Study on Austenitization Kinetics of SA508 Gr.3 Steel Based on Isoconversional Method
Abstract
:1. Introduction
2. Theoretical Background and Experimental Procedures
2.1. General Equation for Kinetics
2.2. JMAK Equation
2.3. Isoconversional Method—Model-Free Method
2.4. Material and Dilatometric Testing
| Element | C | Mn | Si | Ni | Cr | Mo | V | Al | N | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| Composition (wt. %) | 0.20 | 1.47 | 0.17 | 0.89 | 0.13 | 0.51 | 0.001 | 0.039 | 0.014 | Bal. |
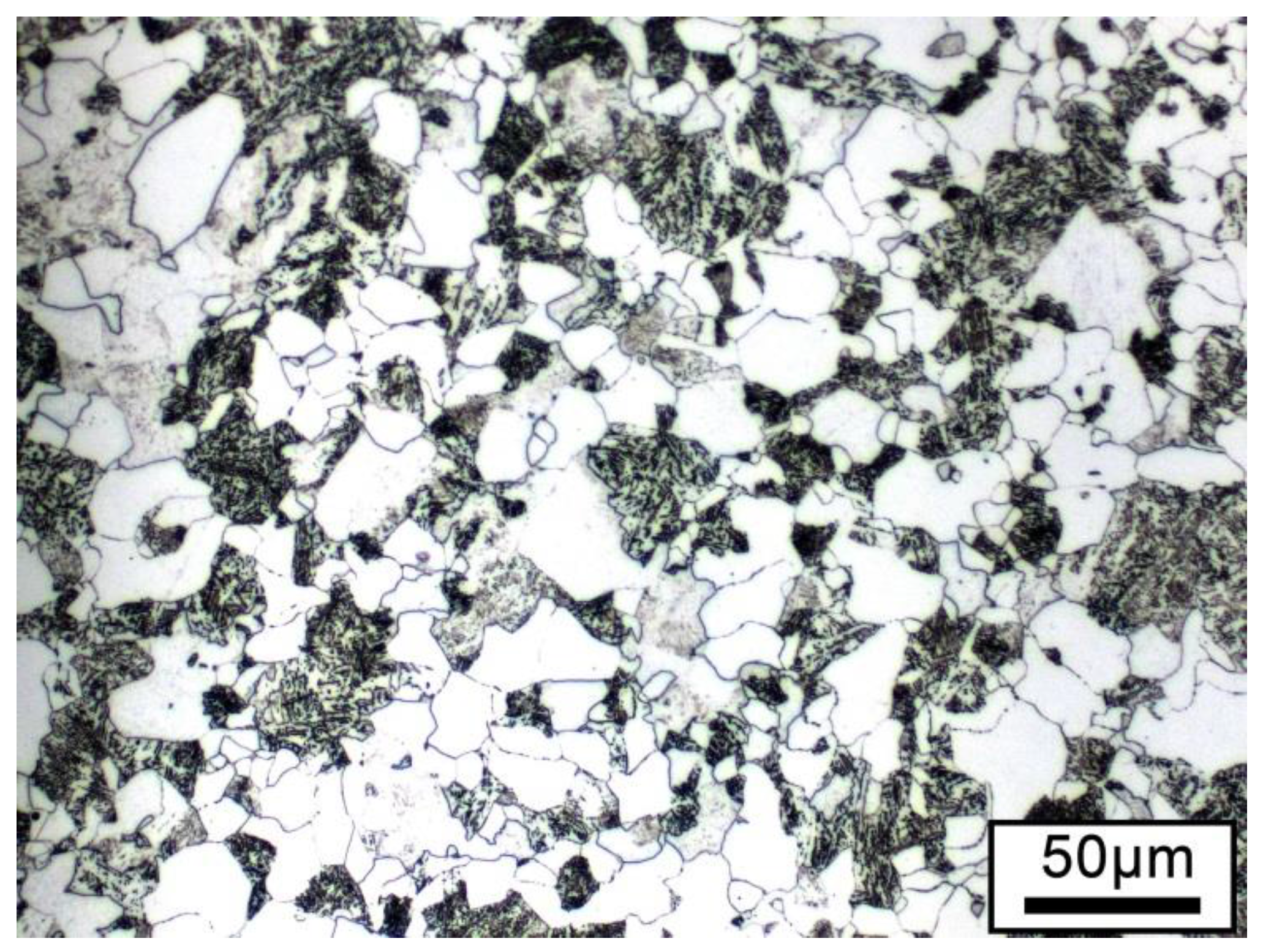
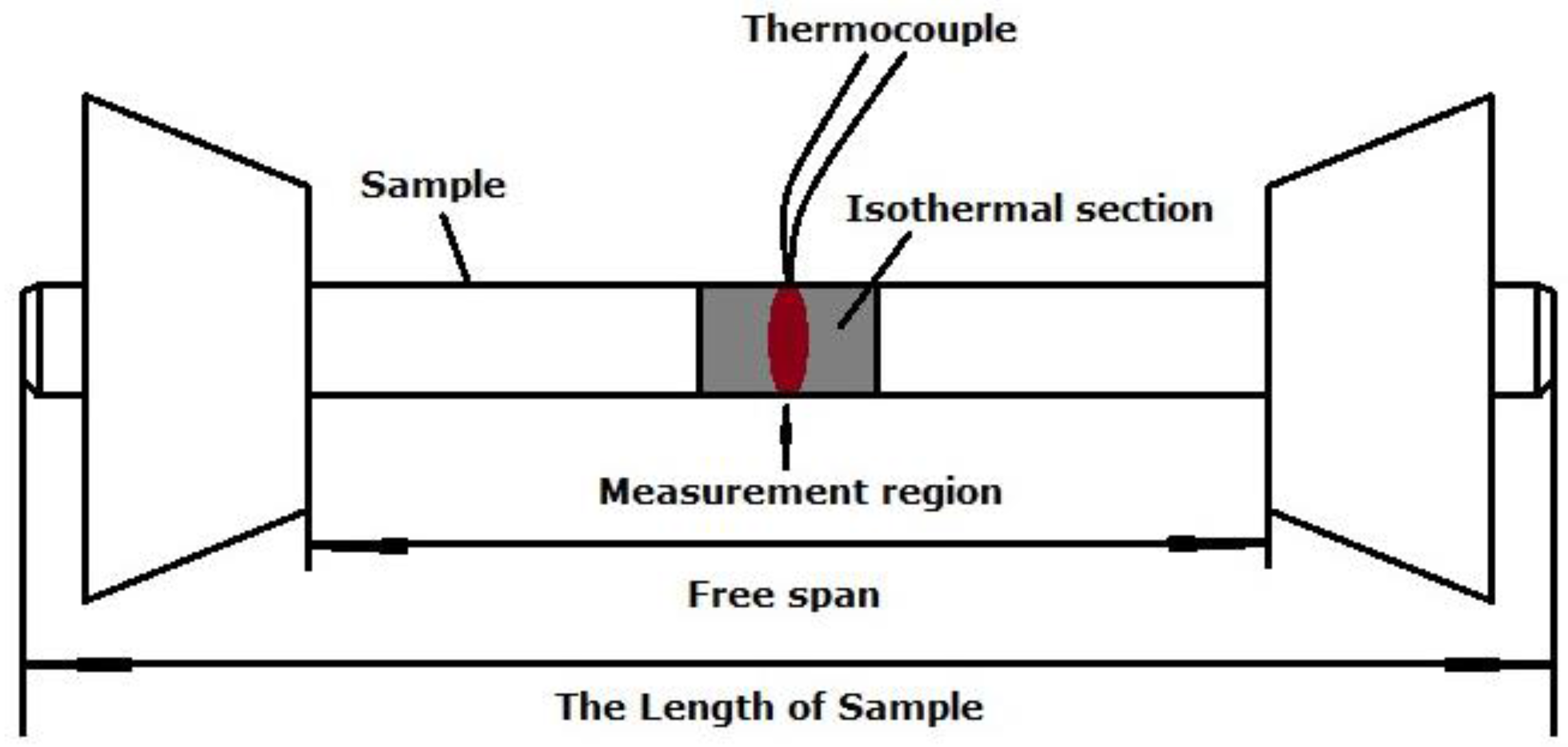
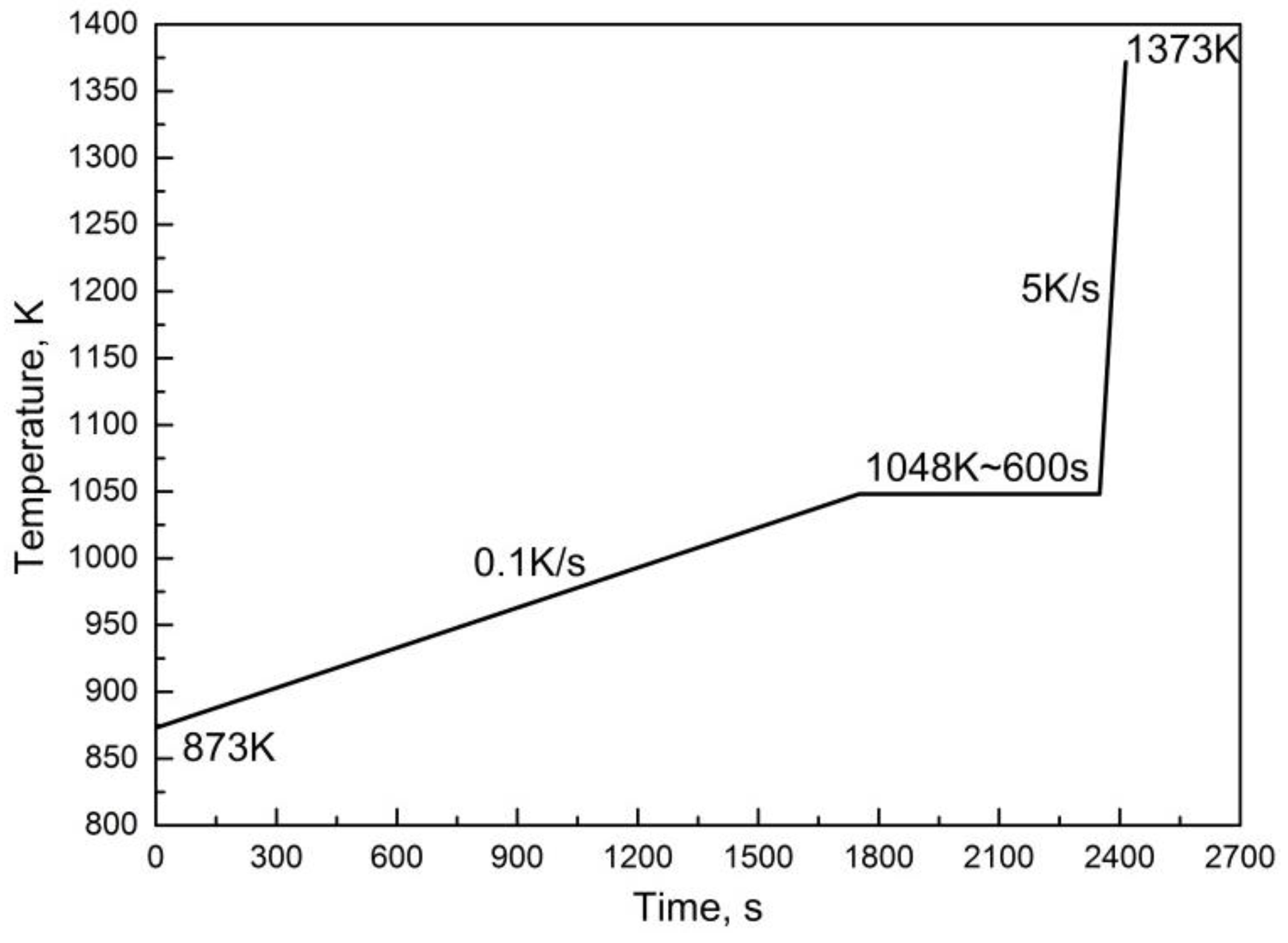
