Tribocorrosion Study of Ordinary and Laser-Melted Ti6Al4V Alloy
Abstract
:1. Introduction
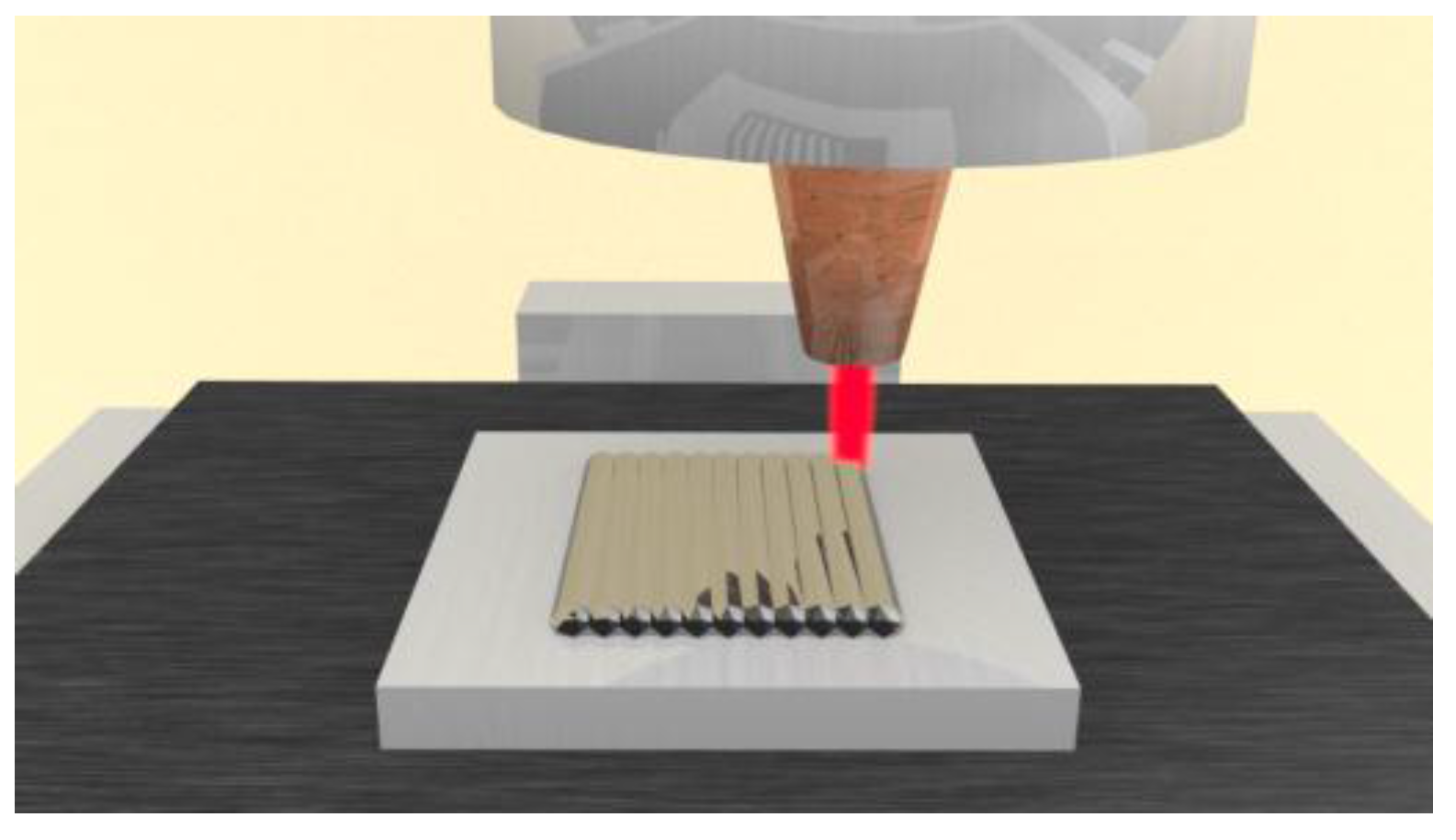
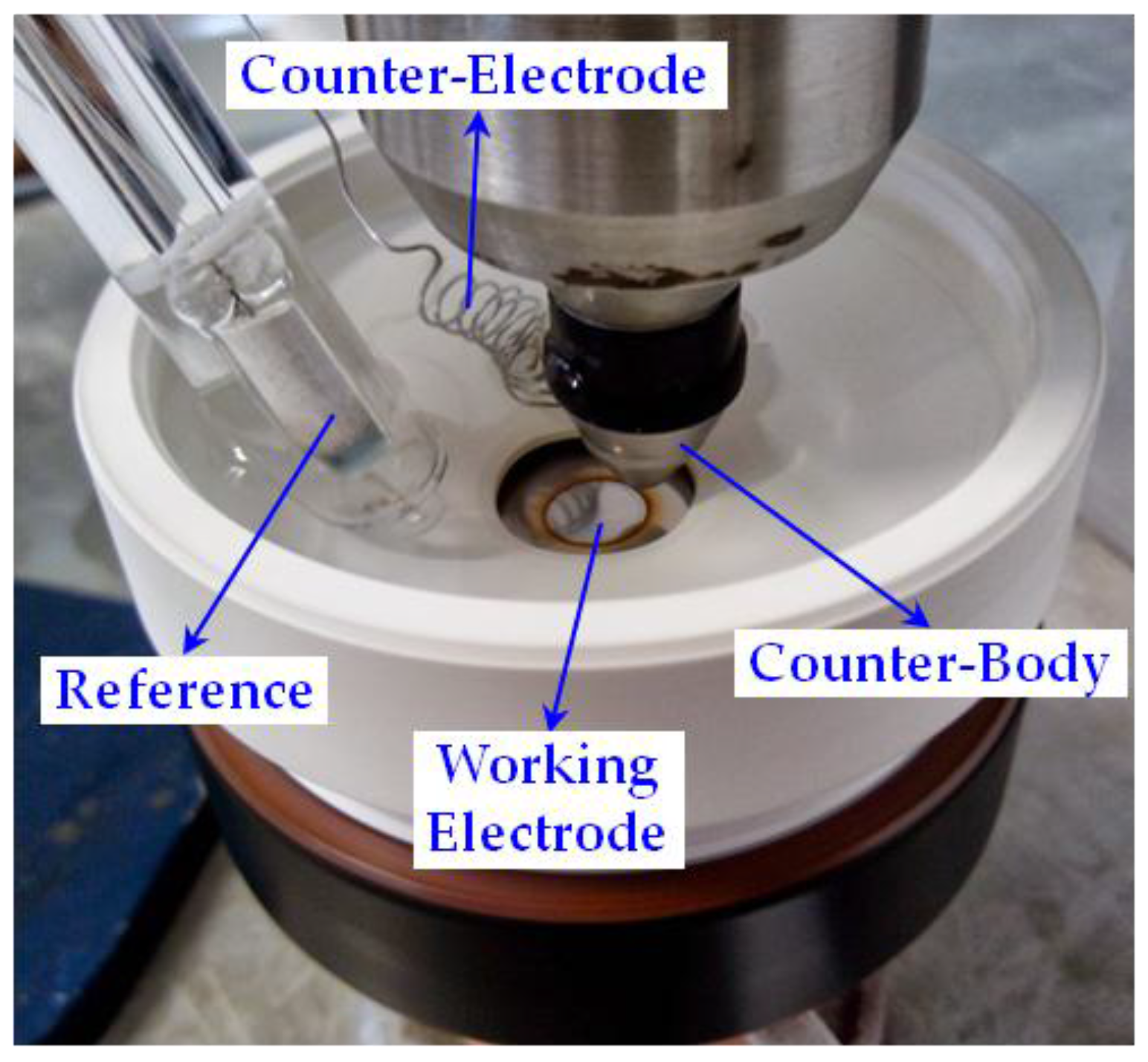
