Study on the Thermal Conductivity Characteristics of Graphene Prepared by the Planetary Ball Mill
Abstract
:1. Introduction
2. Equipment and Materials
2.1. Materials
2.2. Ultrasonic Generator
2.3. Analysis Methods
3. Experimental Methods
4. Results and Discussion
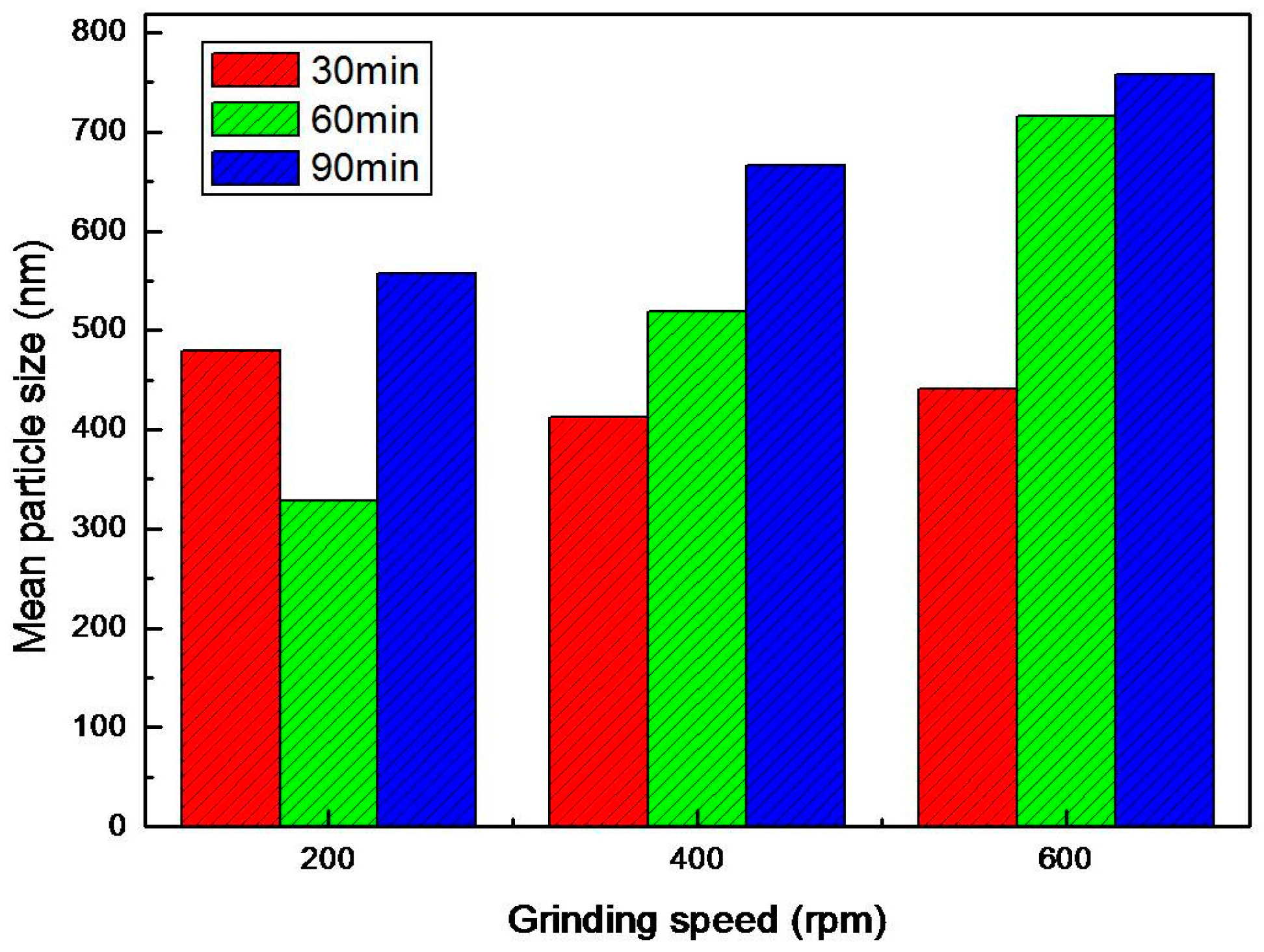
4.1. Results of PSD Measurement
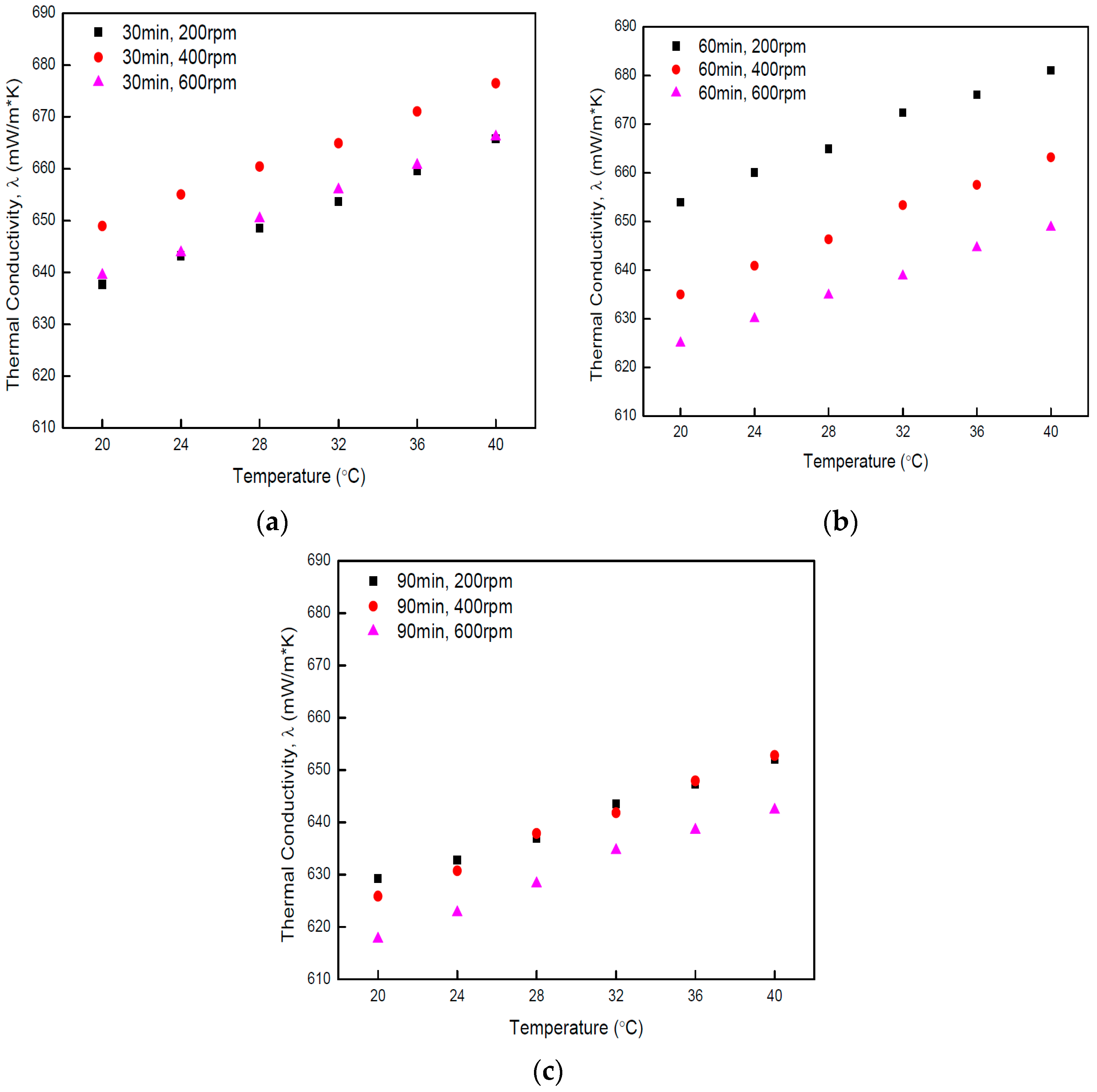
