In Situ Observation of Crystal Rain and Its Effect on Columnar to Equiaxed Transition
Abstract
:1. Introduction
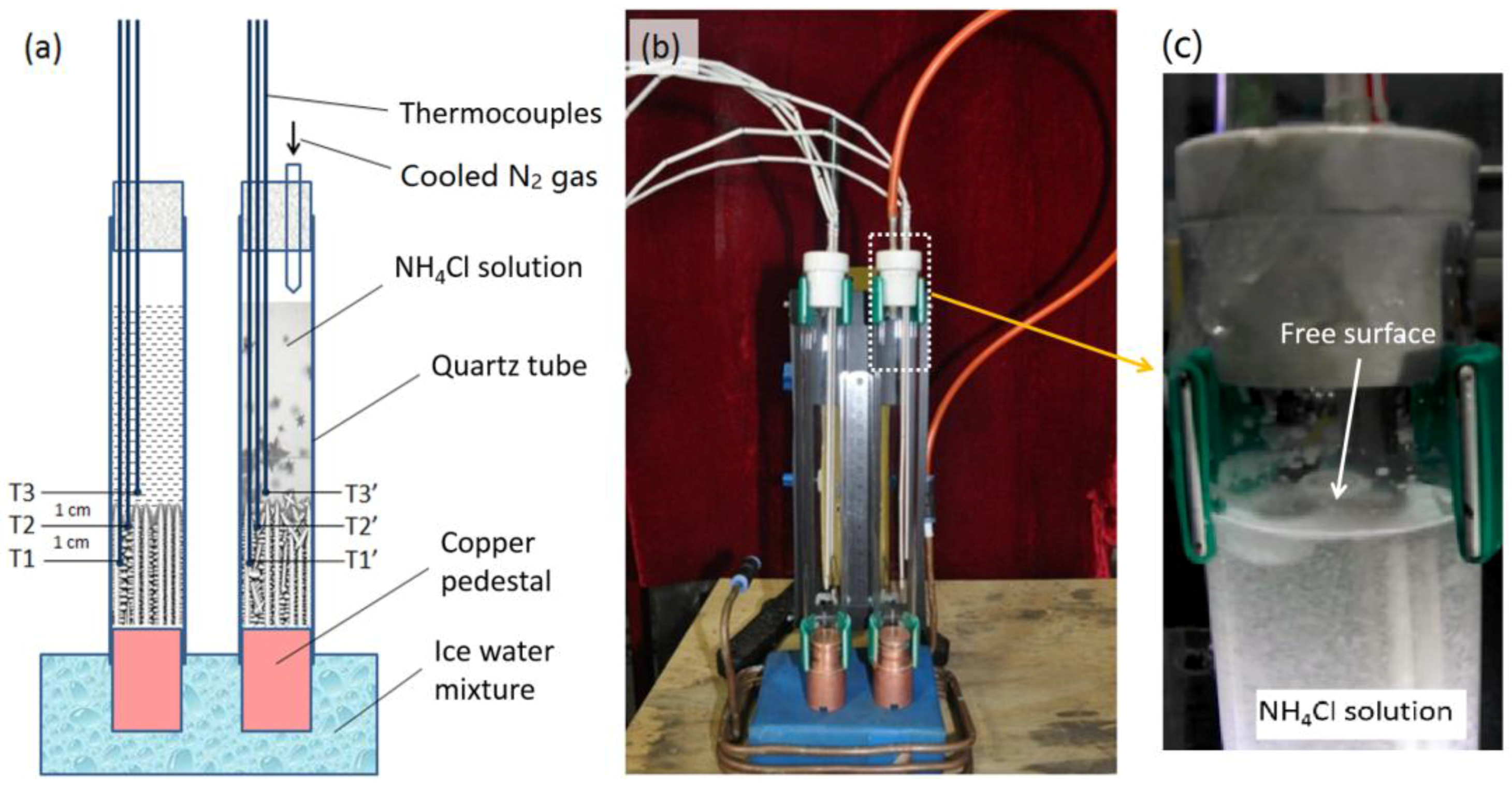
2. Experimental Procedure
3. Results and Discussion
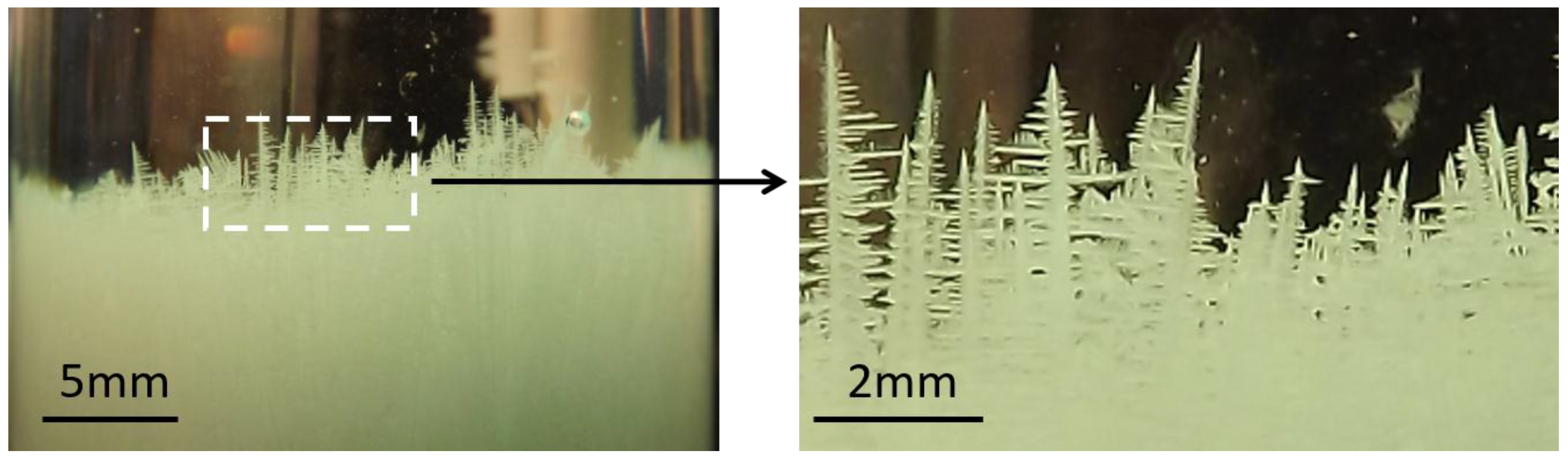
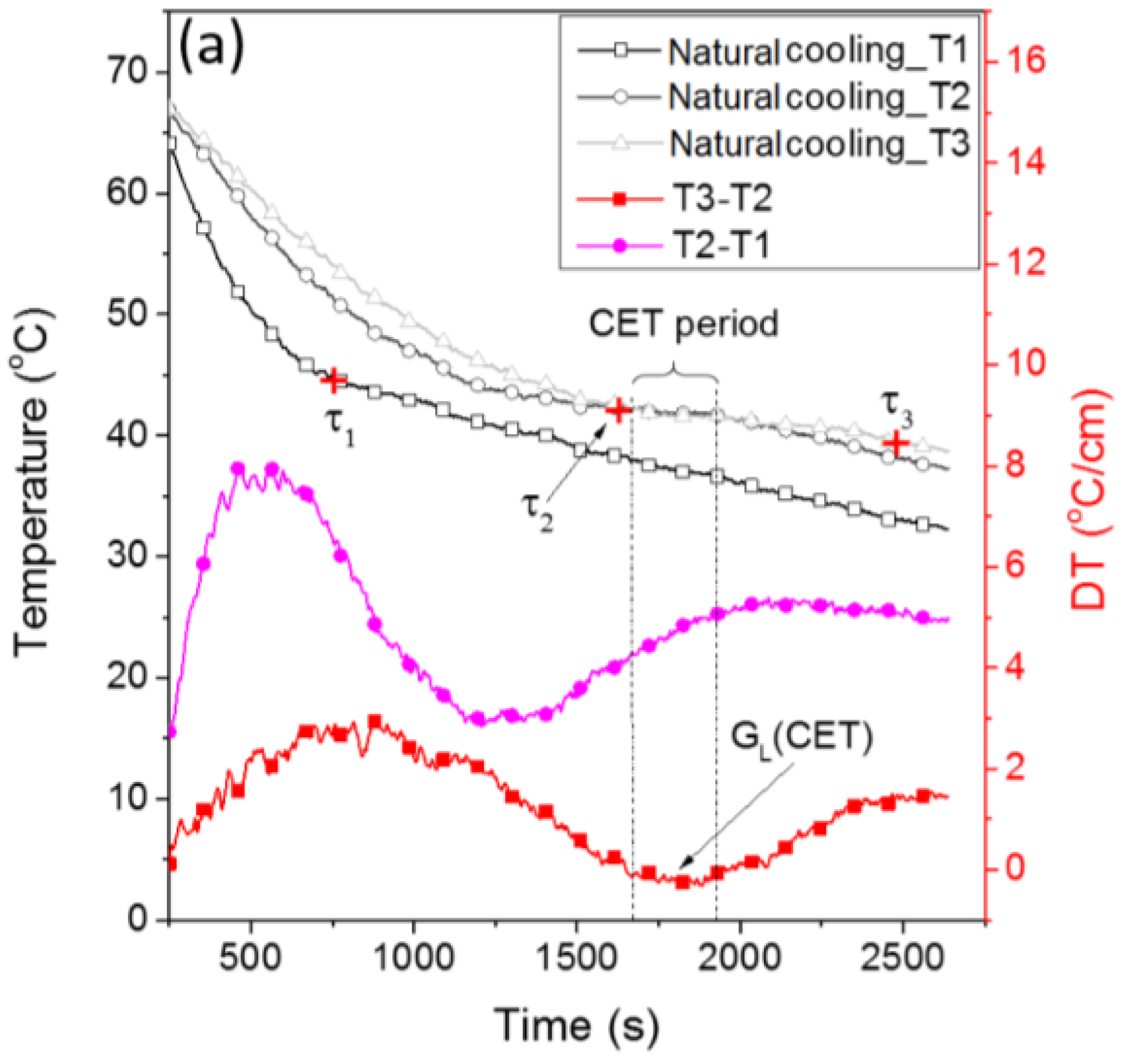
3.1. Crystal Growth and the CET in the Reference Sample
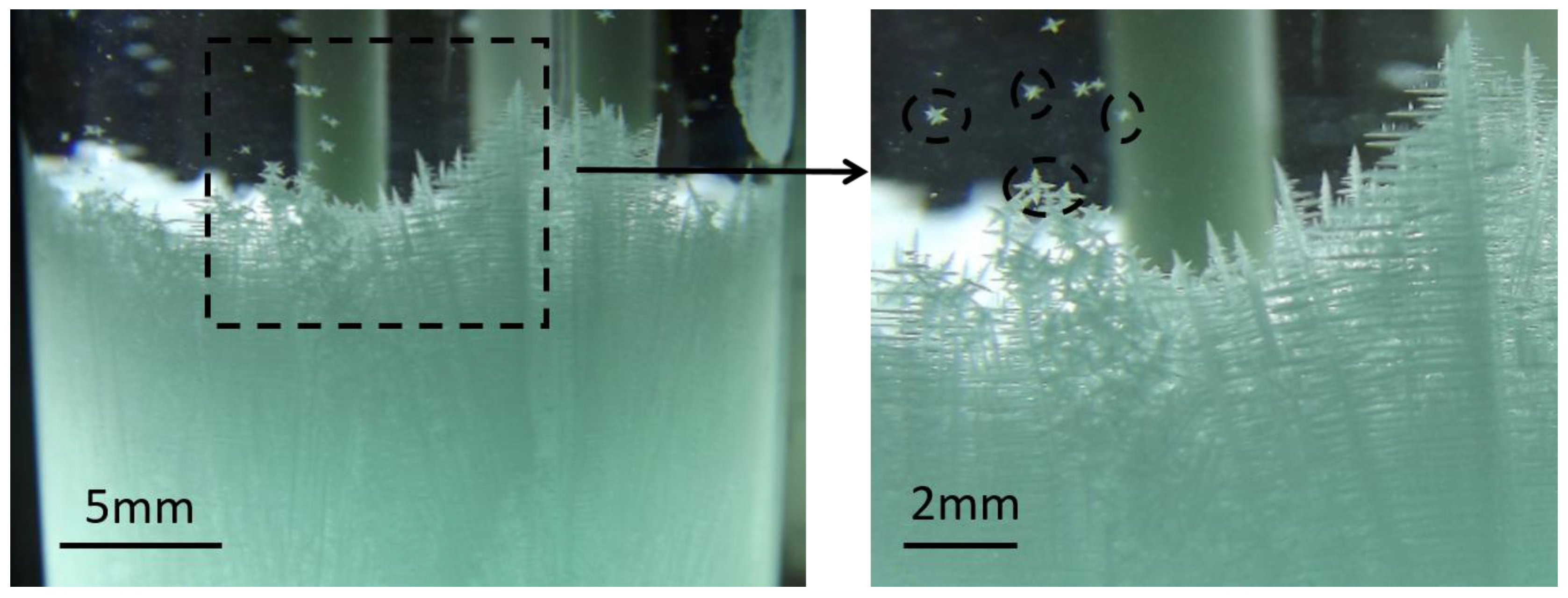
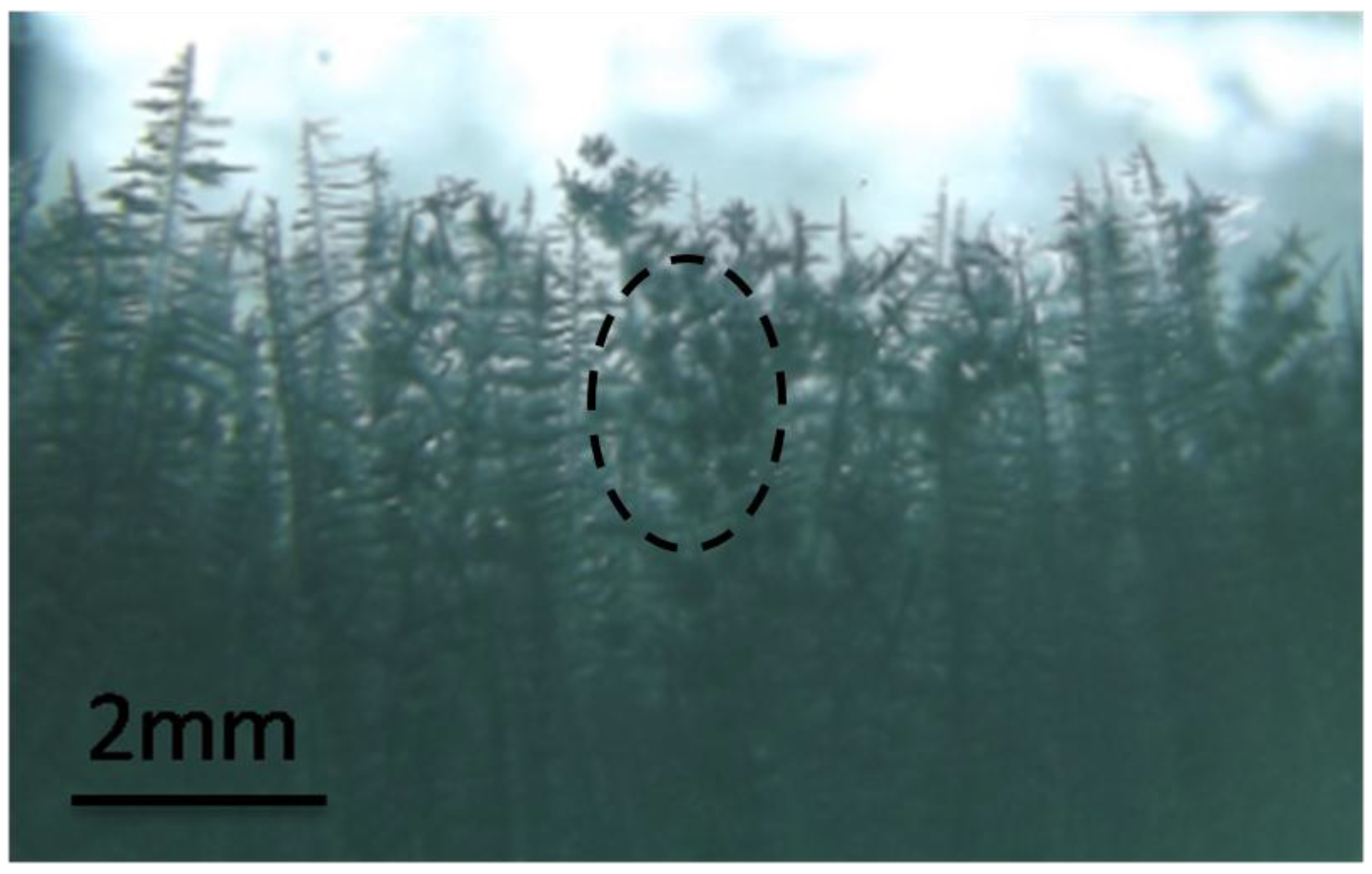
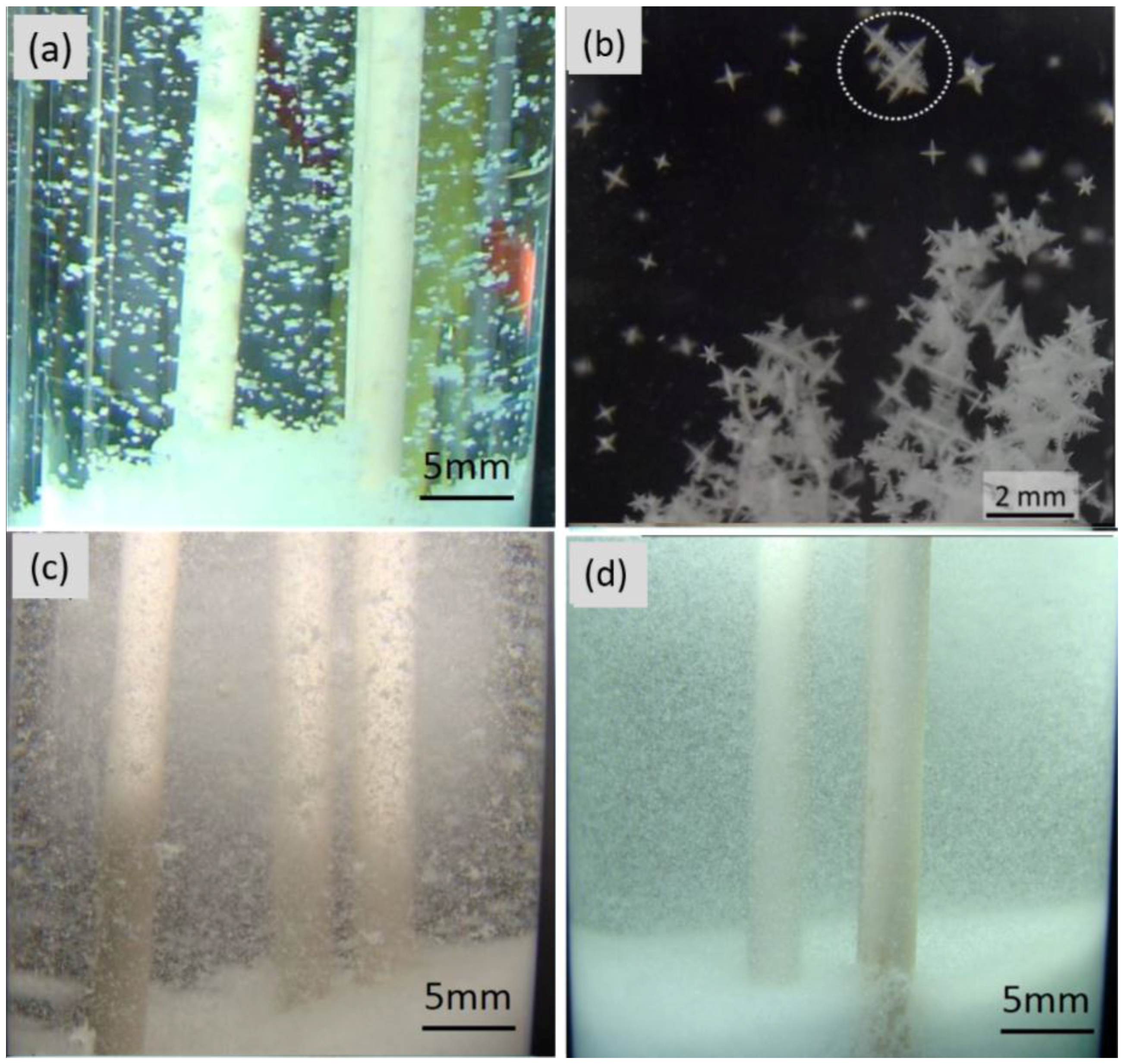
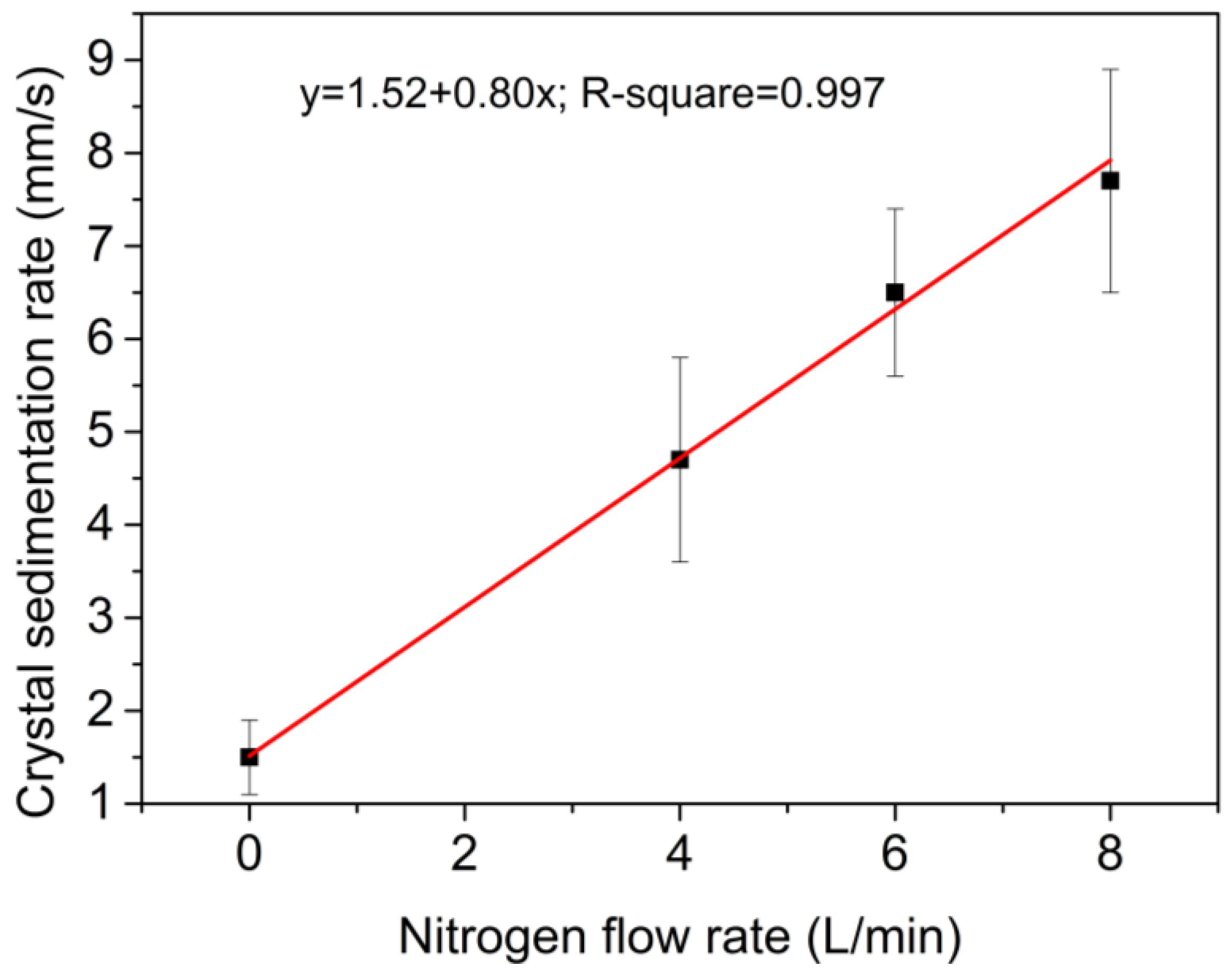
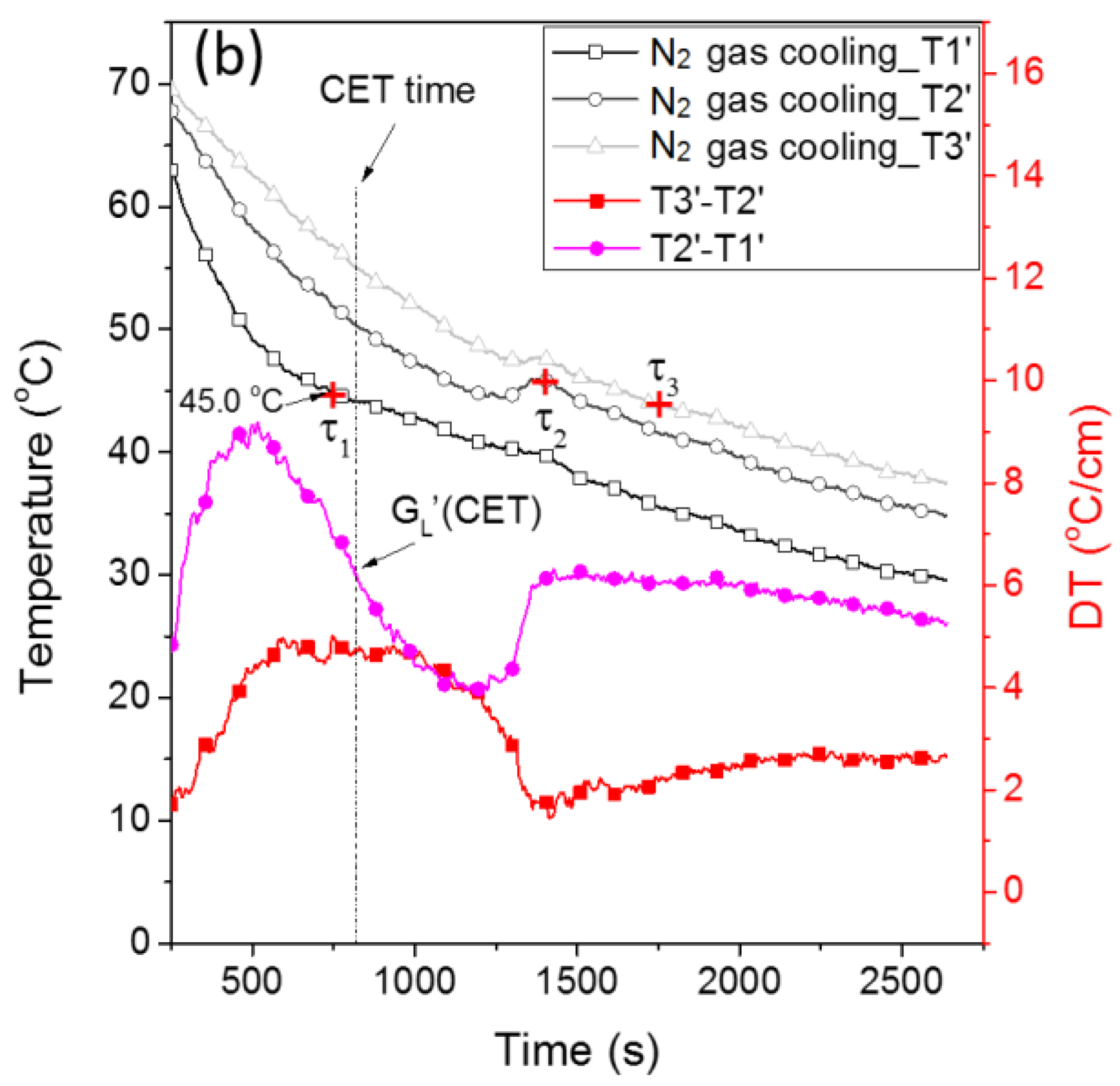
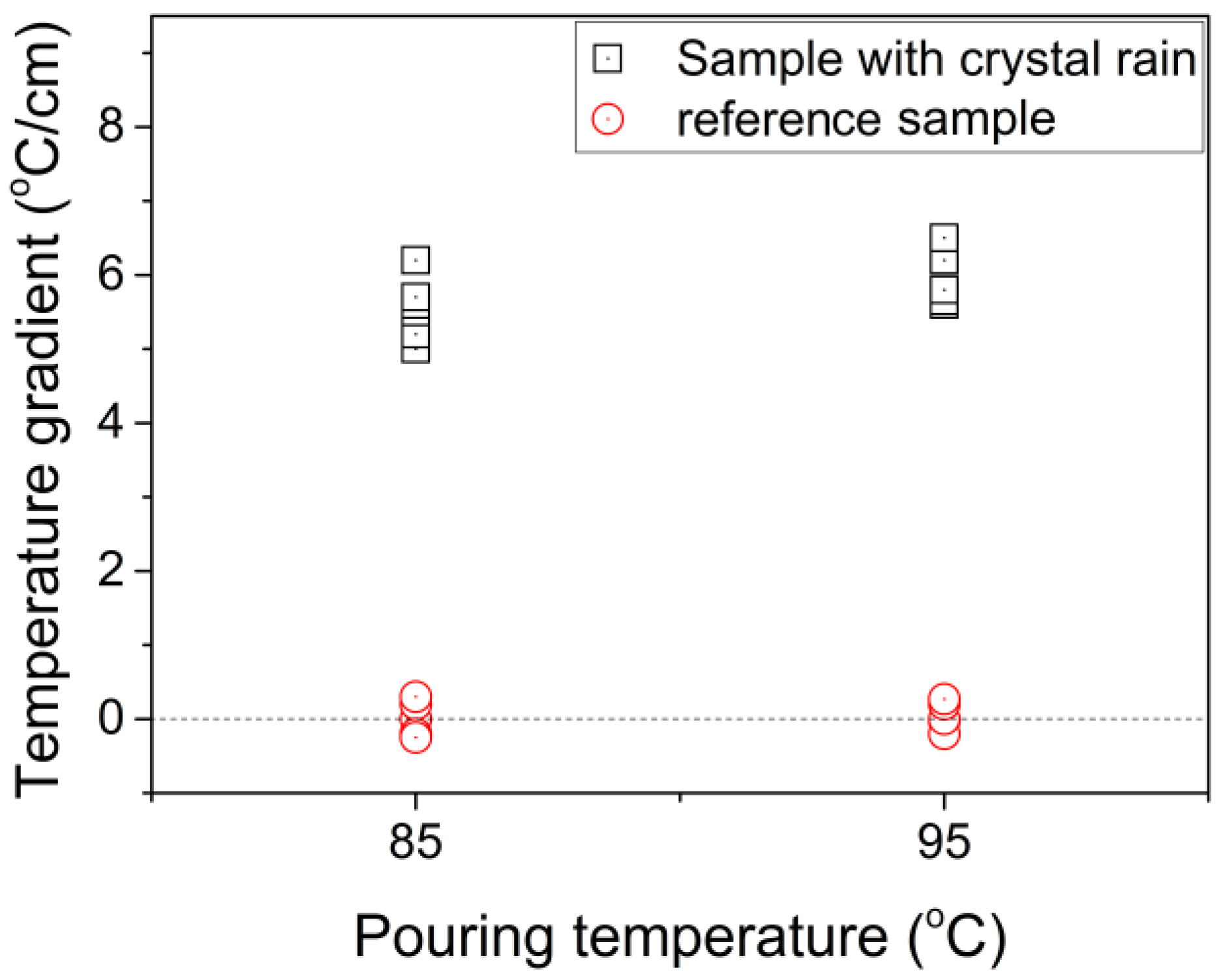
3.2. Crystal Rain and Its Effect on the CET
3.3. The Mechanism of the CET with Crystal Rain
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ohno, A.; Motegi, T.; Soda, H. Origin of the equiaxed crystals in castings. Tran. Iron Steel Inst. Jpn. 1971, 11, 18–23. [Google Scholar]
- Southin, R.T. Nucleation of the equiaxed zone in cast metals. Trans. Metall. Soc. AIME 1967, 239, 220–225. [Google Scholar]