2. Materials and Methods
3. Results and Discussion
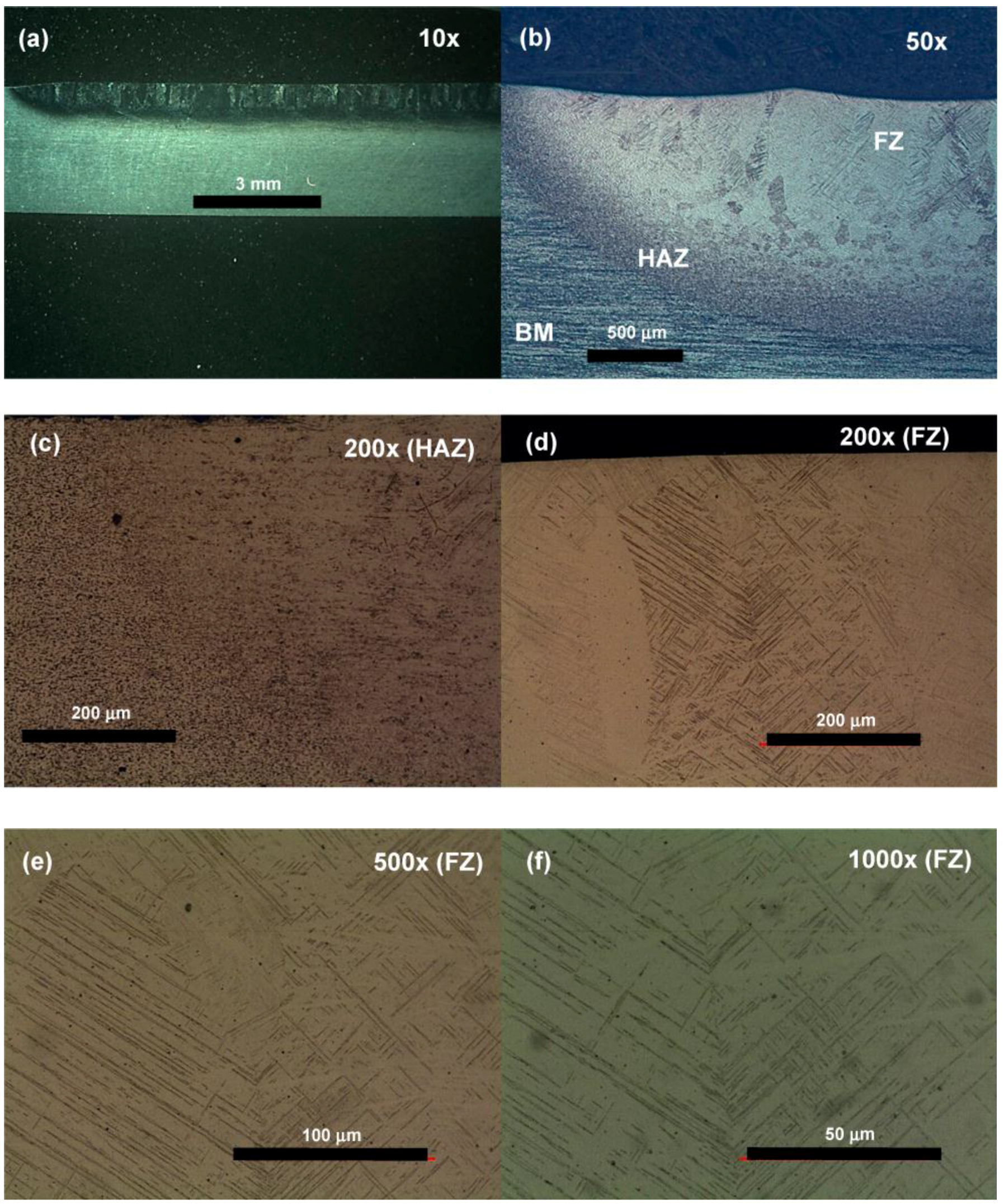
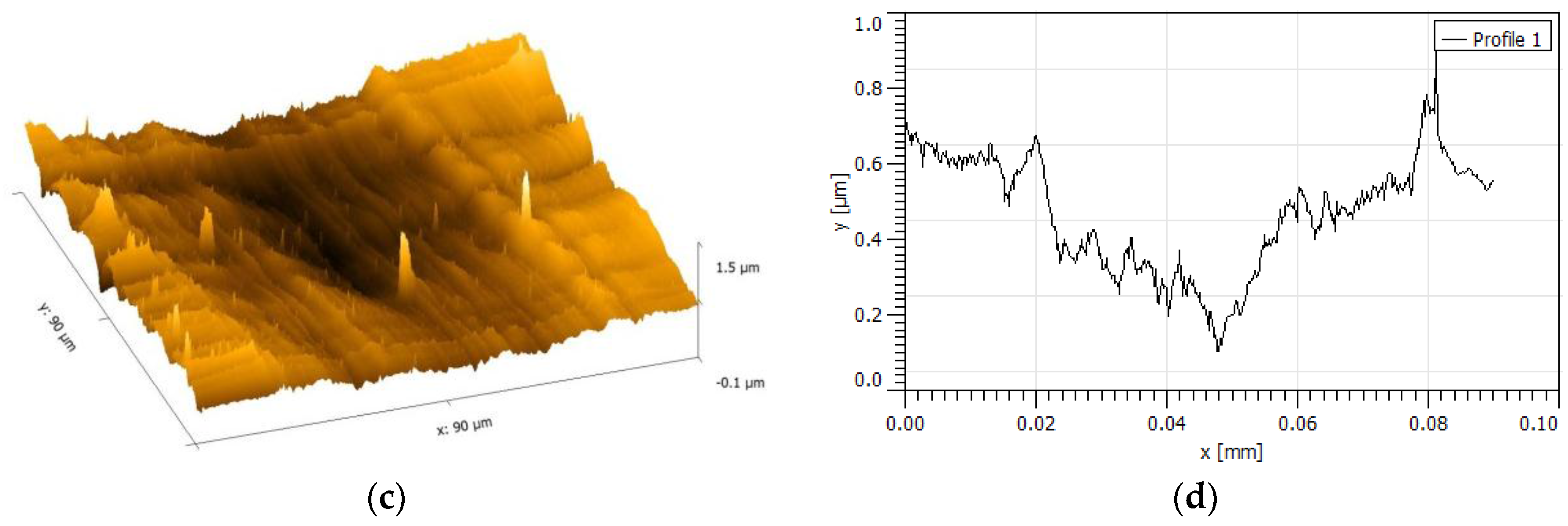
3.1. Microstructure and Microhardness