3. Results and Discussion
3.1. Data Processing
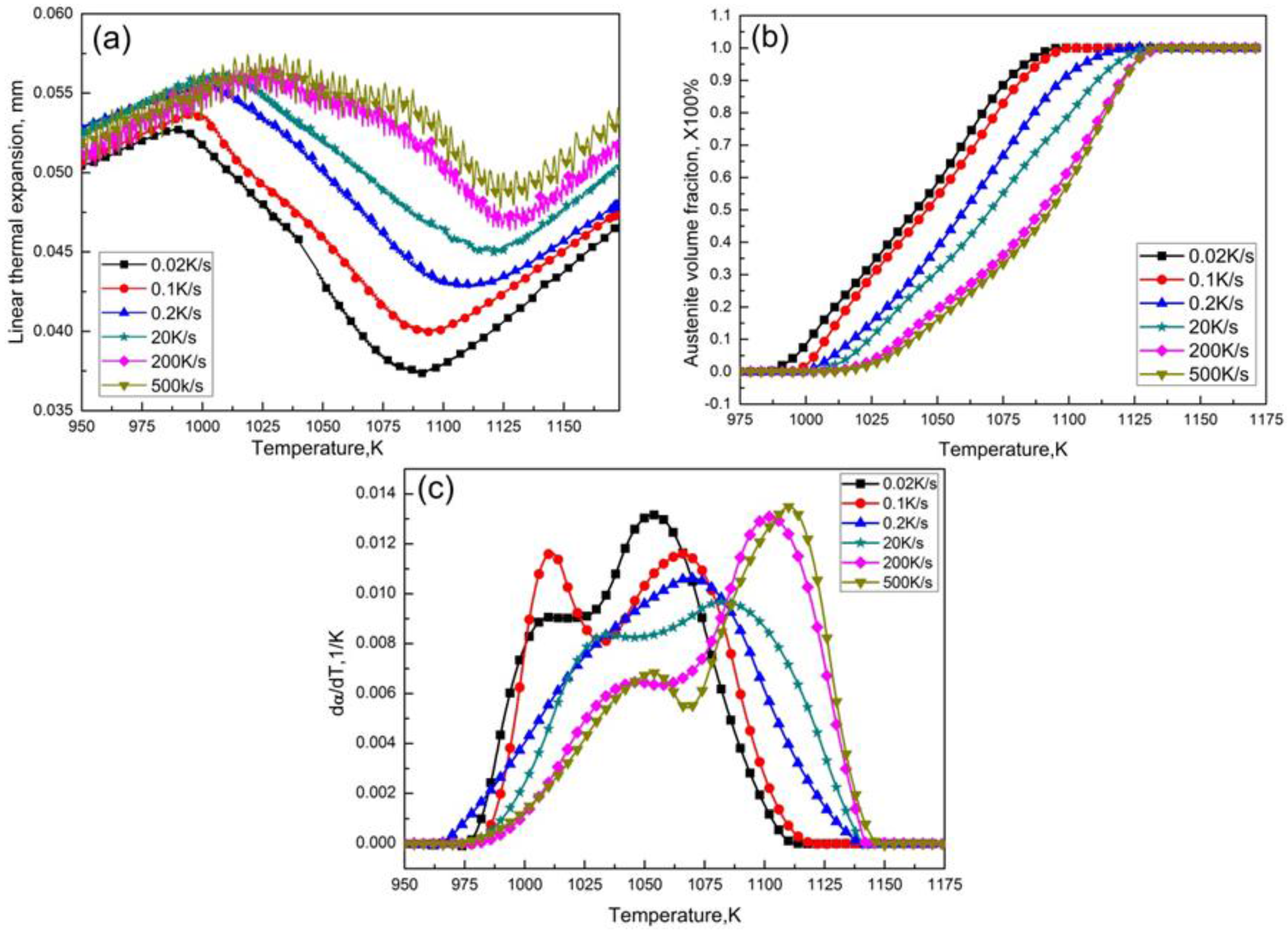
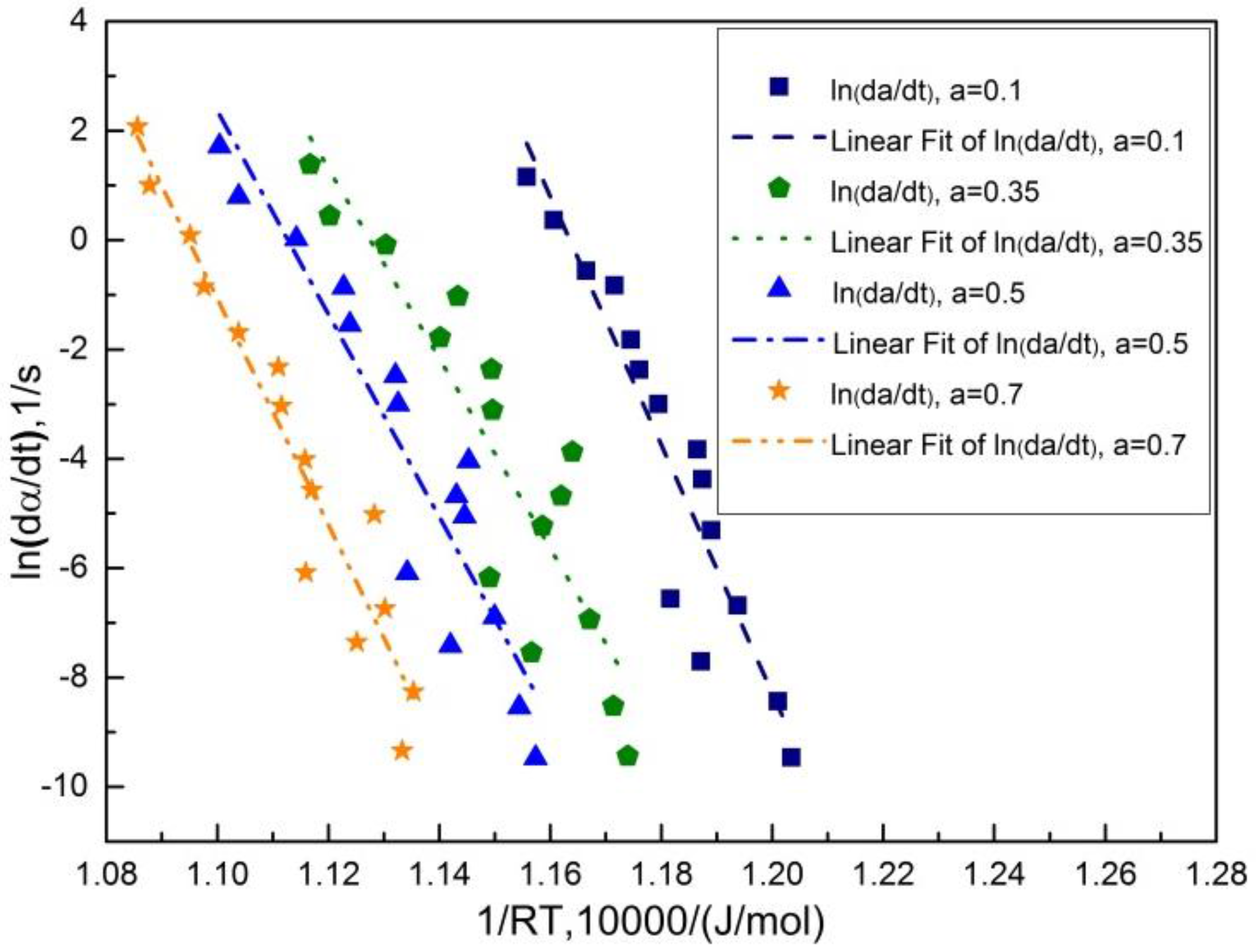
