Combustion Synthesis of MoSi2-Al2O3 Composites from Thermite-Based Reagents
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
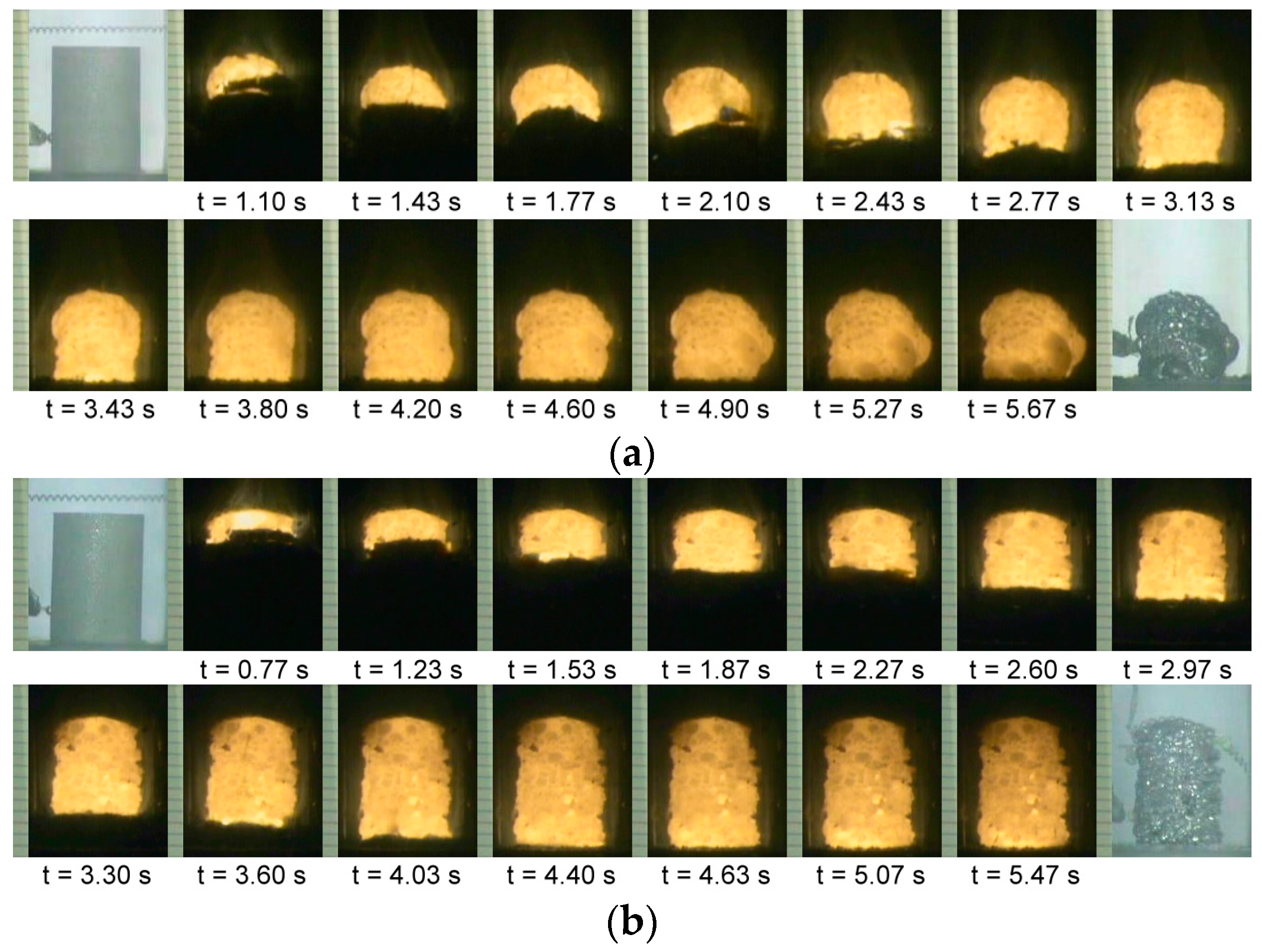
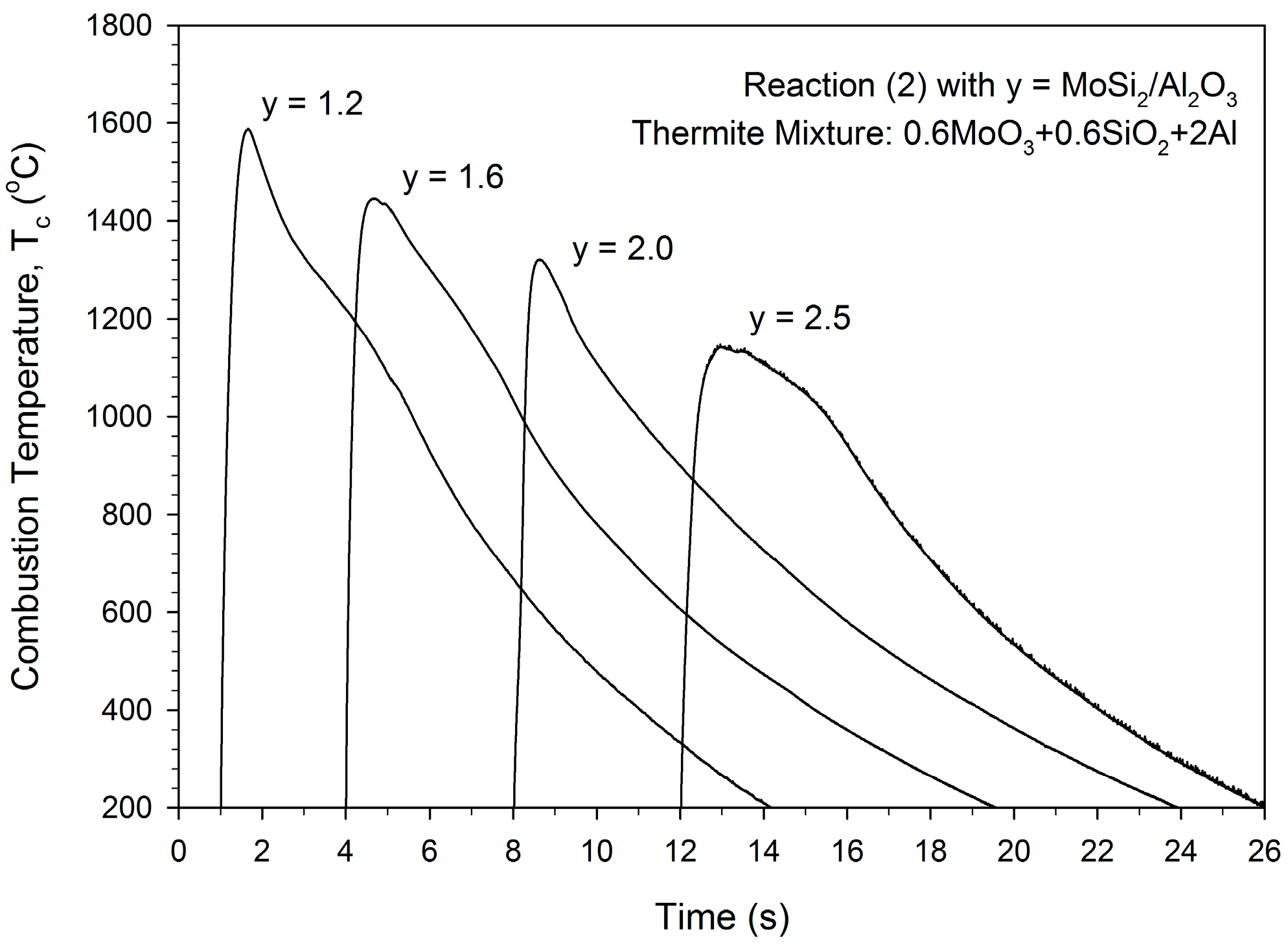
3.1. Combustion Front Velocity and Combustion Temperature
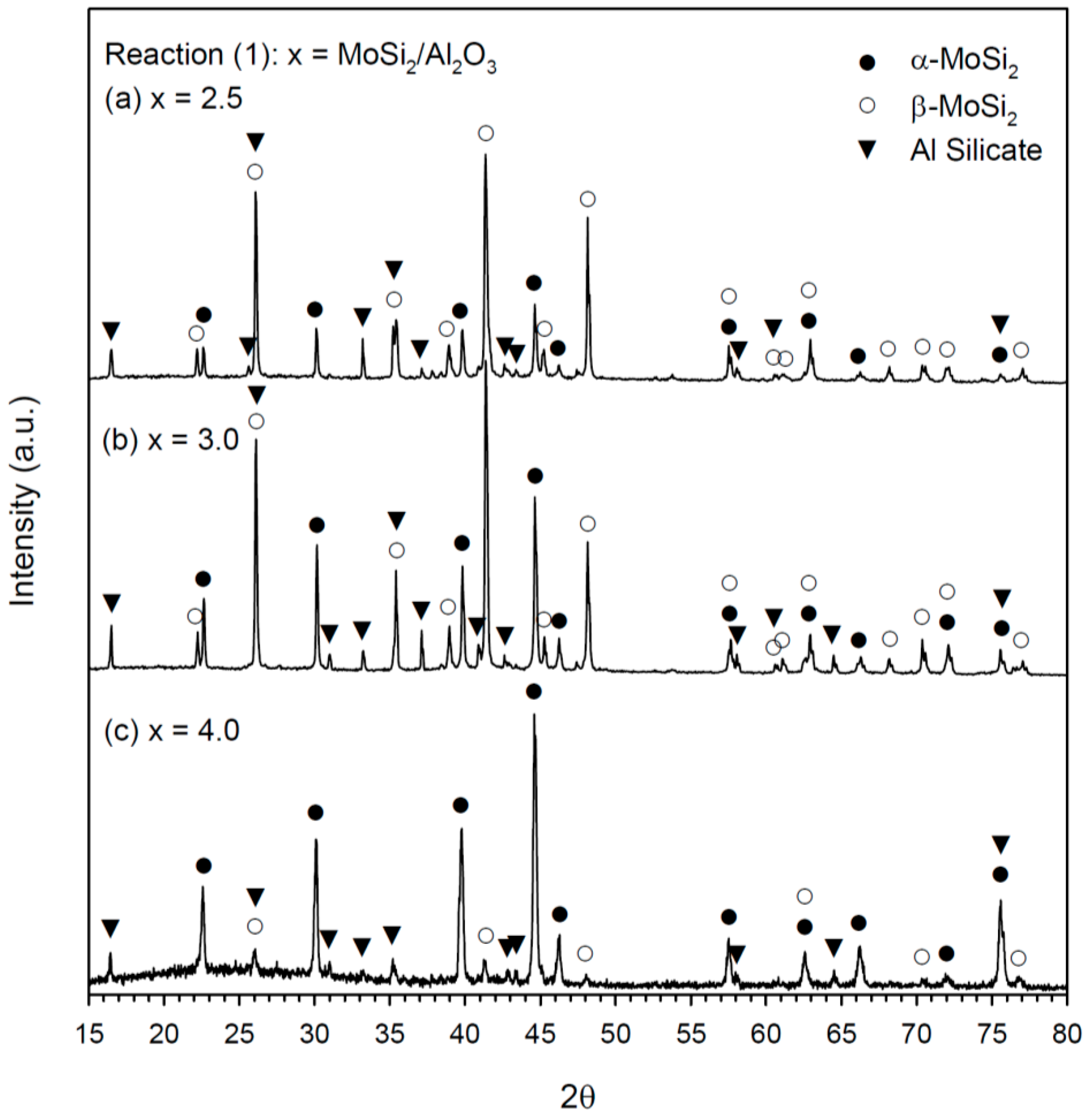
3.2. Phase Constituents of Synthesized Composites
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Meschter, P.J.; Schwartz, D.S. Silicide-matrix materials for high temperature applications. JOM 1989, 41, 52–55. [Google Scholar] [CrossRef]
- Petrovic, J.J.; Vasudevan, A.K. Key developments in high temperature structural silicides. Mater. Sci. Eng. A 1999, 261, 1–5. [Google Scholar] [CrossRef]
- Stoloff, N.S. An overview of powder processing of silicides and their composites. Mater. Sci. Eng. A 1999, 261, 169–180. [Google Scholar] [CrossRef]
- Chen, F.; Xu, J.; Liu, Y.; Cai, L. In situ reactive spark plasma sintering of WSi2/MoSi2 composites. Ceram. Int. 2016, 42, 11165–11169. [Google Scholar] [CrossRef]
- Reddy, V.; Sonber, J.K.; Sairam, K.; Murthy, T.S.R.C.; Kumar, S.; Nageswara Rao, G.V.S.; Srinivasa Rao, T.; Chakravartty, J.K. Densification and mechanical properties of CrB2 + MoSi2 based novel composites. Ceram. Int. 2015, 41, 7611–7617. [Google Scholar] [CrossRef]