- Winegard, W.C.; Chalmers, B. Supercooling and dendritic freezing in alloys. Trans. Am. Soc. Met. 1954, 46, 1214–1224. [Google Scholar]
- Sigworth, G.K. The grain refining of aluminum and phase relationships in the Al–Ti–B system. Metall. Trans. A 1984, 15, 277–282. [Google Scholar] [CrossRef]
- Tzavaras, A.A.; Brody, H.D. Electromagnetic stirring and continuous casting—Achievements, problems, and goals. JOM 1984, 36, 31–37. [Google Scholar] [CrossRef]
- Ma, Q.; Ramirez, A.; Das, A. Ultrasonic refinement of magnesium by cavitation: Clarifying the role of wall crystals. J. Cryst. Growth 2009, 311, 3708–3715. [Google Scholar]
- Liao, X.; Zhai, Q.; Luo, J.; Chen, W.; Gong, Y. Refining mechanism of the electric current pulse on the solidification structure of pure aluminum. Acta Mater. 2007, 55, 3103–3109. [Google Scholar] [CrossRef]
- Nakada, M.; Shiohara, Y.; Flemings, M.C. Modification of solidification structures by pulse electric discharging. Tran. Iron Steel Inst. Jpn. 1990, 30, 27–33. [Google Scholar] [CrossRef]