4.2. Measurements of Thermal Conductivity
4.3. Result of Precipitation of Disintegrated GN
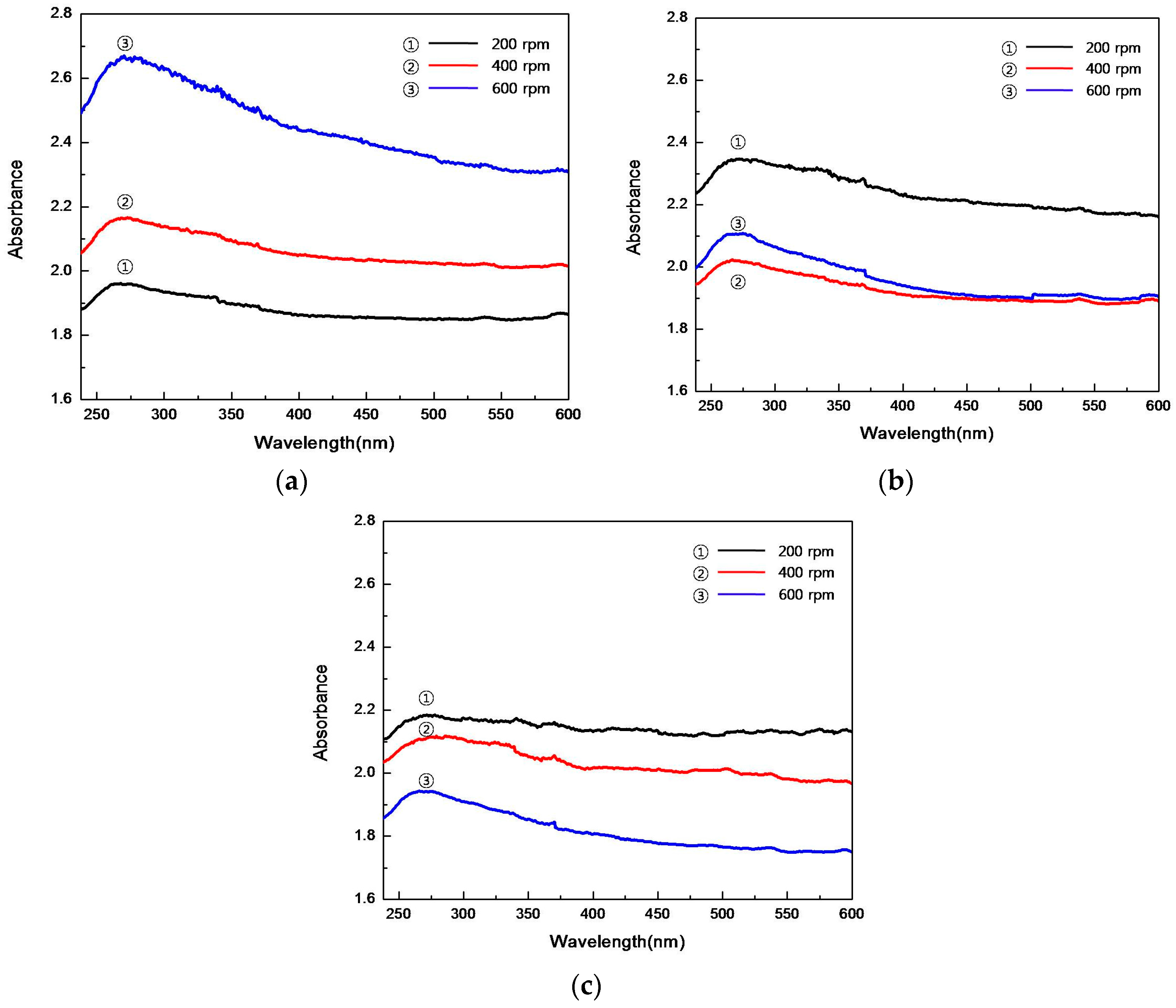
4.4. UV-Vis Spectrum
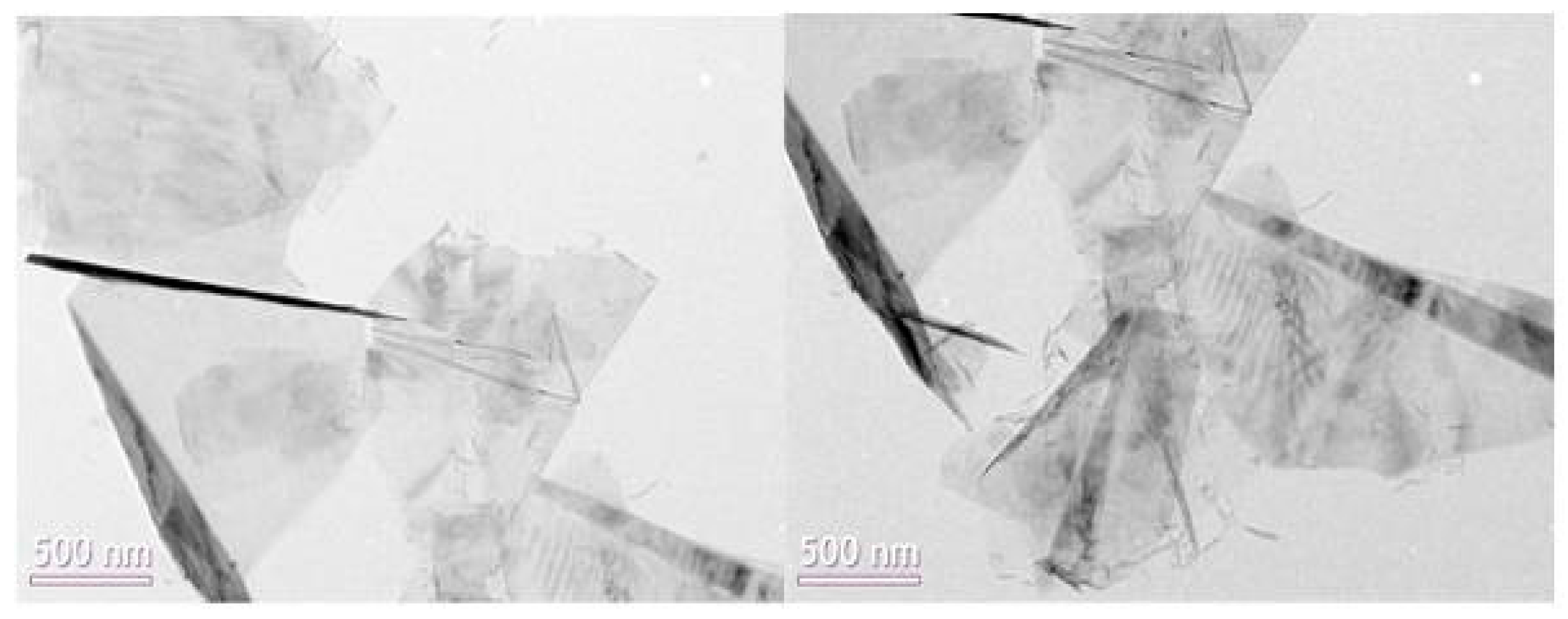
4.5. Result of TEM Measurement
5. Conclusions
- (1)
- Through the process of the disintegration of GN particles, the average particle size of GN decreased and the minimum average particle size of GN was realized from the combination of experimental conditions of 90 min duration of disintegration and 200 rpm of the operation of the planetary ball mill. In contrast, the combined experimental conditions of 90 min duration of disintegration and 600 rpm of the operation of planetary ball mill yielded the biggest average particle size of GN. The cause of these results was attributed to the light weight of the balls (1 mm) attached onto the inner wall of the pot of planetary ball mill by the centrifugal force induced by the high speed rotation that eventually caused the disturbed impact collisions of balls that were supposed to cause the disintegration of GN particles.