- Merzhanov, A.G. Combustion and explosion processes in physical chemistry and technology of inorganic materials. Russ. Chem. Rev. 2003, 72, 289–310. [Google Scholar] [CrossRef]
- Liu, G.; Li, J.; Chen, K. Combustion synthesis of refractory and hard materials: A review. Int. J. Refract. Met. Hard Mater. 2013, 39, 90–102. [Google Scholar] [CrossRef]
- Vicario, I.; Poulon-Quintin, A.; Lagos, M.A.; Silvain, J.F. Effect of material and process atmosphere in the preparation of Al–Ti–B grain refiner by SHS. Metals 2015, 5, 1387–1396. [Google Scholar] [CrossRef] [Green Version]
- Yao, Z.; Stiglich, J.; Sudarshan, T.S. Molybdenum silicide based materials and their properties. J. Mater. Eng. Perform. 1999, 8, 291–304. [Google Scholar] [CrossRef]
- Wang, X.; Feng, P.; Akhtar, F.; Wu, J.; Liu, W.; Qiang, Y.; Wang, Z. Effects of tungsten and aluminum additions on the formation of molybdenum disilicide by mechanically-induced self-propagating reaction. J. Alloy. Compd. 2010, 490, 388–392. [Google Scholar] [CrossRef]
- Samadi, M.; Shamanian, M.; Panjepour, M.; Saidi, A.; Abbasi, M.H.; Asgharzadeh, F. Investigation of MoSi2 formation in the presence of copper by self-propagating high-temperature synthesis. J. Alloy. Compd. 2008, 462, 229–234. [Google Scholar] [CrossRef]