- Kolesnichenko, A.F.; Podoltsev, A.D.; Kucheryavaya, I.N. Action of pulse magnetic field on molten metal. Tran. Iron Steel Inst. Jpn. 1994, 34, 715–721. [Google Scholar] [CrossRef]
- Campbell, J. Grain refinement of solidifying metals by vibration: A review. In Proceedings of the Solidification Technology in the Foundry and Cast House, Proceedings of an International Conference of the Applied Metallurgy & Metals Technology Group, Coventry, UK, 15–17 September 1983; Metals Society: London, UK, 1983; pp. 61–64. [Google Scholar]
- Easton, M.A.; Kaufmann, H.; Fragner, W. The effect of chemical grain refinement and low superheat pouring on the structure of NRC castings of aluminium alloy Al–7Si–0.4Mg. Mater. Sci. Eng. A 2006, 420, 135–143. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Zuo, Y.; Zhao, Z.; Zhu, Q.; Cui, J. Experimental investigation of heat transport and solidification during low frequency electromagnetic hot-top casting of 6063 aluminum alloy. Mater. Sci. Eng. A 2008, 497, 416–420. [Google Scholar] [CrossRef]
- Gong, Y.Y.; Zhai, Q.J.; Li, B.; Li, R.X.; Yin, Z.X. A Method of Refining Solidificaiton Microstructure with Surface Pulsed Magneto-Oscillation. CN Patent 201,010,167,538.3, 5 June 2010. [Google Scholar]
- Zhao, J.; Yu, J.; Li, Q.; Zhong, H.; Song, C.; Zhai, Q. Structure of slowly solidified 30Cr2Ni4MoV casting with surface pulsed magneto-oscillation. Mater. Sci. Technol. 2015, 31, 1589–1594. [Google Scholar] [CrossRef]
- Nguyen-Thi, H.; Salvo, L.; Mathiesen, R.H.; Arnberg, L.; Billia, B.; Suery, M.; Reinhart, G. On the interest of synchrotron X-ray imaging for the study of solidification in metallic alloys. C. R. Phys. 2012, 13, 237–245. [Google Scholar] [CrossRef]
- Mathiesen, R.H.; Arnberg, L.; Nguyen-Thi, H.; Billia, B. In Situ X-ray video microscopy as a tool in solidification science. JOM 2012, 64, 76–82. [Google Scholar] [CrossRef]
- Jackson, K.A.; Hunt, J.D. Transparent compounds that freeze like metals. Acta Metall. 1965, 13, 1212–1215. [Google Scholar] [CrossRef]
- Peppin, S.; Huppert, H.E.; Worster, M.G. Steady-state solidification of aqueous ammonium chloride. J. Fluid Mech. 2008, 599, 465–476. [Google Scholar] [CrossRef]
