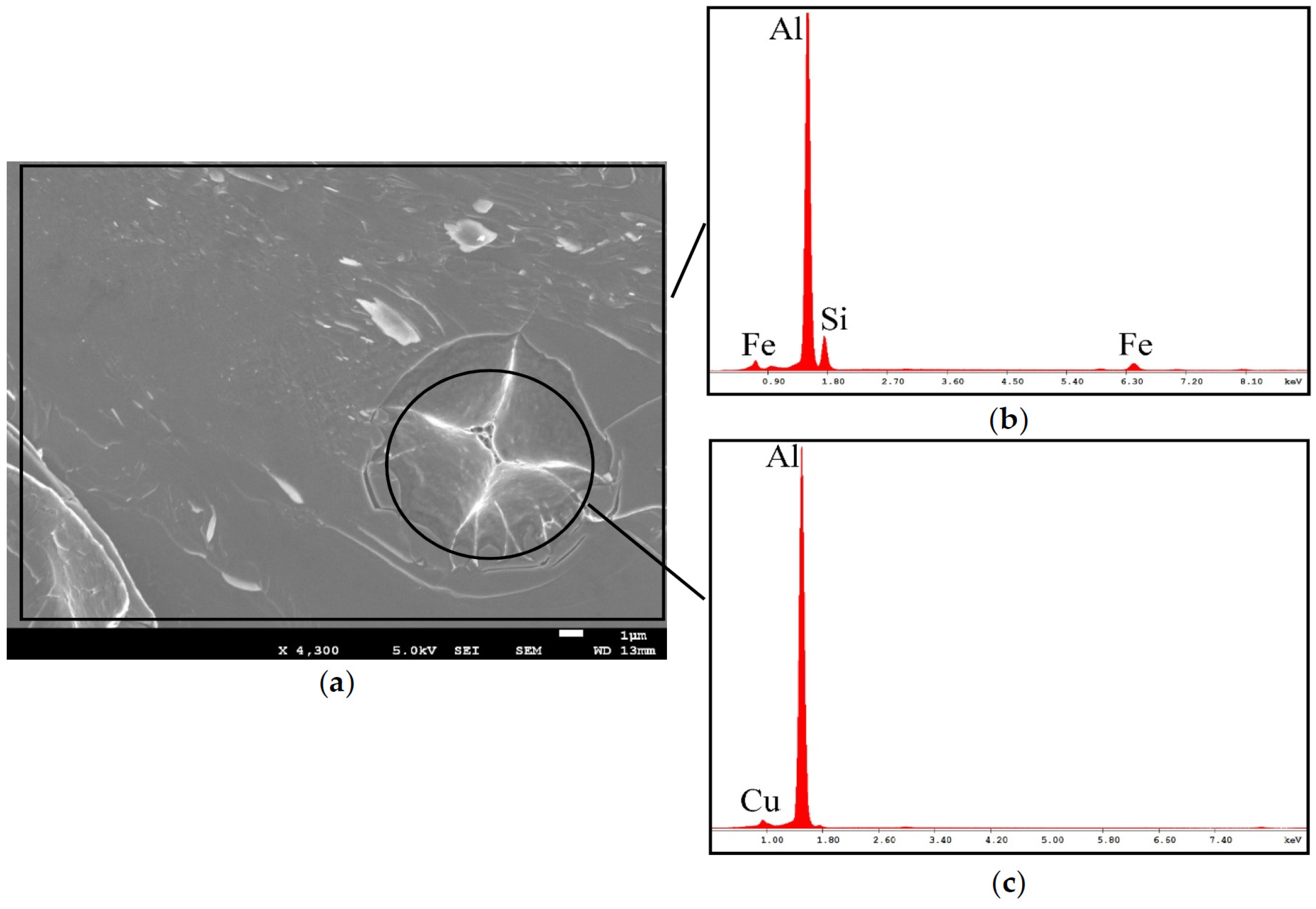
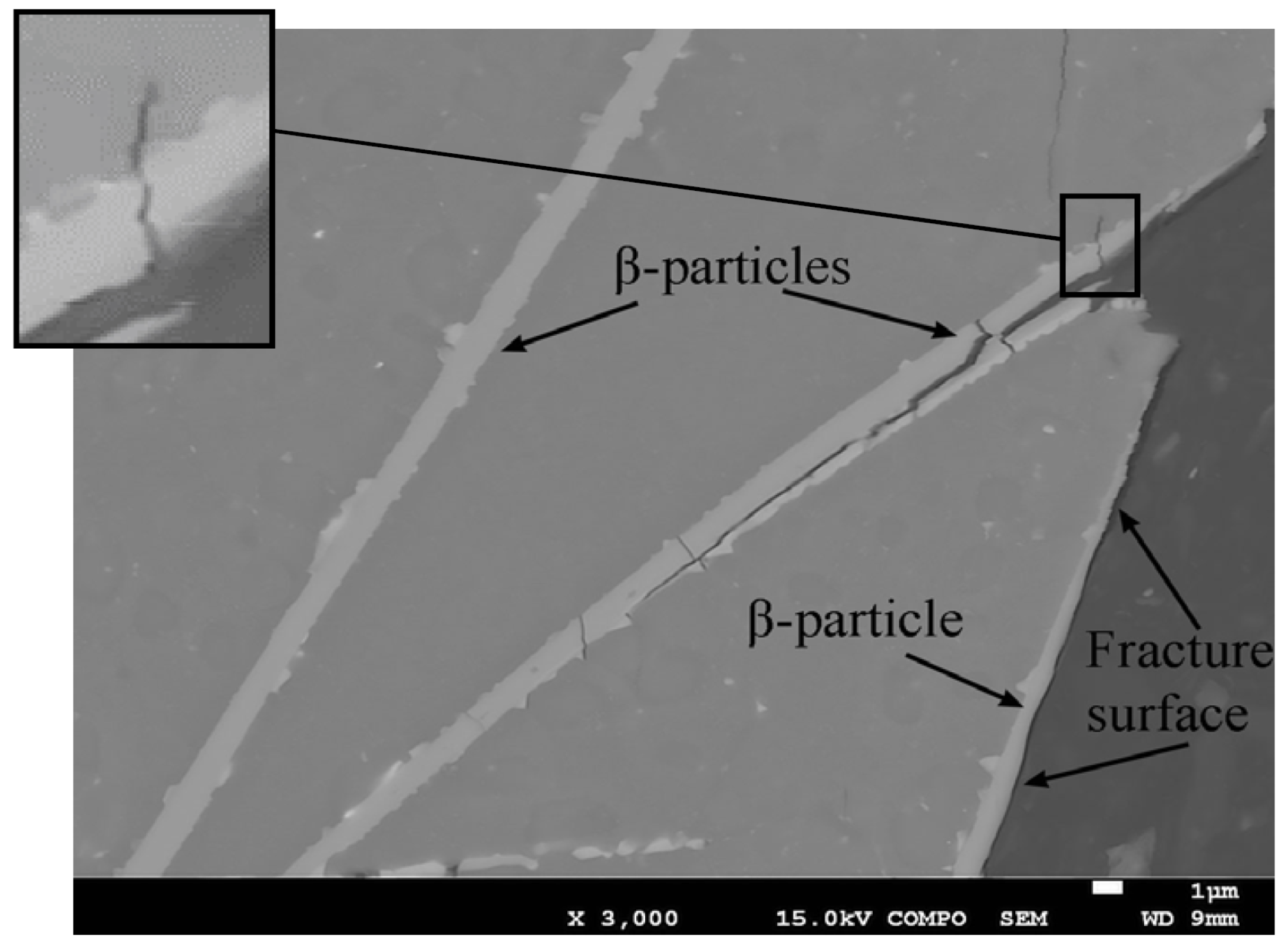
3.3. Fracture Surface Investigation