3.2. Determination of Model-Free Austenitization Kinetics
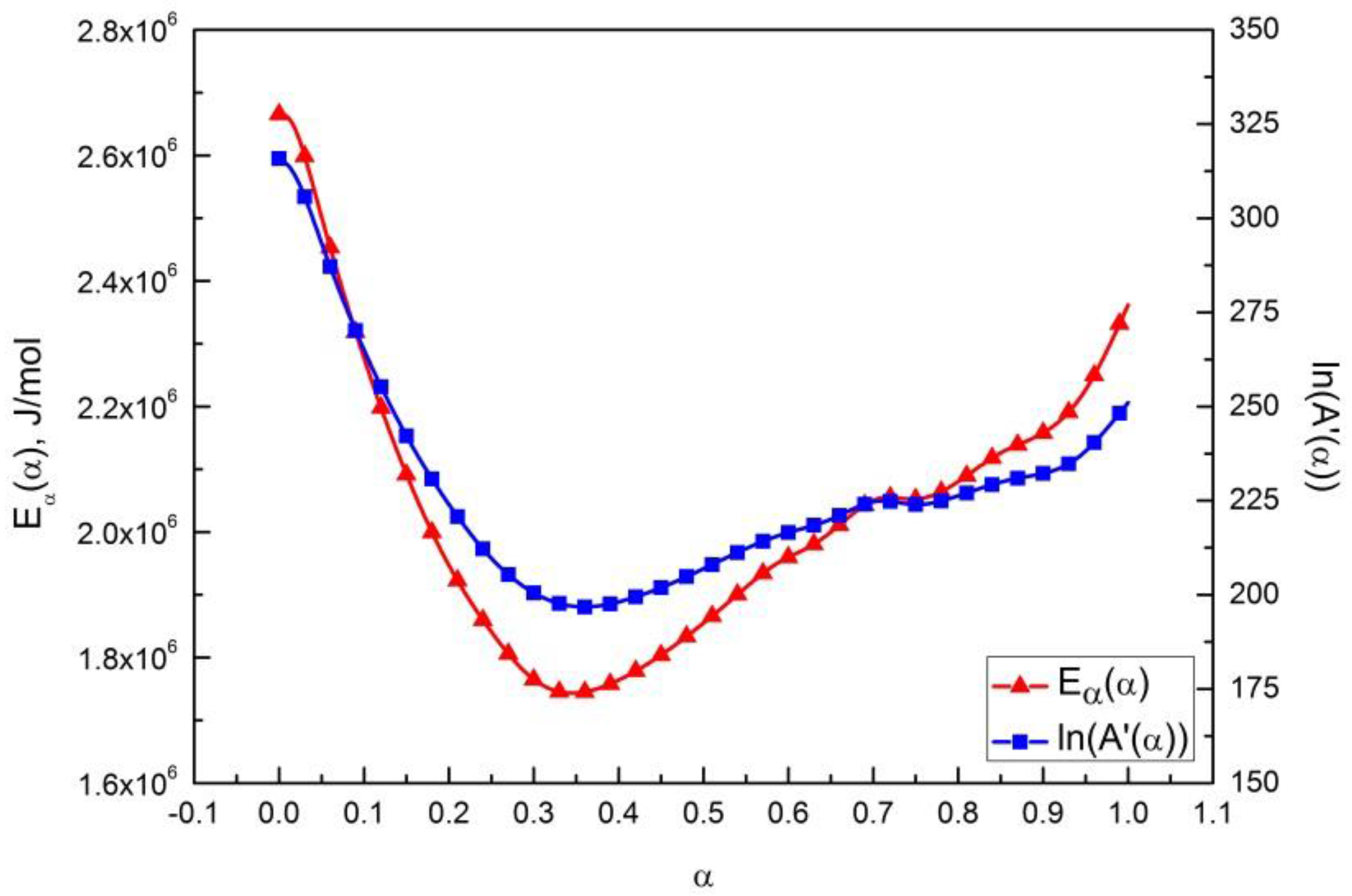
3.3. Regression Validation