- Esmaeily, S.; Kermani, M.; Razavi, M.; Rahimipour, M.R.; Zakeri, M. An investigation on the in situ synthesis-sintering and mechanical properties of MoSi2-xSiC composites prepared by spark plasma sintering. Int. J. Refract. Met. Hard Mater. 2015, 48, 263–271. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, J.; Zhang, H. Effect of Si3N4 content on microstructures and antioxidant properties of MoSi2/Si3N4 composite coatings on Mo substrate. Ceram. Int. 2015, 41, 13903–13907. [Google Scholar] [CrossRef]
- Wang, R.; Li, W. Effects of microstructures and flaw evolution on the fracture strength of ZrB2–MoSi2 composites under high temperatures. J. Alloy. Compd. 2015, 644, 582–588. [Google Scholar] [CrossRef]
- Wang, J.; Feng, P.; Niu, J.; Guo, R.; Liu, Z.; Akhtar, F. Synthesis, microstructure and properties of MoSi2–5 vol % Al2O3 composites. Ceram. Int. 2014, 40, 16381–16387. [Google Scholar] [CrossRef]
- Huang, Z.; Zhou, W.; Tang, X.; Zhu, J. Effects of milling methods on the dielectric and the mechanical properties of hot-pressed sintered MoSi2/Al2O3 composites. J. Alloy. Compd. 2011, 509, 1920–1923. [Google Scholar] [CrossRef]
- Manukyan, K.V.; Aydinyan, S.V.; Kirakosyan, K.G.; Kharatyan, S.L.; Blugan, G.; Müller, U.; Kuebler, J. Molten salt-assisted combustion synthesis and characterization of MoSi2 and MoSi2–Si3N4 composite powders. Chem. Eng. J. 2008, 143, 331–336. [Google Scholar] [CrossRef]
