Thermodynamic Analysis of Possible Chalcopyrite Dissolution Mechanism in Sulfuric Acidic Aqueous Solution
Abstract
:1. Introduction
2. Thermodynamic Analysis of the Possible Reactions
2.1. Possible Oxidative Dissolution Reactions of CuFeS2 in Sulfuric Acid Solution
2.2. Possible Non-Oxidative Dissolution Reactions of CuFeS2 in Sulfuric Acid Solution
2.3. Balanced Concentrations of Cu2+, H2S, Fe2+ and Fe3+ in Aqueous Solution with Simple Sulfides
2.3.1. Balanced Concentrations of Cu2+ and H2S in Aqueous Solution with Copper Sulfides
2.3.2. Balanced Aqueous Concentrations of Fe3+, Fe2+ and H2S with Iron Sulfides
2.4. Dissolution of Secondary Copper Sulfides in Sulfuric Acid Solution
2.4.1. Non-Oxidative Dissolution of Secondary Copper Sulfides in Sulfuric Acid Solution
2.4.2. Oxidative Dissolution of Secondary Copper Sulfides in Sulfuric Acid Solution
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Córdoba, E.; Muñoz, J.; Blázquez, M.; González, F.; Ballester, A. Leaching of chalcopyrite with ferric ion. Part I: General aspects. Hydrometallurgy 2008, 93, 81–87. [Google Scholar] [CrossRef]
- Wang, S. Copper leaching from chalcopyrite concentrates. JOM 2005, 57, 48–51. [Google Scholar] [CrossRef]