- Beckermann, C.; Wang, C.Y. Equiaxed dendritic solidification with convection. 3. Comparisons with NH4Cl–H2O experiments. Metall. Mater. Trans. A 1996, 27, 2784–2795. [Google Scholar] [CrossRef]
- Ramani, A.; Beckermann, C. Dendrite tip growth velocities of settling NH4Cl equiaxed crystals. Scr. Mater. 1997, 36, 633–638. [Google Scholar] [CrossRef]
- Badillo, A.; Ceynar, D.; Beckermann, C. Growth of equiaxed dendritic crystals settling in an undercooled melt, Part 1: Tip kinetics. J. Cryst. Growth 2007, 309, 197–215. [Google Scholar] [CrossRef]
- Nayak, A.K.; Barman, N.; Chattopadhyay, H. Solidification of a binary solution (NH4Cl + H2O) on an inclined cooling plate: A parametric study. Procedia Mater. Sci. 2014, 5, 454–463. [Google Scholar] [CrossRef]
- Mohanty, D.; Nayak, A.K.; Barman, N. Studies on transport phenomena during solidification of a binary solution (NH4Cl + H2O) on an inclined cooling plate. Trans. Indian Inst. Met. 2012, 65, 801–807. [Google Scholar] [CrossRef]
- Liu, S.; Lu, S.; Hellawell, A. Dendritic array growth in the systems NH4Cl–H2O and [CH2CN]2–H2O: The detachment of dendrite side arms induced by deceleration. J. Cryst. Growth 2002, 234, 740–750. [Google Scholar] [CrossRef]
- Wang, W.; Wang, K.; Lin, X. Crystal nucleation and detachment from a chilling metal surface with vibration. Mater. Chem. Phys. 2009, 117, 199–203. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, M.; Lin, X.; Huang, W. Effect of substrate surface microstructure on heterogeneous nucleation behavior. J. Mater. Sci. Technol. 2012, 28, 67–72. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, M.; Lin, X.; Huang, W. Effect of substrate wettability and surface structure on nucleation of crystal. J. Mater. Sci. Technol. 2012, 28, 859–864. [Google Scholar] [CrossRef]
- Ares, A.E.; Gassa, L.M.; Gueijman, S.F.; Schvezov, C.E. Correlation between thermal parameters, structures, dendritic spacing and corrosion behavior of Zn–Al alloys with columnar to equiaxed transition. J. Cryst. Growth 2008, 310, 1355–1361. [Google Scholar] [CrossRef]