by SEM-EDS, As-Cast Samples
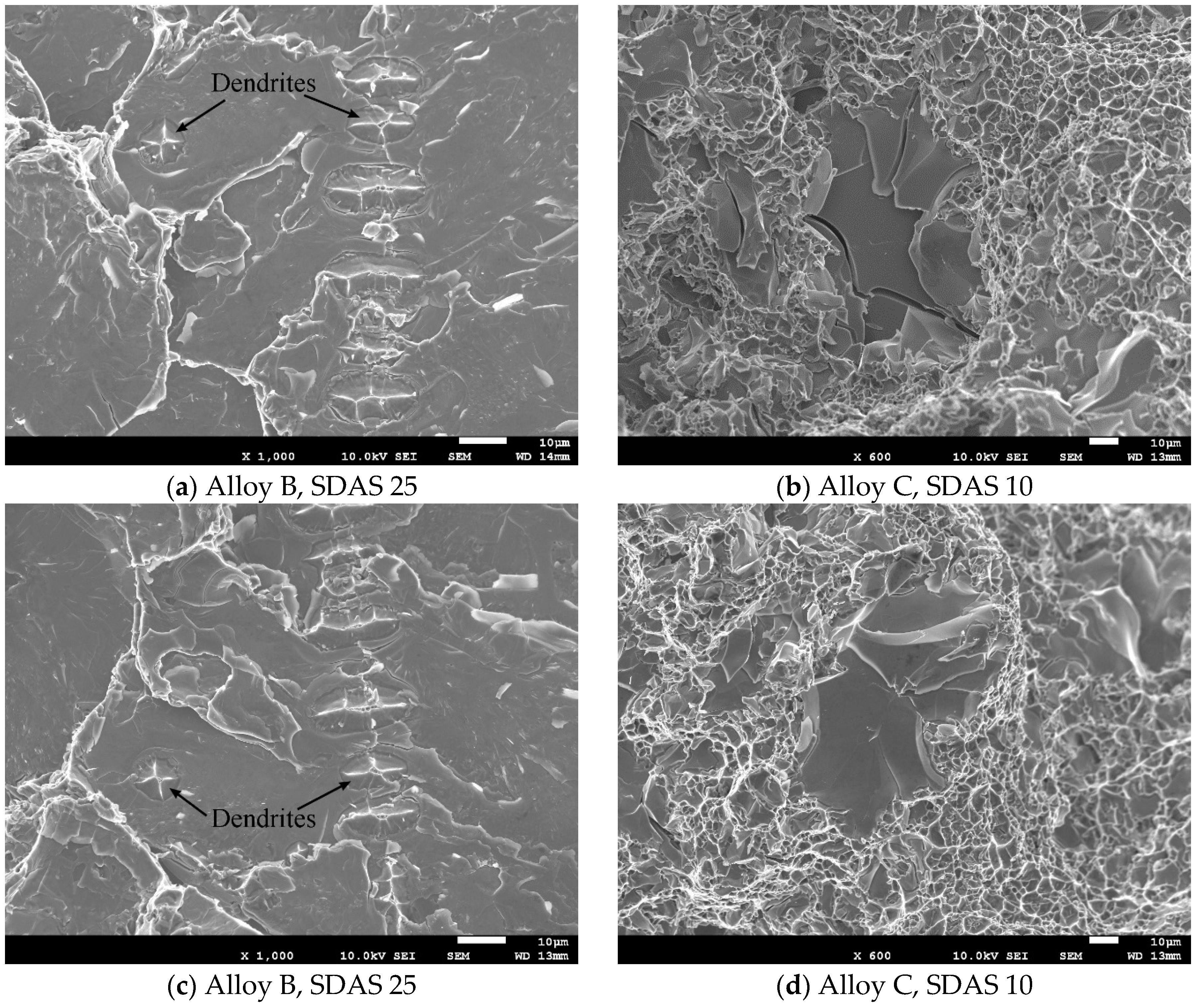
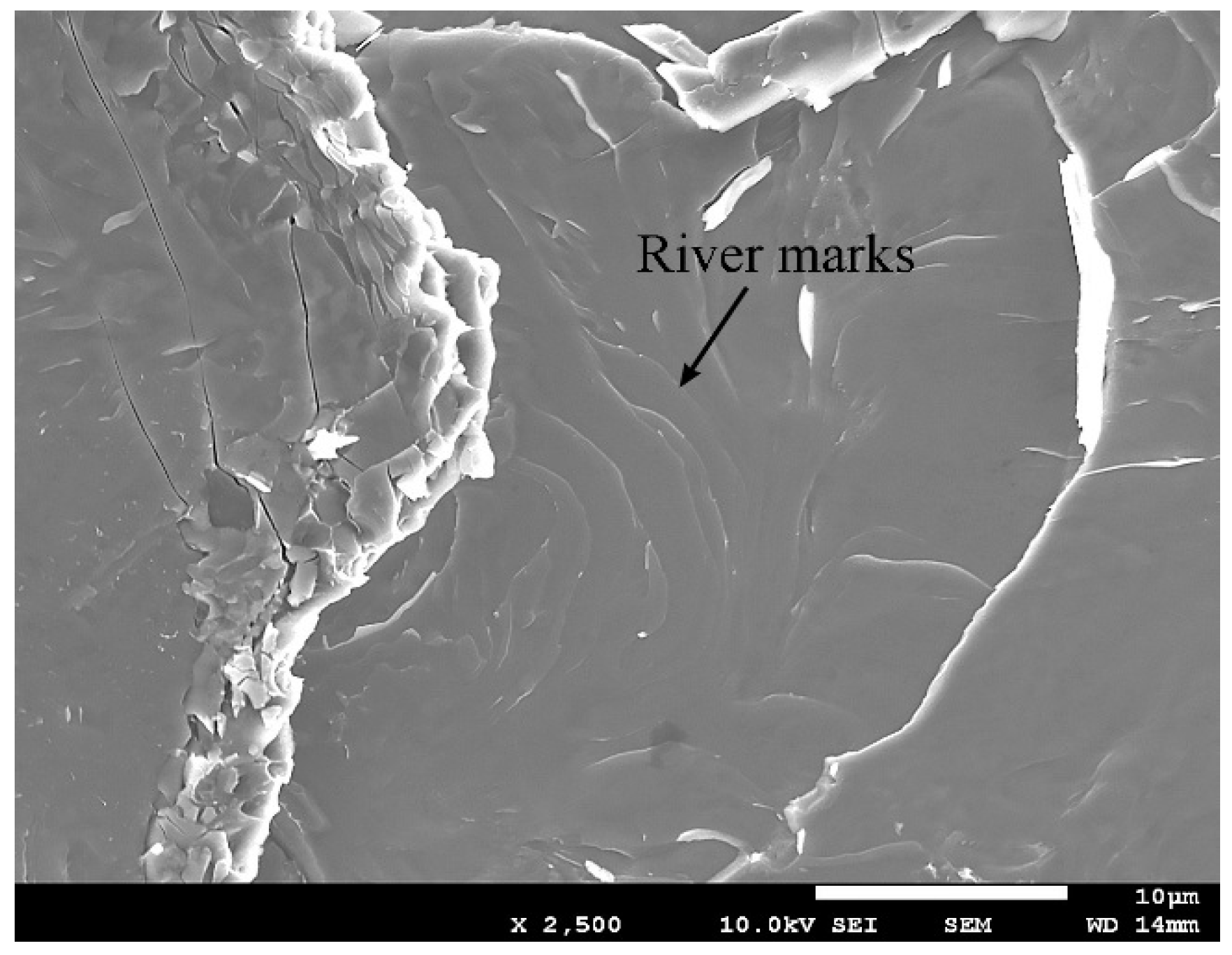