- (2)
- The measurements of thermal conductivity of nano-fluids prepared in this study appeared similar than those of PSD measurements from which the inverse proportionality of average size of particles dispersed in nano-fluid to the level of thermal conductivity of corresponding nano-fluid was identified. The disintegrated GN seen from the captured TEM image showed the average particle size to be smaller than that of the original GN with less coagulation. Thereby, the increased thermal conductivity of nano-fluids was concluded to potentially be attributable to the increased specific surface area of GN particles that resulted from the disintegration that decreased the average particle size.
- (3)
- The degree of precipitation of disintegrated GN particles dispersed in each sample of nano-fluids was visualized according to varied duration of precipitation. An additional application of surfactant to the disintegration of GN particles beyond the sole application of the mechanical method could also improve the degree of dispersion of disintegrated GN particles.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Das, S.K.; Choi, S.U.; Yu, W.; Pradeep, T. Nanofluids: Science and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Choi, S. Enhancing thermal conductivity of fluids with nanoparticles. ASME Publ. FED 1995, 231, 99–106. [Google Scholar]
- Kole, M.; Dey, T.K. Thermal performance of screen mesh wick heat pipes using water-based copper nanofluids. Appl. Therm. Eng. 2013, 50, 763–770. [Google Scholar] [CrossRef]
- Swadzba-Kwasny, M.; Chancelier, L.; Ng, S.; Manyar, H.G.; Hardacre, C.; Nockemann, P. Facile in situ synthesis of nanofluids based on ionic liquids and copper oxide clusters and nanoparticles. Dalton Trans. 2012, 41, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, M.H.; Franzke, U. Improvement of thermosyphon performance by employing nanofluid. Int. J. Refrig. 2014, 40, 416–428. [Google Scholar] [CrossRef]
- Khoshvaght-Aliabadi, M. Influence of different design parameters and Al2O3-water nanofluid flow on heat transfer and flow on heat transfer and flow characteristics of sinusoidal corrugated channels. Energy Convers. Manag. 2014, 88, 96–105. [Google Scholar] [CrossRef]
- Wusiman, K.; Jeong, H.; Tulugan, K.; Afrianto, H.; Chung, H. Thermal performance of multi-walled carbon nanotubes (MWCNTs) in aqueous suspensions with surfactants SDBS and SDS. Int. Commun. Heat Mass Transf. 2013, 41, 28–33. [Google Scholar] [CrossRef]
- Amri, A.; Sadri, R.; Ahmadi, G.; Chew, B.; Kazi, S.; Shanbedi, M. Synthesis of polyethylene glycol-functionalized multi-walled carbon nanotubes with a microwave-assisted approach for improved heat dissipation. RSC Adv. 2015, 5, 35425–35434. [Google Scholar] [CrossRef]
- Ghiadi, B.; Baniadam, M.; Maghrebi, M.; Amiri, A. Rapid, one-pot synthesis of highly-soluble carbon nanotubes functionalized by l-arginine. Russ. J. Phys. Chem. A 2013, 87, 649–653. [Google Scholar] [CrossRef]
- Amiri, A.; Sadri, R.; Shanbedi, M.; Ahmadi, G.; Chew, B.T.; Kazi, S.N.; Dahari, M. Performance dependence of thermosyphon on the functionalization approaches: An experimental study on thermo-physical properties of graphene nanoplatelet-based water nanofluids. Energy Convers. Manag. 2015, 92, 322–330. [Google Scholar] [CrossRef]
- Mehrali, M.; Sadeghinezhad, E.; Latibari, S.T.; Kazi, S.N.; Mehrali, M.; Zubir, M.N.B.M.; Metselaar, H.S.C. Investigation of thermal conductivity and rheological properties of nanofluids containing graphene nanoplatelets. Nanoscale Res. Lett. 2014, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hajjar, Z.; Rashidi, A.M.; Ghozatloo, A. Enhanced thermal conductivities of graphene oxide nanofluids. Int. Commun. Heat Mass Transf. 2014, 57, 128–131. [Google Scholar] [CrossRef]