- Klauber, C. A critical review of the surface chemistry of acidic ferric sulphate dissolution of chalcopyrite with regards to hindered dissolution. Int. J. Miner. Process. 2008, 86, 1–17. [Google Scholar] [CrossRef]
- Hiroyoshi, N.; Arai, M.; Miki, H.; Tsunekawa, M.; Hirajima, T. A new reaction model for the catalytic effect of silver ions on chalcopyrite leaching in sulfuric acid solutions. Hydrometallurgy 2002, 63, 257–267. [Google Scholar] [CrossRef]
- Ahmadi, A.; Schaffie, M.; Petersen, J.; Schippers, A.; Ranjbar, M. Conventional and electrochemical bioleaching of chalcopyrite concentrates by moderately thermophilic bacteria at high pulp density. Hydrometallurgy 2011, 106, 84–92. [Google Scholar] [CrossRef]
- Nicol, M.; Miki, H.; Velásquez-Yévenes, L. The dissolution of chalcopyrite in chloride solutions: Part 3. Mechanisms. Hydrometallurgy 2010, 103, 86–95. [Google Scholar] [CrossRef]
- Majima, H.; Awakura, Y.; Hirato, T.; Tanaka, T. The leaching of chalcopyrite in ferric chloride and ferric sulfate solutions. Can. Metall. Q. 1985, 24, 283–291. [Google Scholar] [CrossRef]
- Jones, D.L.; Peters, E. The Leaching of Chalcopyrite with Ferric Sulphate and Ferric Chloride; American Institute for Mining Engineers: New York, NY, USA, 1976. [Google Scholar]