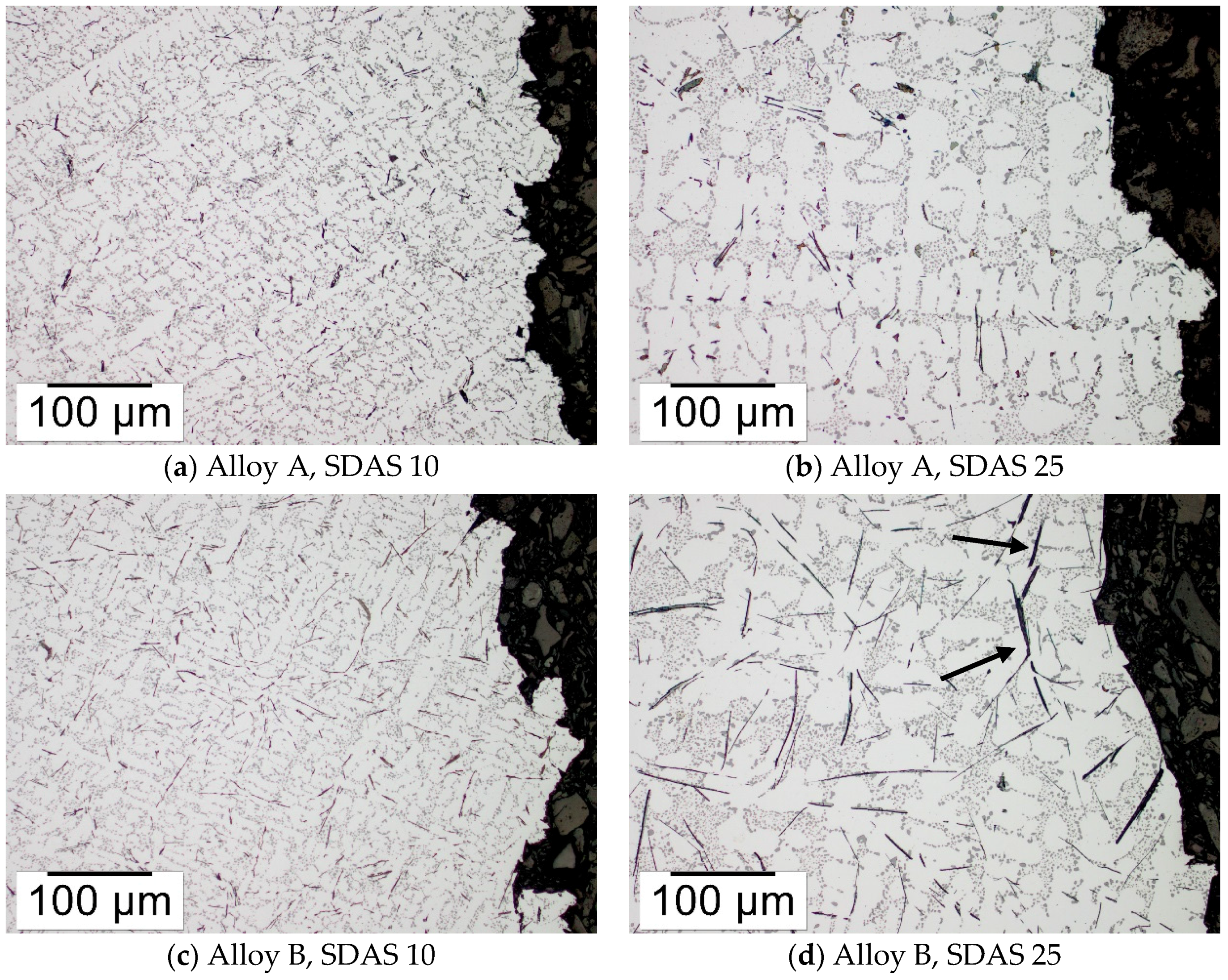
3.4. Microscopy of the Fracture Path
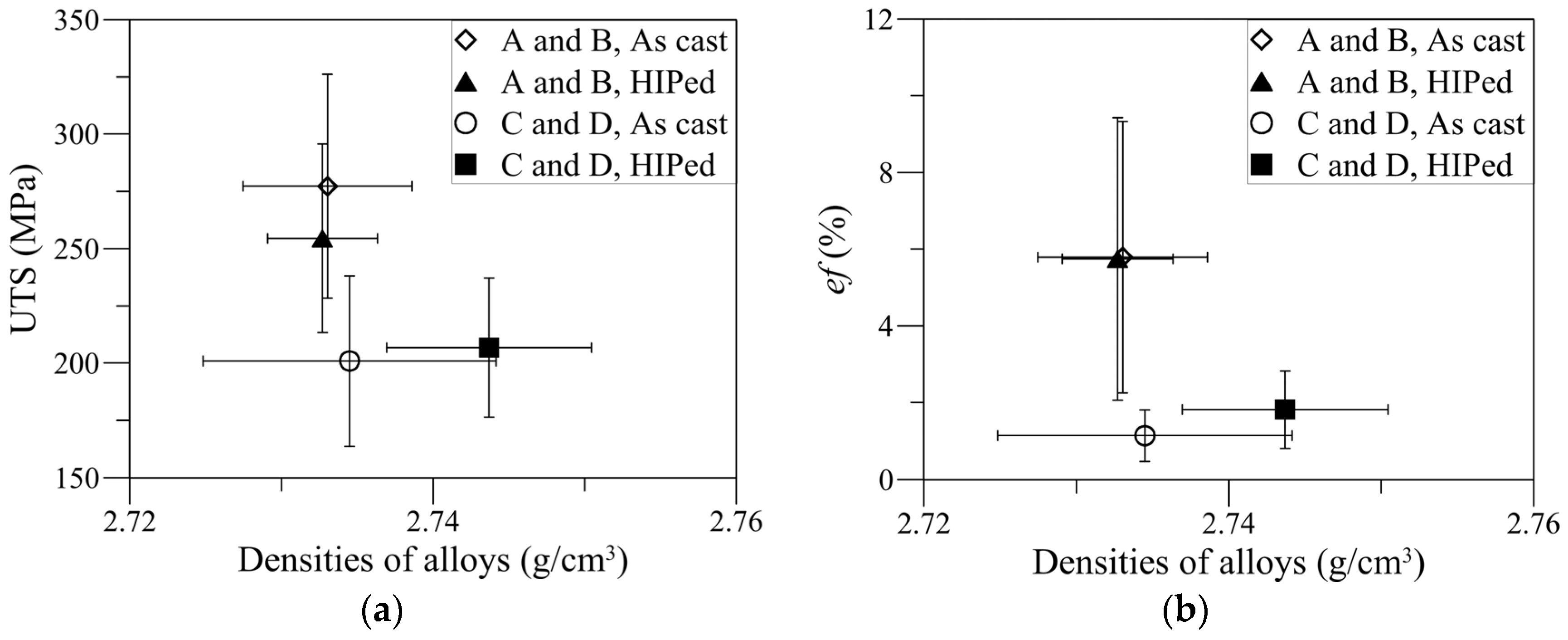
3.5. Effects of HIPing
4. Discussion
5. Conclusions
- Two alloys with different chemical compositions and different solidification rates (SDAS), but with similar β-particles size, exhibited similar UTS and ef.