3.2. Tribocorrosion Behavior at Initial Rubbing
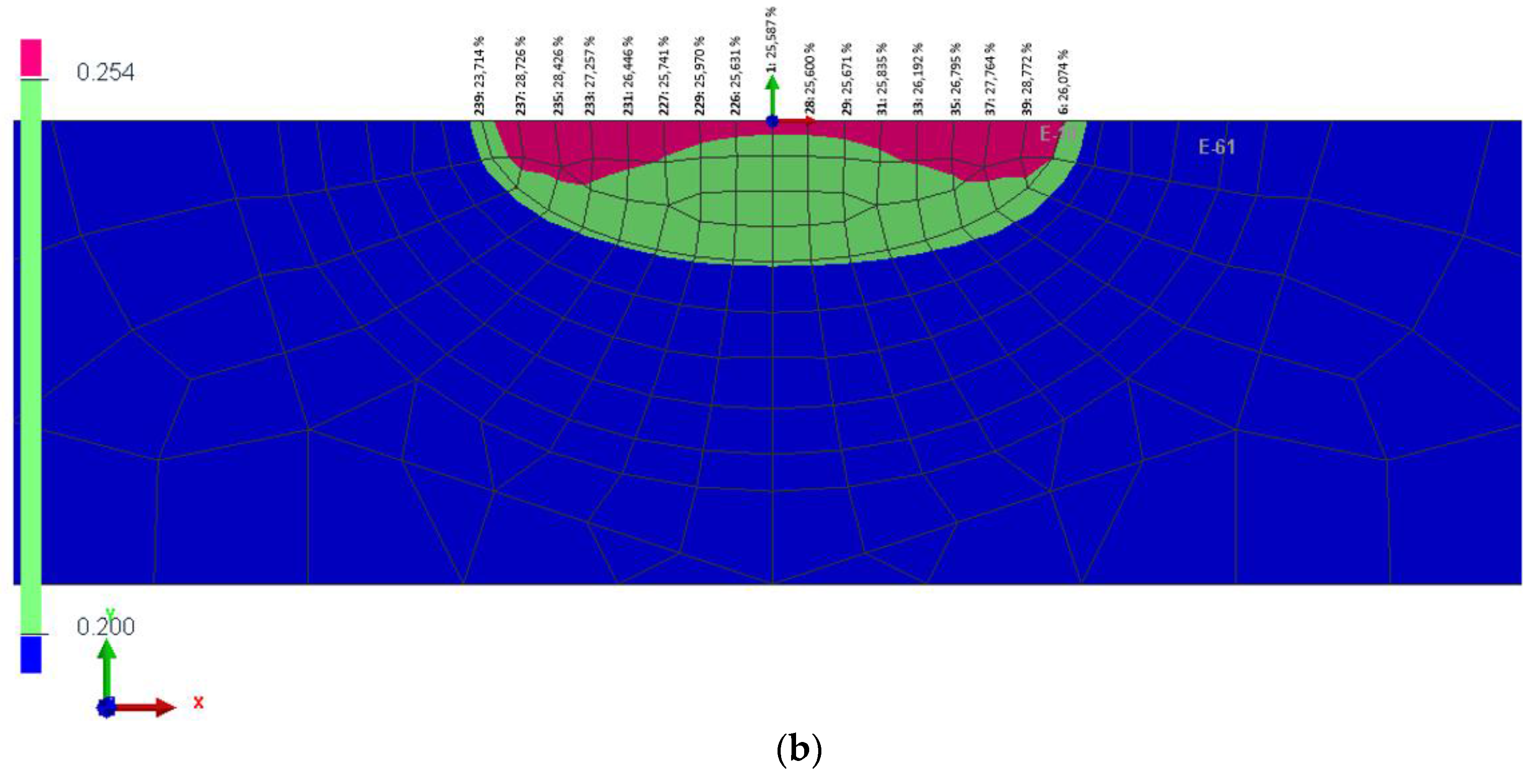
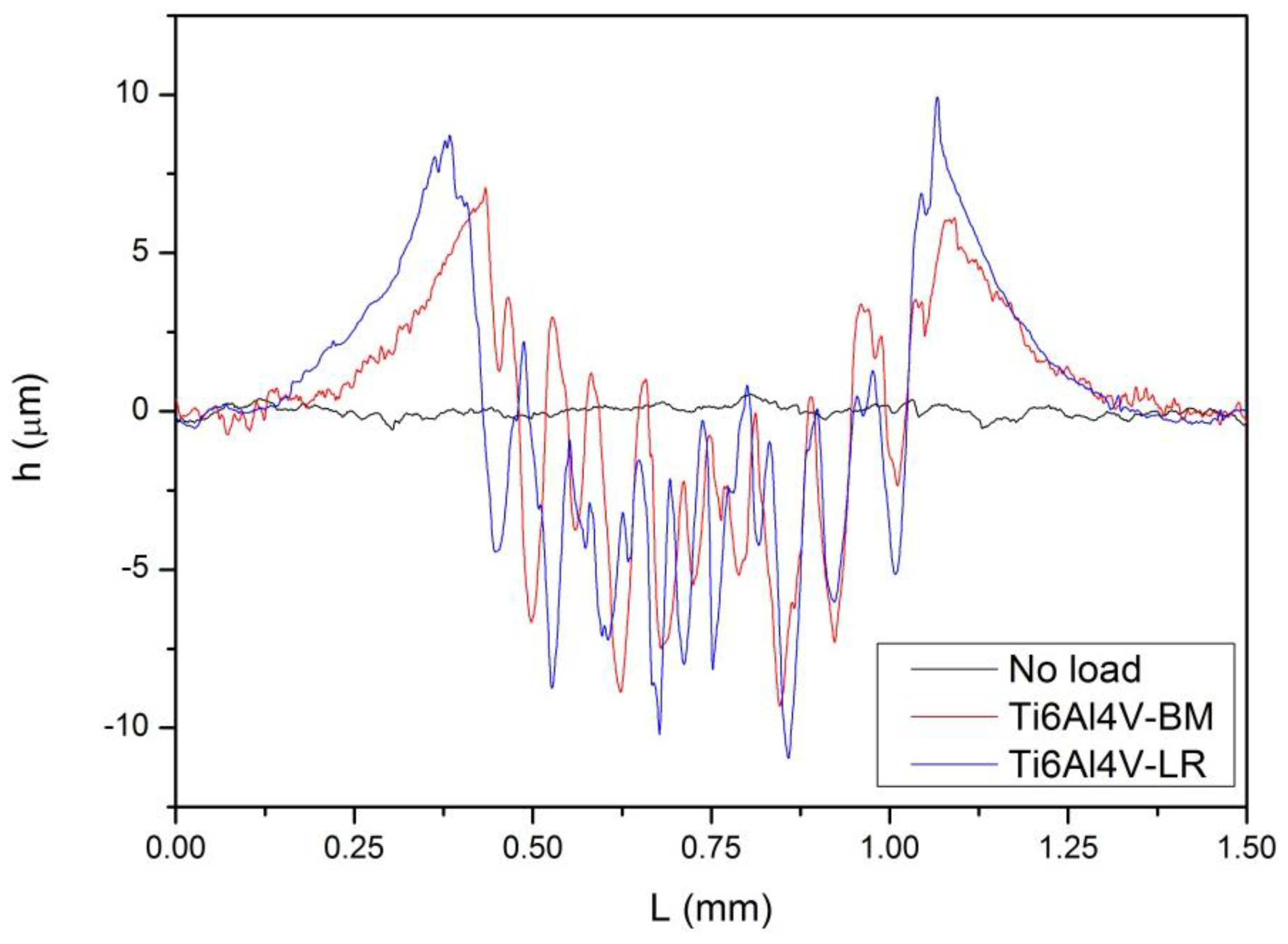
3.3. Tribocorrosion Behavior at Extensive Rubbing
4. Conclusions