- Wang, F.; Han, L.; Zhang, Z.; Fang, X.; Shi, J.; Ma, W. Surfactant-free ionic liquid-based nanofluids with remarkable thermal conductivity enhancement at very low loading of graphene. Nanoscale Res. Lett. 2012, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Baby, T.T.; Ramaprabhu, S. Enhanced convective heat transfer using graphene dispersed nanofluids. Nanoscale Res. Lett. 2011, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yarmand, H.; Gharehkhani, S.; Ahmadi, G.; Shirazi, S.F.S.; Baradaran, S.; Montazer, E.; Zubir, M.N.M.; Alehashem, M.S.; Kazi, S.N.; Dahari, M. Graphene nanoplatelets-silver hybrid nanofluids for enhanced heat transfer. Energy Convers. Manag. 2015, 100, 419–428. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Kotake, N.; Kuboki, M.; Kiya, S.; Kanda, Y. Influence of dry and wet grinding conditions on fineness and shape of particle size distribution of product in a ball mill. Adv. Powder Technol. 2011, 22, 86–92. [Google Scholar] [CrossRef]
- Feng, D.; Aldrich, C. A comparison of the flotation of ore from the Merensky Reef after wet and dry grinding. Int. J. Miner. Process. 2000, 60, 115–129. [Google Scholar] [CrossRef]
- Munkhbayar, B.; Bat-Erdene, M.; Sarangerel, D.; Ochirkhuyag, B. Effect of the collision medium size on thermal performance of silver nanoparticles based aqueous nanofluids. Compos. Part B Eng. 2013, 54, 383–390. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 89th ed.; CRS Press: Boca Raton, FL, USA, 2008; pp. 2–6. [Google Scholar]
- Jang, S.P.; Choi, S.U. Role of Brownian motion in the enhanced thermal conductivity of nanofluids. Appl. Phys. Lett. 2004, 84, 4316–4318. [Google Scholar] [CrossRef]
- Mahbubul, I.M.; Fadhilah, S.A.; Saidur, R.; Leong, K.Y.; Amalina, M.A. Thermophysical properties and heat transfer performance of Al2O3/R-134a nanorefrigerants. Int. J. Heat Mass Transf. 2013, 57, 100–108. [Google Scholar] [CrossRef]
- Rahman, I.A.; Vejayakumaran, P.; Sipaut, C.S.; Ismail, J.; Chee, C.K. Size-dependent physicochemical and optical properties of silica nanoparticles. Mater. Chem. Phys. 2009, 114, 328–332. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Mo, S.; Jia, L.; Shao, X. Effect of surface modification on the stability and thermal conductivity of water-based SiO2-coated graphene nanofluid. Thermochim. Acta 2014, 595, 6–10. [Google Scholar] [CrossRef]






| No. | Parameter | Value |
|---|---|---|
| 1 | Ball size | 1 (mm) |
| 2 | Ball charge volume | 40 (%) |
| 3 | Rotation speeds | 200, 400, 600 (rpm) |
| 4 | Time | 30, 60, 90 (min) |
| 5 | Condition | Wet |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.-N.; Kim, J.-H.; Kim, B.-S.; Jeong, H.-M.; Huh, S.-C. Study on the Thermal Conductivity Characteristics of Graphene Prepared by the Planetary Ball Mill. Metals 2016, 6, 234. https://doi.org/10.3390/met6100234
Kim G-N, Kim J-H, Kim B-S, Jeong H-M, Huh S-C. Study on the Thermal Conductivity Characteristics of Graphene Prepared by the Planetary Ball Mill. Metals. 2016; 6(10):234. https://doi.org/10.3390/met6100234
Chicago/Turabian StyleKim, Gwi-Nam, Ji-Hye Kim, Bo-Sung Kim, Hyo-Min Jeong, and Sun-Chul Huh. 2016. "Study on the Thermal Conductivity Characteristics of Graphene Prepared by the Planetary Ball Mill" Metals 6, no. 10: 234. https://doi.org/10.3390/met6100234