- Miller, J.; McDonough, P.; Portillo, H. Electrochemistry in silver catalyzed ferric sulfate leaching of chalcopyrite. In Process and Fundamental Considerations of Selected Hydrometallurgical Systems; AIME: New York, NY, USA, 1981; Volume 27, pp. 327–328. [Google Scholar]
- Kametani, H.; Aoki, A. Effect of suspension potential on the oxidation rate of copper concentrate in a sulfuric acid solution. Metall. Trans. B 1985, 16, 695–705. [Google Scholar] [CrossRef]
- Hiroyoshi, N.; Miki, H.; Hirajima, T.; Tsunekawa, M. Enhancement of chalcopyrite leaching by ferrous ions in acidic ferric sulfate solutions. Hydrometallurgy 2001, 60, 185–197. [Google Scholar] [CrossRef]
- Hiroyoshi, N.; Miki, H.; Hirajima, T.; Tsunekawa, M. A model for ferrous-promoted chalcopyrite leaching. Hydrometallurgy 2000, 57, 31–38. [Google Scholar] [CrossRef]
- Parker, A.; Paul, R.; Power, G. Electrochemistry of the oxidative leaching of copper from chalcopyrite. J. Electroanal. Chem. 1981, 118, 305–316. [Google Scholar] [CrossRef]
- Nicol, M.; Lazaro, I. The Role of Non-Oxidative Processes in the Leaching of Chalcopyrite; Copper: Santiago, Chile, 2003; pp. 367–381. [Google Scholar]