- Manukyan, K.V.; Kharatyan, S.L.; Blugan, G.; Kocher, P.; Kuebler, J. MoSi2–Si3N4 composites: Influence of starting materials and fabrication route on electrical and mechanical properties. J. Eur. Ceram. Soc. 2009, 29, 2053–2060. [Google Scholar] [CrossRef]
- Yeh, C.L.; Chen, W.H.; Hsu, C.C. Formation of titanium silicides Ti5Si3 and TiSi2 by self-propagating combustion synthesis. J. Alloy. Compd. 2007, 432, 90–95. [Google Scholar] [CrossRef]
- Bertolino, N.; Anselmi-Tamburini, U.; Maglia, F.; Spinolo, G.; Munir, Z.A. Combustion synthesis of Zr–Si intermetallic compounds. J. Alloy. Compd. 1999, 288, 238–248. [Google Scholar] [CrossRef]
- Yeh, C.L.; Chen, W.H. A comparative study on combustion synthesis of Nb–Si compounds. J. Alloy. Compd. 2006, 425, 216–222. [Google Scholar] [CrossRef]
- Yeh, C.L.; Wang, H.J. A comparative study on combustion synthesis of Ta–Si compounds. Intermetallics 2007, 15, 1277–1284. [Google Scholar] [CrossRef]
- Yeh, C.L.; Chen, W.H. Combustion synthesis of MoSi2 and MoSi2–Mo5Si3 composites. J. Alloy. Compd. 2007, 438, 165–170. [Google Scholar] [CrossRef]
- Yeh, C.L.; Chou, C.C.; Hwang, P.W. Experimental and numerical studies on self-propagating high-temperature synthesis of Ta5Si3 intermetallics. Metals 2015, 5, 1580–1590. [Google Scholar] [CrossRef]
- Wang, L.L.; Munir, Z.A.; Maximov, Y.M. Thermite reactions: Their utilization in the synthesis and processing of materials. J. Mater. Sci. 1993, 28, 3693–3708. [Google Scholar] [CrossRef]