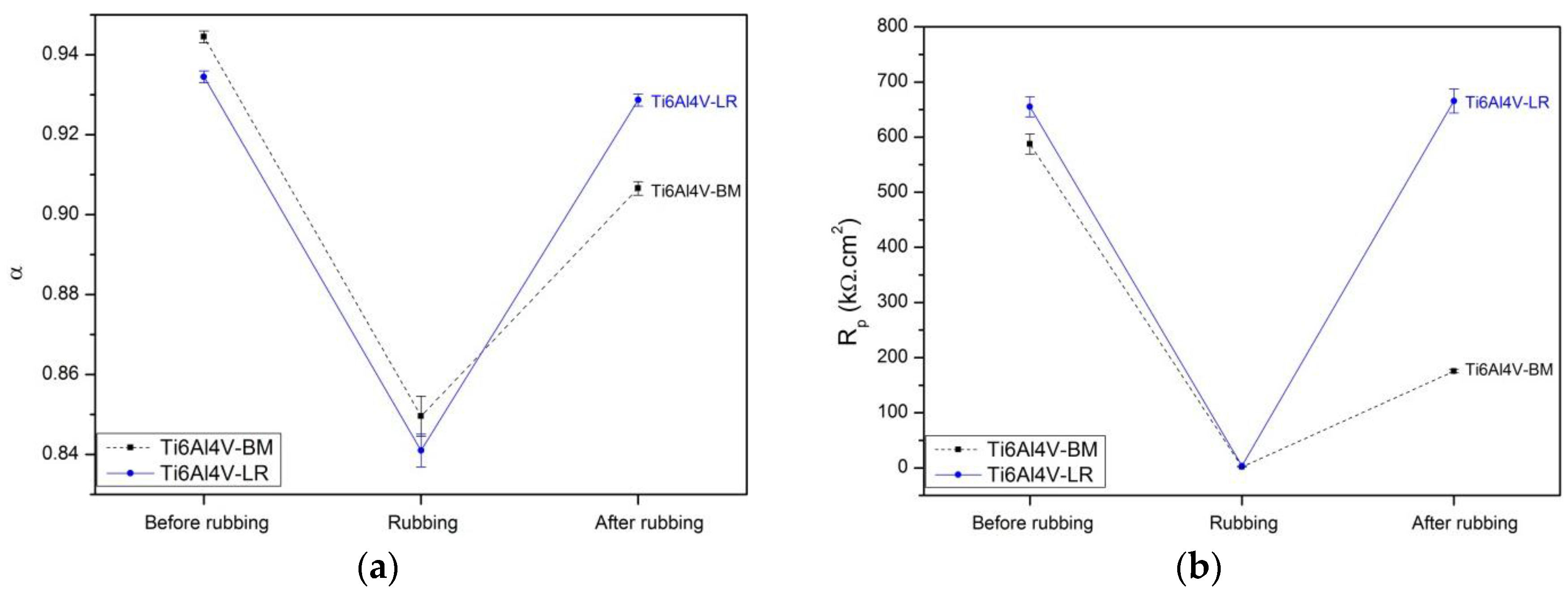
- Initial rubbing reveals that laser-remelted Ti6Al4V samples present better tribocorrosion resistance (shallower track wear) than ordinary non-treated Ti6Al4V-BM samples. This initial wear improvement is associated with the development of a fine α′ martensitic microstructure on the external surface of the laser samples, with an approximate thickness of 10 µm.
- Although Ti6Al4V-BM and Ti6Al4V-LR have different microstructures, they showed similar tribocorrosion behavior in extensive rubbing tests.