- Mn and Cr additions to the Al-Si alloy modified the morphology of β-particles into sludge particles and decreased mechanical properties. Hence, the use of these modifiers should be used with care.
- Sludge governs, to some extent, the formation of porosity in the directional solidified samples. The porosity at the fracture surfaces increased in alloys where the Fe-rich particles were modified into sludge.
- Fe-rich β-particles and sludge fracture preferably through cleavage without influence of oxides, but not by particle-matrix decohesion.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Taylor, J.A. Iron-containing intermetallic phases in Al-Si based casting alloys. Procedia Mater. Sci. 2012, 1, 19–33. [Google Scholar] [CrossRef]
- Backerud, L.; Chai, G.; Tamminen, J. Solidification Characteristics of Aluminum Alloys, Vol. 2: Foundry Alloys; AFS-Skanaluminium: Stockholm, Sweden, 1990; p. 266. [Google Scholar]
- Dinnis, C.M.; Taylor, J.A.; Dahle, A.K. As-cast morphology of iron-intermetallics in Al–Si foundry alloys. Scr. Mater. 2005, 53, 955–958. [Google Scholar] [CrossRef]
- Seifeddine, S.; Svensson, I.L. The influence of Fe and Mn content and cooling rate on the microstructure and mechanical properties of A380-die casting alloys. Metall. Sci. Technol. 2009, 27, 11–20. [Google Scholar]
- Zahedi, H.; Emamy, M.; Razaghian, A.; Mahta, M.; Campbell, J.; Tiryakioǧlu, M. The effect of Fe-rich intermetallics on the Weibull distribution of tensile properties in a cast Al-5 pct Si-3 pct Cu-1 pct Fe-0.3 pct Mg alloy. Metall. Mater. Trans. A 2007, 38, 659–670. [Google Scholar] [CrossRef]
- Wang, L.; Apelian, D.; Makhlouf, M.M. Iron-bearing compounds in Al-Si diecasting alloys: Morphology and conditions under which they form. Trans. Am. Found. Soc. 1999, 107, 231–238. [Google Scholar]
- Hwang, J.Y.; Doty, H.W.; Kaufman, M.J. The effects of Mn additions on the microstructure and mechanical properties of Al–Si–Cu casting alloys. Mater. Sci. Eng. A 2008, 488, 496–504. [Google Scholar] [CrossRef]
- Crepeau, P.N. Effect of iron in Al-Si casting alloys: A critical review. AFS Trans. 1995, 103, 361–366. [Google Scholar]
- Shabestari, S.G. The effect of iron and manganese on the formation of intermetallic compounds in aluminum–silicon alloys. Mater. Sci. Eng. A 2004, 383, 289–298. [Google Scholar] [CrossRef]
- Dunn, R. Aluminum melting problems and their influence on furnace selection. Die Cast. Eng. B 1965, 9, 8–30. [Google Scholar]
- Ji, S.; Yang, W.; Gao, F.; Watson, D.; Fan, Z. Effect of iron on the microstructure and mechanical property of Al–Mg–Si–Mn and Al–Mg–Si diecast alloys. Mater. Sci. Eng. A 2012, 564, 130–139. [Google Scholar] [CrossRef]
- Vorren, O.; Evensen, J.; Pedersen, T. Microstructure and mechanical properties of AlSi(Mg) casting alloys. AFS Trans. 1984, 92, 84–462. [Google Scholar]
- Samuel, A.M.; Samuel, F.H.; Doty, H.W. Observations on the formation of β-Al5FeSi phase in 319 type Al-Si alloys. J. Mater. Sci. 1996, 31, 5529–5539. [Google Scholar] [CrossRef]