- Harmer, S.L. Surface Layer Control for Improved Copper Recovery for Chalcopyrite Leaching. Master’s Thesis, Ian Wark Research Institute, University of South Australia, Adelaide, Australia, 2002. [Google Scholar]
- Dutrizac, J. Elemental sulphur formation during the ferric sulphate leaching of chalcopyrite. Can. Metall. Q. 1989, 28, 337–344. [Google Scholar] [CrossRef]
- Linge, H.G. Reactivity comparison of australian chalcopyrite concentrates in acidified ferric solution. Hydrometallurgy 1977, 2, 219–233. [Google Scholar] [CrossRef]
- Yin, Q.; Kelsall, G.H.; Vaughan, D.J.; England, K.E.R. Atmospheric and electrochemical oxidation of the surface of chalcopyrite (CuFeS2). Geochim. Cosmochim. Acta 1995, 59, 1091–1100. [Google Scholar] [CrossRef]
- Klauber, C.; Parker, A.; van Bronswijk, W.; Watling, H. Sulphur speciation of leached chalcopyrite surfaces as determined by X-ray photoelectron spectroscopy. Int. J. Miner. Process. 2001, 62, 65–94. [Google Scholar] [CrossRef]
- Buckley, A.N.; Woods, R. An X-ray photoelectron spectroscopic study of the oxidation of chalcopyrite. Aust. J. Chem. 1984, 37, 2403–2413. [Google Scholar] [CrossRef]
- Brion, D. Photoelectron spectroscopic study of the surface degradation of pyrite (FeS2), chalcopyrite (CuFeS2), sphalerite (ZnS), and galena (PbS) in air and water. Appl. Surf. Sci. 1980, 5, 133–152. [Google Scholar] [CrossRef]