- Ares, A.E.; Gueijman, S.F.; Schvezov, C.E. An experimental investigation of the columnar-to-equiaxed grain transition in aluminum-copper hypoeutectic and eutectic alloys. J. Cryst. Growth 2010, 312, 2154–2170. [Google Scholar] [CrossRef]
- Liu, D.R.; Mangelinck-Noël, N.; Gandin, C.A.; Zimmermann, G.; Sturz, L.; Nguyen-Thi, H.; Billia, B. Structures in directionally solidified Al–7 wt.% Si alloys: Benchmark experiments under microgravity. Acta Mater. 2014, 64, 253–265. [Google Scholar] [CrossRef]
- Nguyen-Thi, H.; Reinhart, G.; Mangelinck-NoёL, N.; Jung, H.; Billia, B.; Schenk, T.; Gastaldi, J.; Härtwig, J.; Baruchel, J. In-situ and real-time investigation of columnar-to-equiaxed transition in metallic alloy. Metall. Mater. Trans. A 2007, 38, 1458–1464. [Google Scholar] [CrossRef]
- Martorano, M.; Beckermann, C.; Gandin, C. A solutal interaction mechanism for the columnar-to-equiaxed transition in alloy solidification. Metall. Mater. Trans. A 2003, 34, 1657–1674. [Google Scholar] [CrossRef]