- Manukyan, K.; Davtyan, D.; Bossert, J.; Kharatyan, S. Direct reduction of ammonium molybdate to elemental molybdenum by combustion reaction. Chem. Eng. J. 2011, 168, 925–930. [Google Scholar] [CrossRef]
- Binnewies, M.; Milke, E. Thermochemical Data of Elements and Compounds; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2002. [Google Scholar]
- Yeh, C.L.; Lin, J.Z. Combustion synthesis of Cr–Al and Cr–Si intermetallics with Al2O3 additions from Cr2O3–Al and Cr2O3–Al–Si reaction systems. Intermetallics 2013, 33, 126–133. [Google Scholar] [CrossRef]
- Yamada, T.; Yamane, H. Crystal structure and thermoelectric properties of β-MoSi2. Intermetallics 2011, 19, 908–912. [Google Scholar] [CrossRef]



© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeh, C.-L.; Peng, J.-A. Combustion Synthesis of MoSi2-Al2O3 Composites from Thermite-Based Reagents. Metals 2016, 6, 235. https://doi.org/10.3390/met6100235
Yeh C-L, Peng J-A. Combustion Synthesis of MoSi2-Al2O3 Composites from Thermite-Based Reagents. Metals. 2016; 6(10):235. https://doi.org/10.3390/met6100235
Chicago/Turabian StyleYeh, Chun-Liang, and Je-An Peng. 2016. "Combustion Synthesis of MoSi2-Al2O3 Composites from Thermite-Based Reagents" Metals 6, no. 10: 235. https://doi.org/10.3390/met6100235