- Hiroyoshi, N.; Kuroiwa, S.; Miki, H.; Tsunekawa, M.; Hirajima, T. Synergistic effect of cupric and ferrous ions on active-passive behavior in anodic dissolution of chalcopyrite in sulfuric acid solutions. Hydrometallurgy 2004, 74, 103–116. [Google Scholar] [CrossRef]
- Okamoto, H.; Nakayama, R.; Kuroiwa, S.; Hiroyoshi, N.; Tsunekawa, M. Normalized redox potential used to assess chalcopyrite column leaching. J. MMIJ 2005, 121, 246–254. [Google Scholar] [CrossRef]
- Petersen, J.; Dixon, D.G. Competitive bioleaching of pyrite and chalcopyrite. Hydrometallurgy 2006, 83, 40–49. [Google Scholar] [CrossRef]
- Holliday, R.I.; Richmond, W.R. An electrochemical study of the oxidation of chalcopyrite in acidic solution. J. Electroanal. Chem. Interfacial Electrochem. 1990, 288, 83–98. [Google Scholar] [CrossRef]
- Biegler, T.; Swift, D. Anodic electrochemistry of chalcopyrite. J. Appl. Electrochem. 1979, 9, 545–554. [Google Scholar] [CrossRef]
- Barin, I.; Knacke, O.; Kubaschewski, O. Thermochemical Properties of Inorganic Substances, Supplement; Springer: Berlin, Germany, 1977; pp. 2558–2560. [Google Scholar]