- Cao, X.; Campbell, J. The nucleation of Fe-rich phases on oxide films in Al-11.5Si-0.4Mg cast alloys. Metall. Mater. Trans. A 2003, 34A, 1409–1420. [Google Scholar] [CrossRef]
- Cao, X.; Campbell, J. Effect of precipitation and sedimentation of primary α-Fe phase on liquid metal quality of cast Al-11.1Si-0.4Mg alloy. Int. J. Cast Met. Res. 2004, 17, 1–11. [Google Scholar] [CrossRef]
- Campbell, J. Complete Casting Handbook: Metal Casting Processes, Metallurgy, Techniques and Design; Butterworth-Heinemann: Oxford, UK, 2011. [Google Scholar]
- Terzi, S.; Taylor, J.A.; Cho, Y.H.; Salvo, L.; Suéry, M.; Boller, E.; Dahle, A.K. In situ study of nucleation and growth of the irregular α-Al/β-Al5FeSi eutectic by 3-d synchrotron X-ray microtomography. Acta Mater. 2010, 58, 5370–5380. [Google Scholar] [CrossRef]
- Narayanan, L.A.; Samuel, F.H.; Gruzleski, J.E. Crystallization behavior of iron-containing intermetallic compounds in 319 aluminum alloy. Metall. Mater. Trans. A 1994, 25, 1761–1773. [Google Scholar] [CrossRef]
- Mbuya, T.O.; Odera, B.O.; Ng'ang'a, S.P. Influence of iron on castability and properties of aluminium silicon alloys: Literature review. Int. J. Cast Met. Res. 2003, 16, 451–465. [Google Scholar] [CrossRef]
- Dinnis, C.M.; Taylor, J.A.; Dahle, A.K. Iron-related porosity in Al-Si-(Cu) foundry alloys. Mater. Sci. Eng. A 2006, 425, 286–296. [Google Scholar] [CrossRef]
- Roy, N.; Samuel, A.M.; Samuel, F.H. Porosity formation in Al-9 wt pet Si-3 wt pet Cu alloy systems: Metallographic observations. Metall. Mater. Trans. A 1996, 27, 415–429. [Google Scholar] [CrossRef]
- Ferraro, S.; Timelli, G. Influence of sludge particles on the tensile properties of die-cast secondary aluminum alloys. Metall. Mater. Trans. B 2015, 46, 1022–1034. [Google Scholar] [CrossRef]
- Gruzleski, J.E.; Closset, B.M. The Treatment of Liquid Aluminum-Silicon Alloys; American Foundrymen’s Society, Inc.: Des Plaines, IL, USA, 1990. [Google Scholar]
- Seifeddine, S. Characteristics of Cast Aluminium-Silicon Alloys—Microstructures and Mechanical Properties. Ph.D. Thesis, Linköping Studies in Science and Technology, Sweden, 2006; p. 138. [Google Scholar]
- Sjölander, E.; Seifeddine, S. The heat treatment of Al-Si-Cu-Mg casting alloys. J. Mater. Process. Technol. 2010, 210, 1249–1259. [Google Scholar] [CrossRef]
- Dispinar, D. Determination of Metal Quality of Aluminium and Its Alloys. Ph.D. Thesis, The University of Birmingham, Birmingham, UK, January 2005. [Google Scholar]
- Makhlouf, M.M.; Apelian, D. Casting Characteristics of Aluminum Die Casting Alloys; Advanced Casting Research Center Worcester Polytechnic Institute: Worcester, MA, USA, 2002; pp. 1–46. [Google Scholar]
- Ramakrishnan, N. An analytical study on strengthening of particulate reinforced metal matrix composites. Acta Mater. 1996, 44, 69–77. [Google Scholar] [CrossRef]
- Dai, L.H.; Ling, Z.; Bai, Y.L. Size-dependent inelastic behavior of particle-reinforced metal-matrix composites. Compos. Sci. Technol. 2001, 61, 1057–1063. [Google Scholar] [CrossRef]