- Willers, B.; Eckert, S.; Michel, U.; Haase, I.; Zouhar, G. The columnar-to-equiaxed transition in Pb–Sn alloys affected by electromagnetically driven convection. Mater. Sci. Eng. A 2005, 402, 55–65. [Google Scholar] [CrossRef]
- Banaszek, J.; Browne, D.J. Modelling columnar dendritic growth into an undercooled metallic melt in the presence of convection. Mater. Trans. 2005, 46, 1378–1387. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, H.; Zhang, Y.; Chen, X.; Wu, C.; Wei, Z.; Zhai, Q. In Situ Observation of Crystal Rain and Its Effect on Columnar to Equiaxed Transition. Metals 2016, 6, 271. https://doi.org/10.3390/met6110271
Zhong H, Zhang Y, Chen X, Wu C, Wei Z, Zhai Q. In Situ Observation of Crystal Rain and Its Effect on Columnar to Equiaxed Transition. Metals. 2016; 6(11):271. https://doi.org/10.3390/met6110271
Chicago/Turabian StyleZhong, Honggang, Yunhu Zhang, Xiangru Chen, Congsen Wu, Zhiqiang Wei, and Qijie Zhai. 2016. "In Situ Observation of Crystal Rain and Its Effect on Columnar to Equiaxed Transition" Metals 6, no. 11: 271. https://doi.org/10.3390/met6110271