- Knacke, O.; Kubaschewski, O.; Hesselman, K. Thermochemical Properties of Inorganic Substances, 2nd ed.; Springer: Berlin, Germany, 1991; pp. 602–631. [Google Scholar]
- Landolt-Börnstein. Thermodynamic Properties of Inorganic Material; Springer: Berlin, Germany, 1999. [Google Scholar]
- Warren, G.; Wadsworth, M.; El-Raghy, S. Passive and transpassive anodic behavior of chalcopyrite in acid solutions. Metall. Trans. B 1982, 13, 571–579. [Google Scholar] [CrossRef]
- Majuste, D.; Ciminelli, V.; Osseo-Asare, K.; Dantas, M.; Magalhães-Paniago, R. Electrochemical dissolution of chalcopyrite: Detection of bornite by synchrotron small angle X-ray diffraction and its correlation with the hindered dissolution process. Hydrometallurgy 2012, 111, 114–123. [Google Scholar] [CrossRef]
- Silvester, E.; Grieser, F.; Healy, T.W.; Meisel, D.; Sullivan, J.C. Thermodynamics and kinetics of the reaction of copper(II) and iron(III) with ultra-small colloidal chalcopyrite (CuFeS2). J. Chem. Soc. Faraday Trans. 1994, 90, 3301–3307. [Google Scholar] [CrossRef]
- Woods, R.; Yoon, R.; Young, C. Eh-pH diagrams for stable and metastable phases in the copper-sulfur-water system. Int. J. Miner. Process. 1987, 20, 109–120. [Google Scholar] [CrossRef]