- Electrochemical impedance diagrams are similar for Ti6Al4V-BM and Ti6Al4V-LR samples during the rubbing. However, after this phase, and without counter-body contact, a higher EIS modulus is observed for the laser-remelted samples.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sun, Z.; Annergren, I.; Pan, D.; Mai, T.A. Effect of laser surface remelting on the corrosion behavior of commercially pure titanium sheet. Mater. Sci. Eng. A 2003, 345, 293–300. [Google Scholar] [CrossRef]
- Akman, E.; Demir, A.; Canel, T.; Sınmazçelik, T. Laser welding of Ti6Al4V titanium alloys. J. Mater. Process. Technol. 2009, 209, 3705–3713. [Google Scholar] [CrossRef]
- Prasad, S.; Ehrensberger, M.; Gibson, M.P.; Kim, H.; Monaco, E.A., Jr. Biomaterial properties of titanium in dentistry. J. Oral Biosci. 2015, 57, 192–199. [Google Scholar] [CrossRef]
- Bastos, I.N.; Platt, G.M.; Andrade, M.C.; Soares, G.D. Theoretical study of Tris and Bistris effects on simulated body fluids. J. Mol. Liq. 2008, 139, 121–130. [Google Scholar] [CrossRef]
- Resende, C.X.; Dille, J.; Platt, G.M.; Bastos, I.N.; Soares, G.D. Characterization of coating produced on titanium surface by a designed solution containing calcium and phosphate ions. Mater. Chem. Phys. 2008, 108, 429–435. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Runa, M.J.; Mathew, M.T.; Rocha, L.A. Tribocorrosion response of the Ti6Al4V alloys commonly used in femoral stems. Tribol. Int. 2013, 68, 85–93. [Google Scholar] [CrossRef]
- Niinomi, M. Mechanical properties of biomedical titanium alloys. Mater. Sci. Eng. A 1998, 243, 231–236. [Google Scholar] [CrossRef]
- Vieira, A.; Ribeiro, A.; Rocha, L.; Celis, J.P. Influence of pH and corrosion inhibitors on the tribocorrosion of titanium in artificial saliva. Wear 2006, 261, 994–1001. [Google Scholar] [CrossRef]
- Weng, F.; Chen, C.; Yu, H. Research status of laser cladding on titanium and its alloys: A review. Mater. Des. 2014, 58, 412–425. [Google Scholar] [CrossRef]
- Landolt, D. Corrosion and Surface Chemistry of Metals, 1st ed.; EPFL Press: Lausanne, Switzerland, 2007. [Google Scholar]
- Achanta, S.; Liskiewicz, T.; Drees, D.; Celis, J.-P. Friction mechanisms at the micro-scale. Tribol. Int. 2009, 42, 1792–1799. [Google Scholar] [CrossRef]
- Johnson, K.L. Contact mechanics and the wear of metals. Wear 1995, 190, 162–170. [Google Scholar] [CrossRef]
- Yamamoto, T.; Fushimi, K.; Habazaki, H.; Konno, H. Depassivation-repassivation behavior of a pure iron surface investigated by micro-indentation. Electrochim. Acta 2010, 55, 1232–1238. [Google Scholar] [CrossRef]
- Manhabosco, T.M.; Tamborim, S.M.; dos Santos, C.B.; Müller, I.L. Tribological, electrochemical and tribo-electrochemical characterization of bare and nitrided Ti6Al4V in simulated body fluid solution. Corros. Sci. 2011, 53, 1786–1793. [Google Scholar] [CrossRef]
- Vanzillotta, P.S.; Sader, M.S.; Bastos, I.N.; Soares, G.A. Improvement of in vitro titanium bioactivity by three different surface treatments. Dent. Mater. 2006, 22, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Fazel, M.; Salimijazi, H.R.; Golozar, M.A.; GarsivazJazi, M.R. A comparison of corrosion, tribocorrosion and electrochemical impedance properties of pure Ti and Ti6Al4V alloy treated by micro-arc oxidation process. Appl. Surf. Sci. 2015, 324, 751–756. [Google Scholar] [CrossRef]
- Sánchez-Amaya, J.M.; Vazquez, M.R.A.; Botana, F.J. Chapter 8 Laser Welding of Light Metal Alloys: Aluminium and Titanium Alloys. In Handbook of Laser Welding Technologies; Katayama, S., Ed.; Woodhead Publishing Series in Electronic and Optical Materials; Osaka University: Suita, Japan, 2013; No. 41; pp. 215–254. [Google Scholar]
- Janasekaran, S.; Tan, A.W.; Yusof, F.; Shukor, M.H.A. Influence of the overlapping factor and welding speed on T-joint welding of Ti6Al4V and Inconel 600 using low-power fiber laser. Metals 2016, 6, 134. [Google Scholar] [CrossRef]