- Ceschini, L.; Dahle, A.; Gupta, M.; Jarfors, A.E.W.; Jayalakshmi, S.; Morri, A.; Rotundo, F.; Toschi, S.; Singh, R.A. Aluminium and Magnesium Metal Matrix Nanocomposites; Springer Nature: Singapore, 2017. [Google Scholar]
- Ferraro, S.; Bjurenstedt, A.; Seifeddine, S. On the Formation of Sludge Intermetallic Particles in Secondary Aluminum Alloys. Metall. Mater. Trans. A 2015, 46, 3713–3722. [Google Scholar] [CrossRef]
- Surappa, M.K.; Blank, E.; Jaquet, J.C. Effect of macro-porosity on the strength and ductility of cast Al7Si0.3Mg alloy. Scr. Metall. 1986, 20, 1281–1286. [Google Scholar] [CrossRef]




| Alloy | Si | Fe | Cu | Mn | Mg | Cr | Ni | Zn | Pb | Sn | Sr | Al | SF | Fe/Mn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 9.30 | 0.74 | 2.79 | 0.25 | 0.05 | 0.03 | 0.04 | 0.96 | 0.06 | 0.03 | 0.022 | Bal. | 1.3 | 3.0 |
| B | 9.40 | 1.17 | 2.77 | 0.25 | 0.05 | 0.03 | 0.04 | 0.91 | 0.05 | 0.03 | 0.027 | Bal. | 1.8 | 4.7 |
| C | 9.23 | 1.29 | 2.65 | 0.53 | 0.04 | 0.15 | 0.04 | 0.86 | 0.05 | 0.03 | 0.025 | Bal. | 2.8 | 2.4 |
| D | 9.30 | 1.59 | 2.64 | 0.80 | 0.04 | 0.18 | 0.04 | 0.80 | 0.05 | 0.03 | 0.029 | Bal. | 3.7 | 2.0 |
| Measured Feature | Alloy A | Alloy B | ||
|---|---|---|---|---|
| SDAS 10 | SDAS 25 | SDAS 10 | SDAS 25 | |
| Average β-particle length (µm) 1 | 40 ± 12 | 79 ± 26 | 74 ± 19 | 160 ± 45 |
| UTS (MPa) | 297 ± 14 | 258 ± 24 | 262 ± 50 | 228 ± 9 |
| ef (%) | 7.5 ± 2.6 | 3.4 ± 1.1 | 3.5 ± 2.4 | 1.1 ± 0.1 |
| Area% β-particles | 1.12 ± 0.24 | 1.21 ± 0.58 | 2.08 ± 0.27 | 2.91 ± 0.57 |
| Measured Feature | As-Cast (SDAS 10) | HIPed (SDAS 10) | ||
|---|---|---|---|---|
| A and B | C and D | A and B | C and D | |
| No of samples with porosity | 1 of 12 | 9 of 11 | 2 of 9 | 4 of 10 |
| Porosity area (%) | 1.9 ± n/a | 7.4 ± 5.7 | 1.5 ± 1.6 | 1.5 ± 0.5 |
| Average No of pores | 4 ± n/a | 7.9 ± 5.3 | 1 ± 0 | 1.5 ± 0.6 |
| Average size of pores (mm2) | 0.13 ± 0.06 | 0.27 ± 0.26 | 0.42 ± 0.44 | 0.29 ± 0.16 |
| No of samples with oxides | 2 of 12 | 5 of 11 | 2 of 9 | 5 of 10 |
| Oxides area (%) | 15.7 ± 11.5 | 12.7 ± 12.7 | 29.9 ± 20.6 | 23.6 ± 12.5 |
| Tensile Property | As-Cast (SDAS 10) | HIPed (SDAS 10) | ||
|---|---|---|---|---|
| A and B | C and D | A and B | C and D | |
| UTS (MPa) | 295 ± 14 | 221 ± 22 | 273 ± 19 | 229 ± 14 |
| ef (%) | 6.4 ± 2.5 | 1.6 ± 0.6 | 7.0 ± 3.1 | 2.6 ± 0.8 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bjurenstedt, A.; Seifeddine, S.; Jarfors, A.E.W. The Effects of Fe-Particles on the Tensile Properties of Al-Si-Cu Alloys. Metals 2016, 6, 314. https://doi.org/10.3390/met6120314
Bjurenstedt A, Seifeddine S, Jarfors AEW. The Effects of Fe-Particles on the Tensile Properties of Al-Si-Cu Alloys. Metals. 2016; 6(12):314. https://doi.org/10.3390/met6120314
Chicago/Turabian StyleBjurenstedt, Anton, Salem Seifeddine, and Anders E. W. Jarfors. 2016. "The Effects of Fe-Particles on the Tensile Properties of Al-Si-Cu Alloys" Metals 6, no. 12: 314. https://doi.org/10.3390/met6120314