- Koch, D.; McIntyre, R. The application of reflectance spectroscopy to a study of the anodic oxidation of cuprous sulphide. J. Electroanal. Chem. Interfacial Electrochem. 1976, 71, 285–296. [Google Scholar] [CrossRef]
- Liu, J.; Liu, D. Spectroscopic characterization of dissolubility and surface properties of chalcopyrite in aqueous solution. Spectrosc. Spectr. Anal. 2012, 32, 519–524. [Google Scholar]
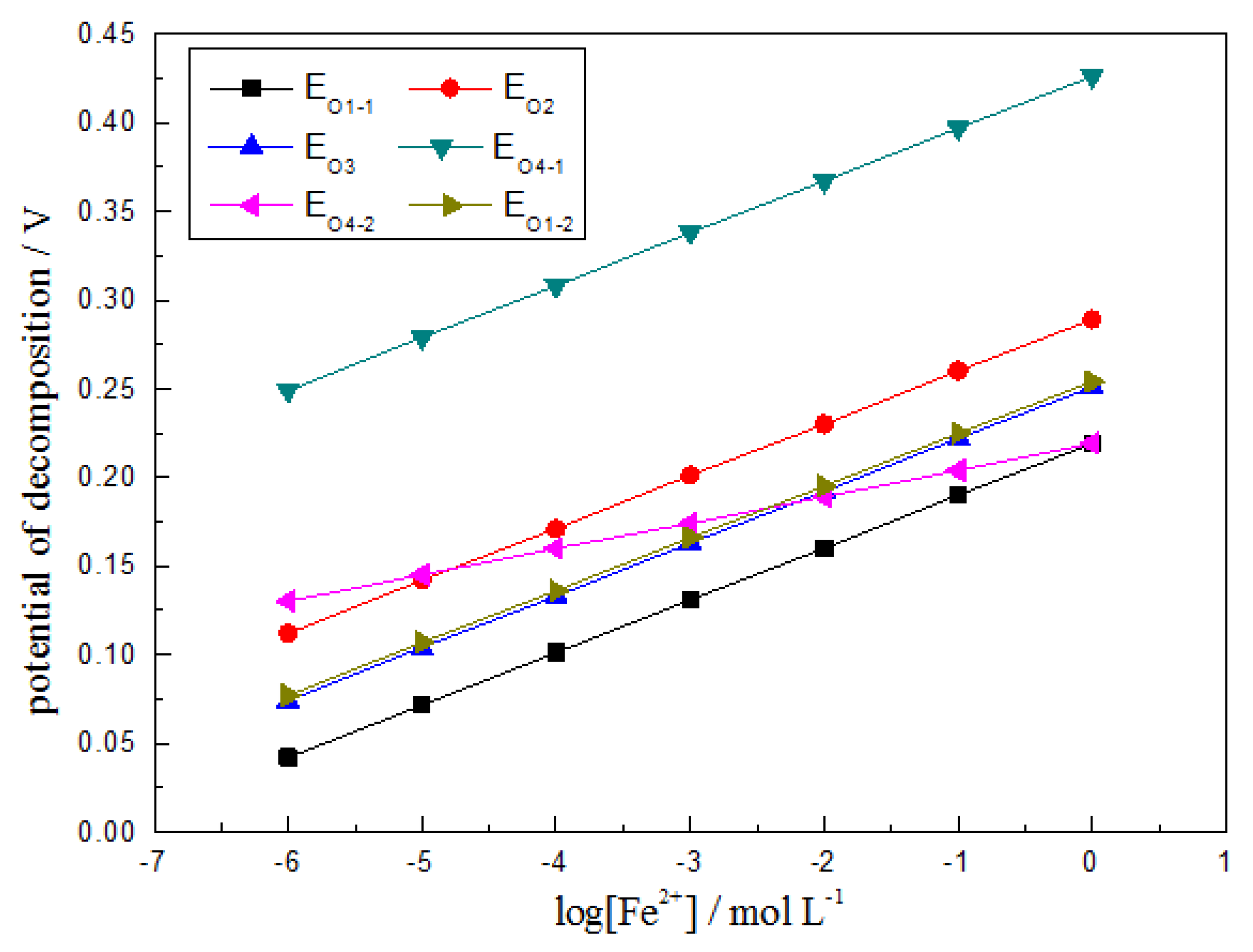
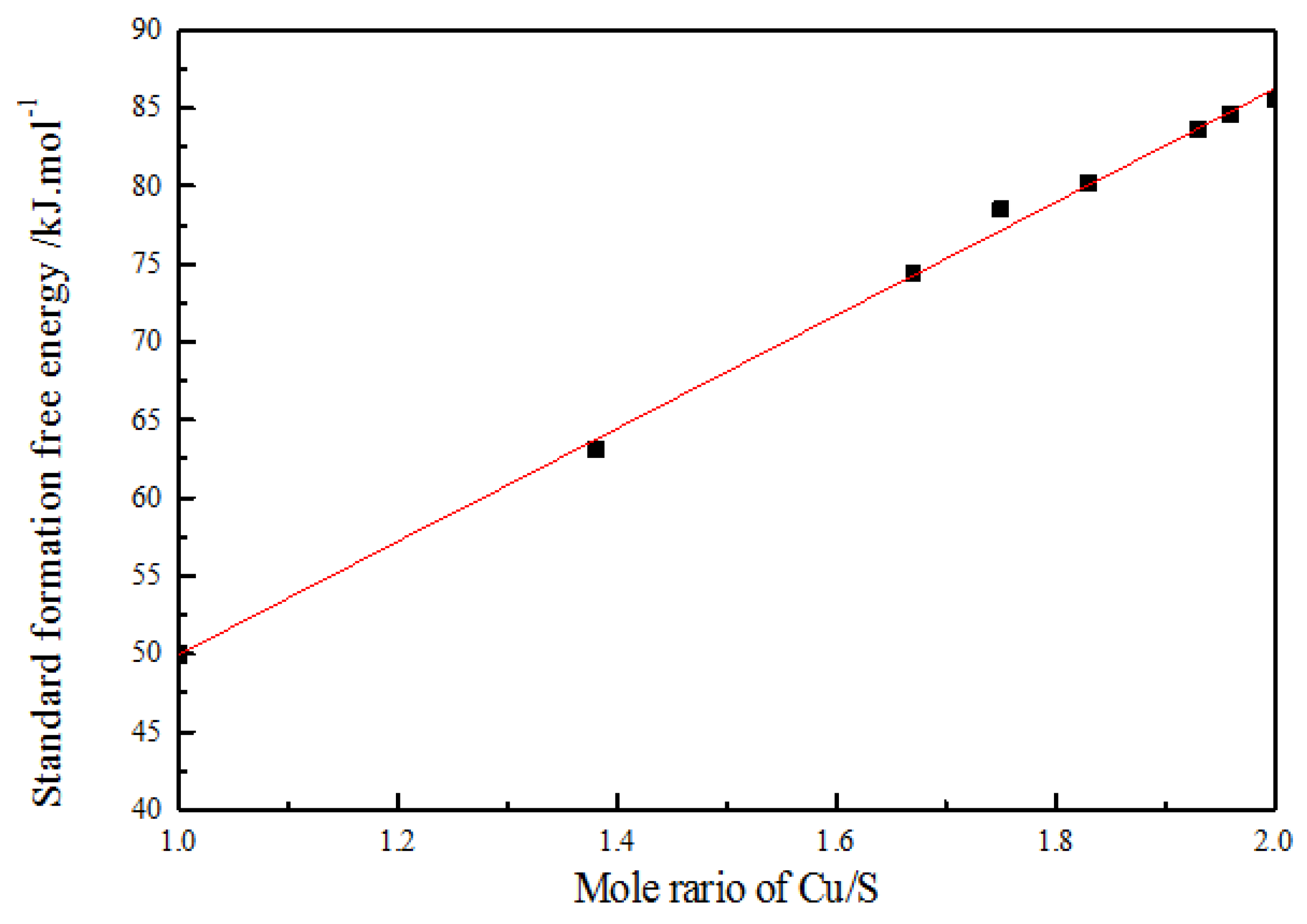

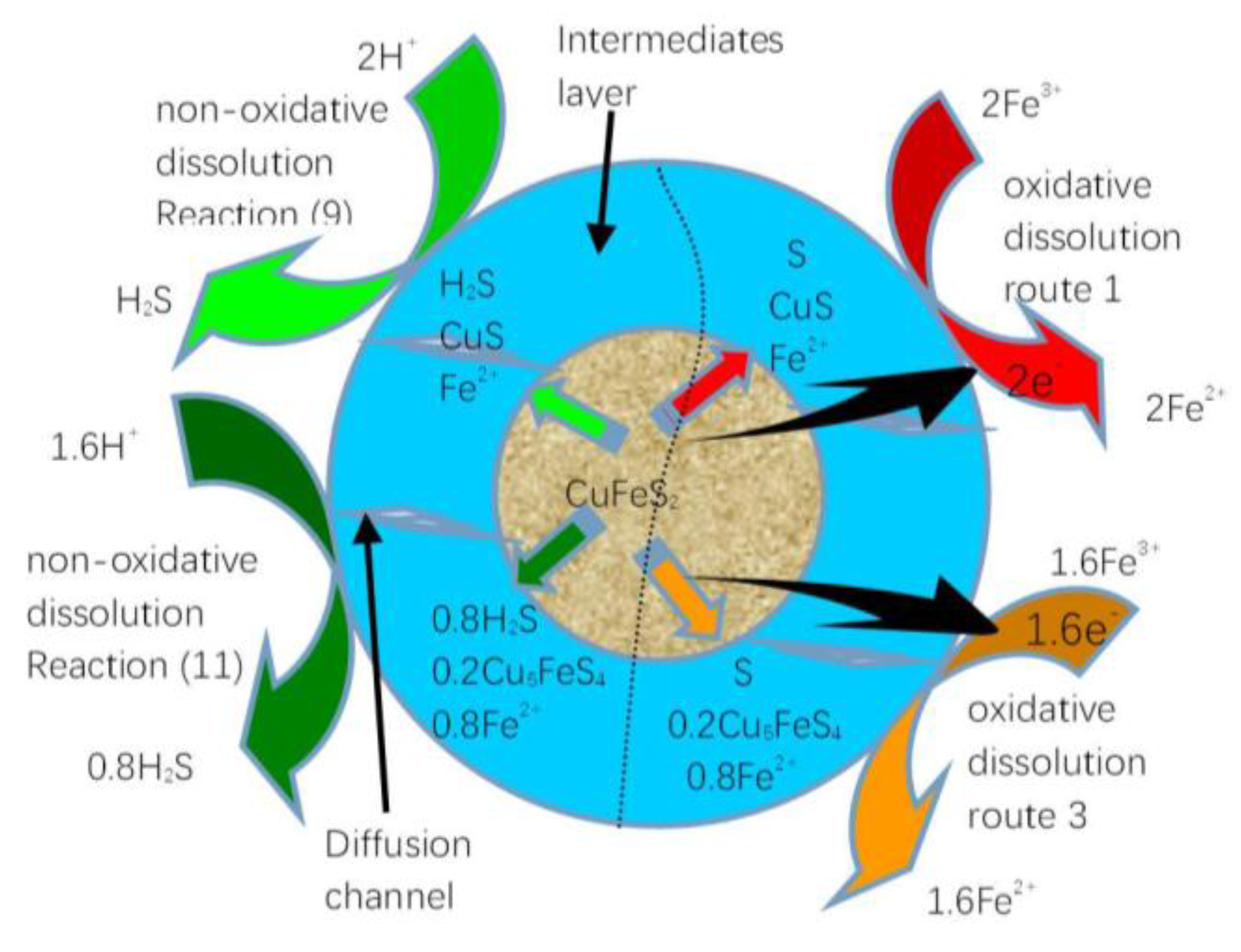

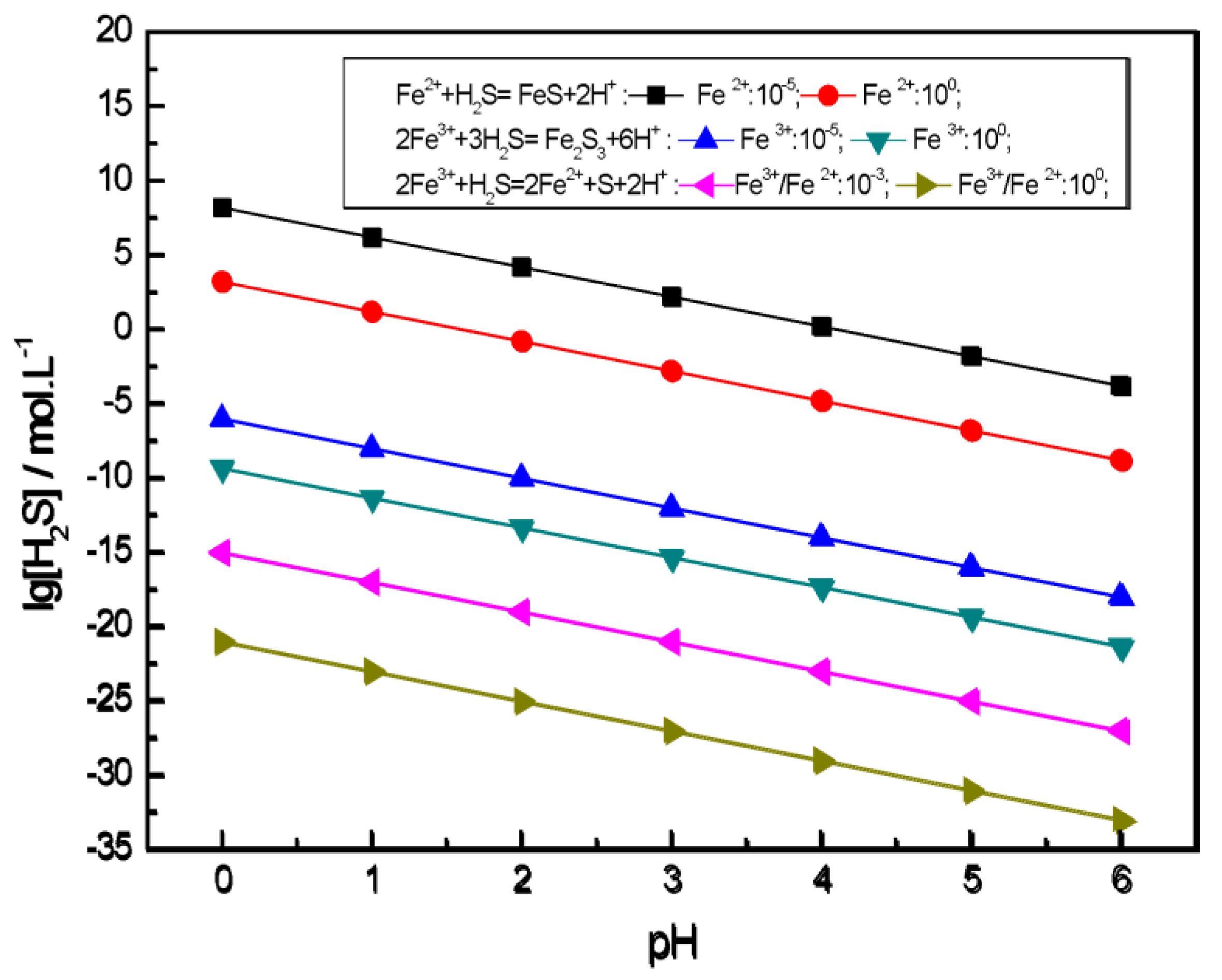
| Routes | Reactions | Equilibrium Potentials |
|---|---|---|
| 1 | CuFeS2 = CuS + S + Fe2+ + 2e | Eo1 = 0.219 + 0.0295lg[Fe2+] |
| 2 | 2CuFeS2 = Cu2S + 3S + 2Fe2+ + 4e | Eo2 = 0.289 + 0.0295lg[Fe2+] |
| 3 | 10CuFeS2 = 2Cu5FeS4 + 12S + 8Fe2+ + 16e | Eo3 = 0.251 + 0.0295lg[Fe2+] |
| 4 | CuFeS2 = Cu2+ + 2S + Fe2+ + 4e | Eo4 = 0.426 + 0.0148lg[Fe2+] + 0.0148lg[Cu2+] |
| Formula | Cu2S | Cu1.96S | Cu1.93S | Cu1.83S | Cu1.75S | Cu1.67S | Cu1.38S | CuS |
|---|---|---|---|---|---|---|---|---|
| ΔGf0 | −85.52 | −84.60 | −83.60 | −80.25 | −78.50 | −74.40 | −63.11 | −48.93 |
| Atmosphere | Solution Acidity | Leaching Time (min) | Ratio of Cu/Fe |
|---|---|---|---|
| Ar | Pure water | 420 | 1.04 |