- Kim, J.-M.; Ha, T.-H.; Park, J.-S.; Kim, H.-G. Effect of laser surface treatment on the corrosion behavior of FeCrAl-coated TZM alloy. Metals 2016, 6, 29. [Google Scholar] [CrossRef]
- Amaya-Vazquez, M.R.; Sánchez-Amaya, J.M.; Boukha, Z.; Botana, F.J. Microstructure, microhardness and corrosion resistance of remelted TiG2 and Ti6Al4V by a high power diode laser. Corros. Sci. 2012, 56, 36–48. [Google Scholar] [CrossRef]
- Amaya, J.M.S.; Vazquez, M.R.A.; Rovira, L.Z.; Galvin, M.B.; Botana, F.J. Influence of surface pre-treatments on laser welding of Ti6Al4V alloy. J. Mater. Eng. Perform. 2014, 23, 1568–1575. [Google Scholar] [CrossRef]
- Silva, R.C.C.; Nogueira, R.P.; Bastos, I.N. Tribocorrosion of UNS S32750 in chloride medium: Effect of the load level. Electrochim. Acta 2011, 56, 8839–8845. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 6507-1:2005; ISO: Geneva, Switzerland, 2005. [Google Scholar]
- Churiaque, C.; Vazquez, M.R.A.; Botana, F.J.; Amaya, J.M.A. FEM simulation and experimental validation of LBW under conduction regime of Ti6Al4V alloy. J. Mater. Eng. Perform. 2016, 25, 3260–3269. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 3290-2:2014; ISO: Geneva, Switzerland, 2014. [Google Scholar]
- Zaveri, N.; Mahapatra, M.; Deceuster, A.; Peng, Y.; Li, L.; Zhou, A. Corrosion resistance of pulsed laser-treated Ti-6Al-4V implant in simulated biofluids. Electrochim. Acta 2008, 53, 5022–5032. [Google Scholar] [CrossRef]
- Barril, S.; Mischler, S.; Landolt, D. Influence of fretting regimes on the tribocorrosion behavior of Ti6Al4V in 0.9 wt. % sodium chloride solution. Wear 2004, 256, 963–972. [Google Scholar] [CrossRef]
- Alexander, C.L.; Tribollet, B.; Orazem, M.E. Contribution of surface distributions to constant-phase-element (CPE) behavior: 1. Influence of roughness. Electrochim. Acta 2015, 173, 416–424. [Google Scholar] [CrossRef]
- Kumar, D.; Akhtar, S.N.; Patel, A.K.; Ramkumar, L.; Balani, K. Tribological performance of laser peened Ti-6Al-4V. Wear 2015, 322, 203–217. [Google Scholar] [CrossRef]









| Al | V | Fe | C | Other | Ti |
|---|---|---|---|---|---|
| 5.67 | 4.50 | 0.18 | 0.10 | <0.1 | Balance |
| FZ (Ti6Al4V-LR) | HAZ (Ti6Al4V-LR) | BM (Ti6Al4V-LR) | Ti6Al4V-BM |
|---|---|---|---|
| 410 ± 8 | 392 ± 11 | 372 ± 10 | 353 ± 5 |
| Sample | Test Condition | Corrosion Potential (mV vs. SCE) | Passivation Current Density (µA/cm2) |
|---|---|---|---|
| Ti6Al4V-BM | No rubbing | −320 ± 30 | 2 ± 1 |
| Rubbing | −420 ± 20 | 90 ± 40 | |
| Ti6Al4V-LR | No rubbing | −630 ± 30 | 2 ± 1 |
| Rubbing | −630 ± 20 | 90 ± 40 |
| Sample | Test Condition | Re (Ω·cm2) | α (×10−3) | Q (µS·sα/cm2) | Rp (kΩ·cm2) |
|---|---|---|---|---|---|
| Ti6Al4V-BM | Before rubbing | 69.7 ± 0.5 | 945 ± 2 | 224 ± 10 | 587.00 ± 18.00 |
| Rubbing | 75.7 ± 0.5 | 850 ± 5 | 721 ± 18 | 1.65 ± 0.02 | |
| After rubbing | 66.1 ± 0.5 | 907 ± 2 | 246 ± 20 | 175.00 ± 3.00 | |
| Ti6Al4V-LR | Before rubbing | 84.7 ± 0.5 | 935 ± 2 | 279 ± 20 | 655.00 ± 18.00 |
| Rubbing | 93.1 ± 0.6 | 841 ± 4 | 1007 ± 17 | 3.61 ± 0.04 | |
| After rubbing | 79.7 ± 0.5 | 929 ± 2 | 270 ± 20 | 666.00 ± 22.00 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, D.P.; Churiaque, C.; Bastos, I.N.; Sánchez-Amaya, J.M. Tribocorrosion Study of Ordinary and Laser-Melted Ti6Al4V Alloy. Metals 2016, 6, 253. https://doi.org/10.3390/met6100253
Silva DP, Churiaque C, Bastos IN, Sánchez-Amaya JM. Tribocorrosion Study of Ordinary and Laser-Melted Ti6Al4V Alloy. Metals. 2016; 6(10):253. https://doi.org/10.3390/met6100253
Chicago/Turabian StyleSilva, Danillo P., Cristina Churiaque, Ivan N. Bastos, and José María Sánchez-Amaya. 2016. "Tribocorrosion Study of Ordinary and Laser-Melted Ti6Al4V Alloy" Metals 6, no. 10: 253. https://doi.org/10.3390/met6100253