| pH 2.09 | 180 | 1.75 | |
| O2 | Pure water | 420 | 1.02 |
| pH 2.09 | 180 | 1.23 |
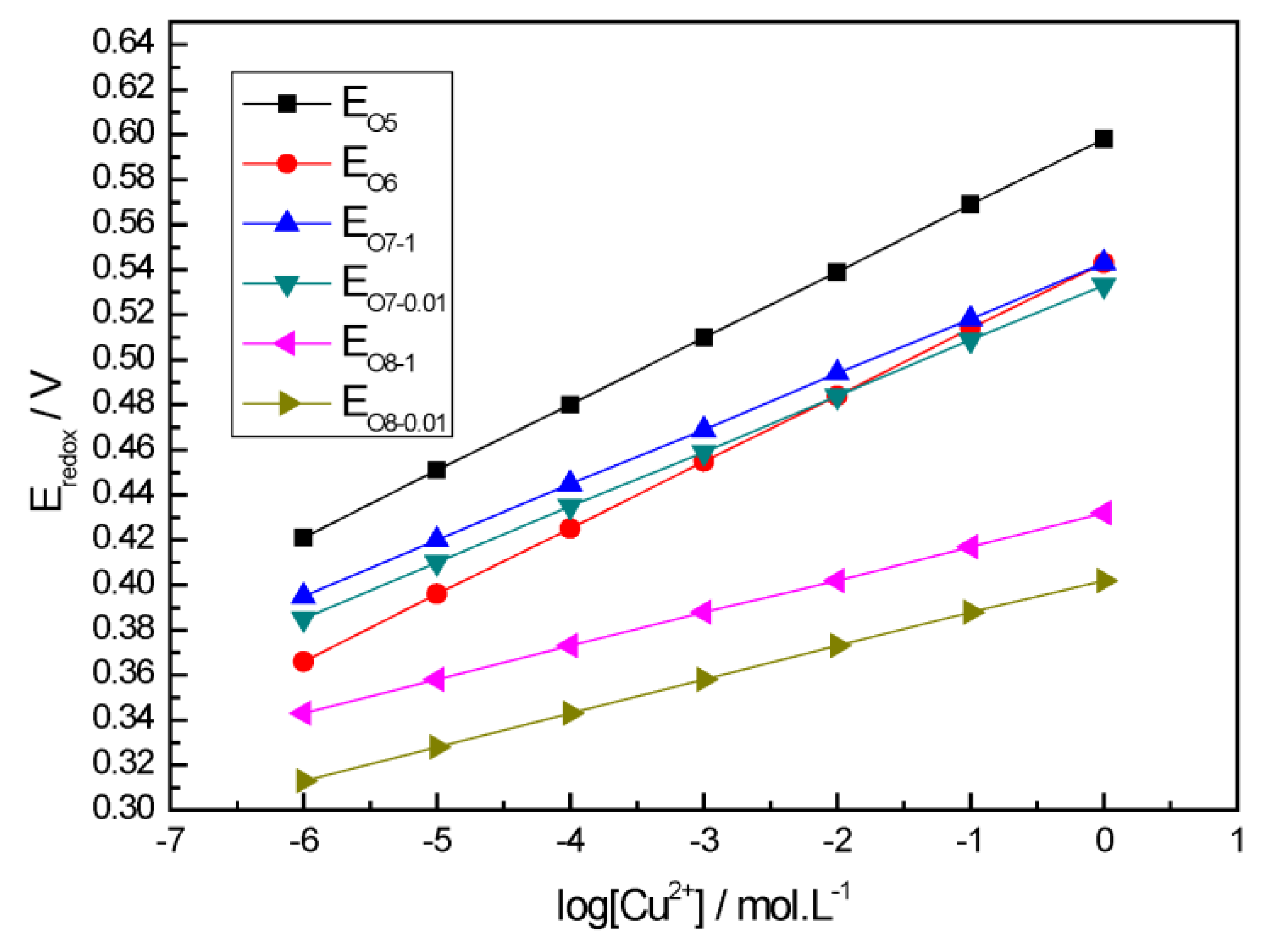
| Equation Number | Reactions | Potentials |
|---|---|---|
| 5 | CuS = S + Cu2+ + 2e | Eo5 = 0.598 + 0.0295lg[Cu2+] |
| 6 | Cu5FeS4 = Cu3FeS4 + 2Cu2+ + 4e | Eo6 = 0.543 + 0.0295lg[Cu2+] |
| 7 | Cu5FeS4 = 5Cu2+ + Fe2+ + 4S + 12e | Eo7 = 0.543 + 0.0246lg[Cu2+] + 0.00492lg[Fe2+] |
| 8 | Cu5FeS4 = 4CuS + Fe2+ + Cu2+ + 4e | Eo8 = 0.432 + 0.0148lg[Cu2+] + 0.0148lg[Fe2+] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, D.; Wang, W.; Chang, Y.; Xie, F.; Jiang, K. Thermodynamic Analysis of Possible Chalcopyrite Dissolution Mechanism in Sulfuric Acidic Aqueous Solution. Metals 2016, 6, 303. https://doi.org/10.3390/met6120303
Lu D, Wang W, Chang Y, Xie F, Jiang K. Thermodynamic Analysis of Possible Chalcopyrite Dissolution Mechanism in Sulfuric Acidic Aqueous Solution. Metals. 2016; 6(12):303. https://doi.org/10.3390/met6120303
Chicago/Turabian StyleLu, Diankun, Wei Wang, Yongfeng Chang, Feng Xie, and Kaixi Jiang. 2016. "Thermodynamic Analysis of Possible Chalcopyrite Dissolution Mechanism in Sulfuric Acidic Aqueous Solution" Metals 6, no. 12: 303. https://doi.org/10.3390/met6120303