3.4. Calculation of TTA Diagram
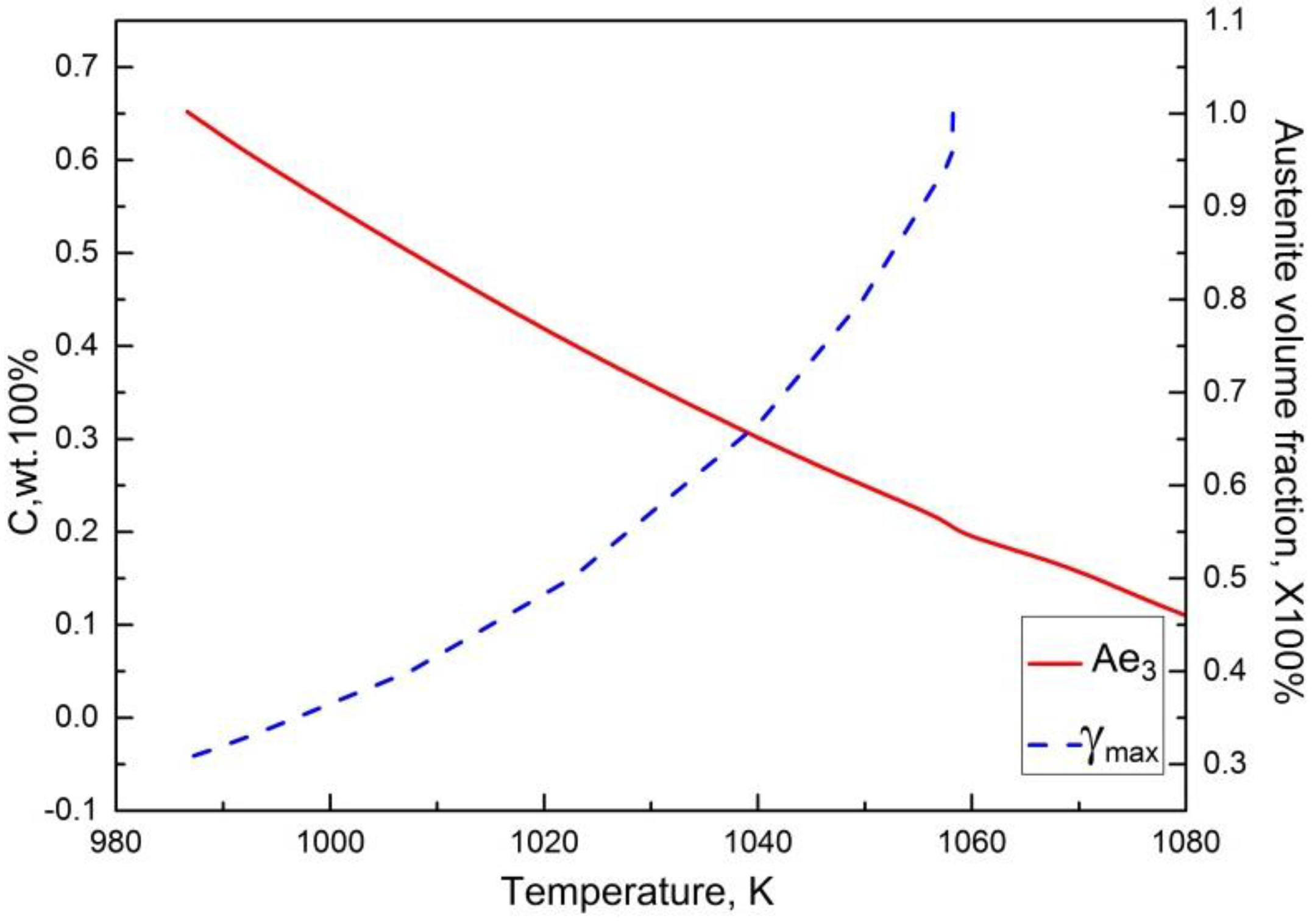
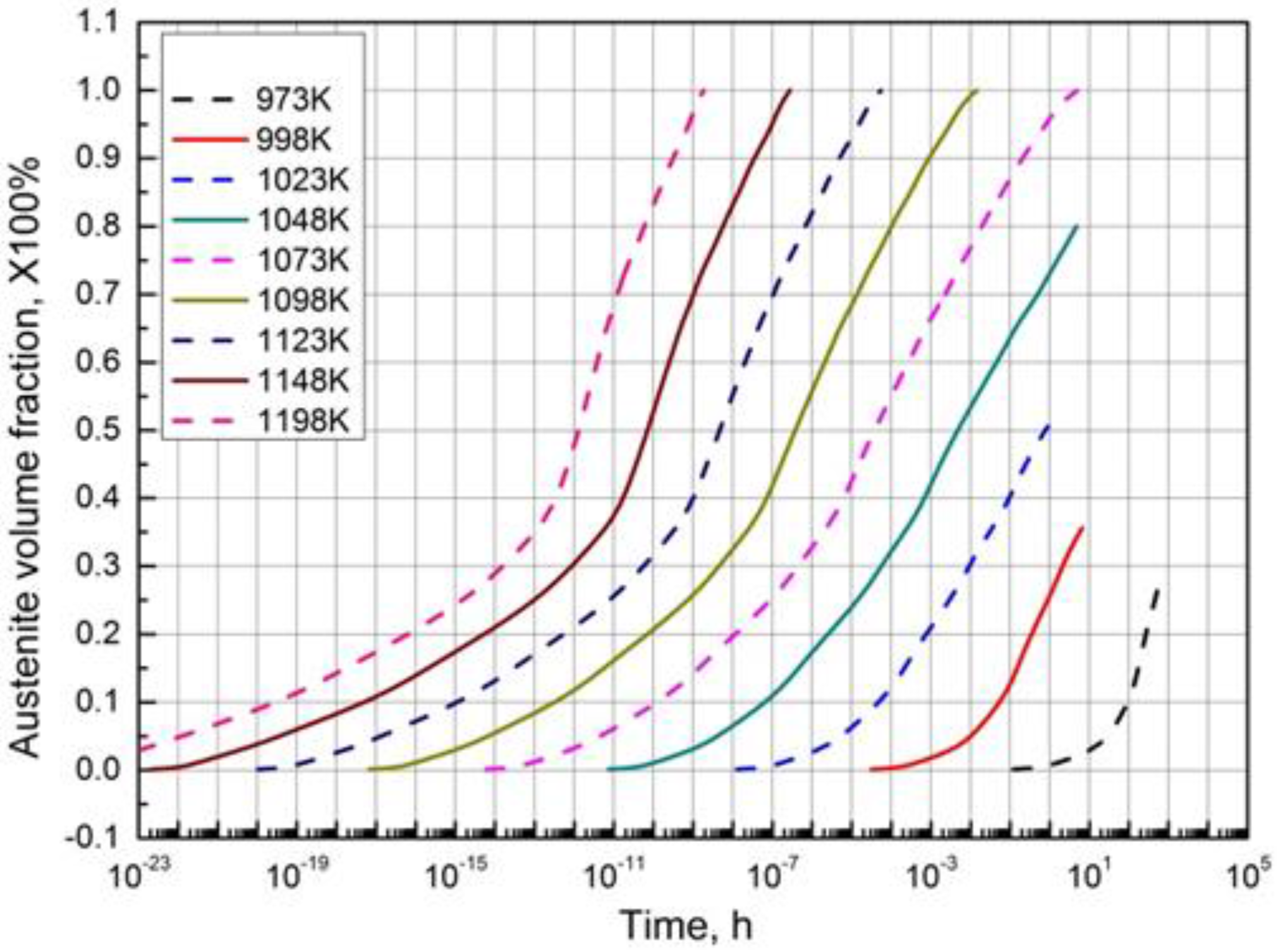
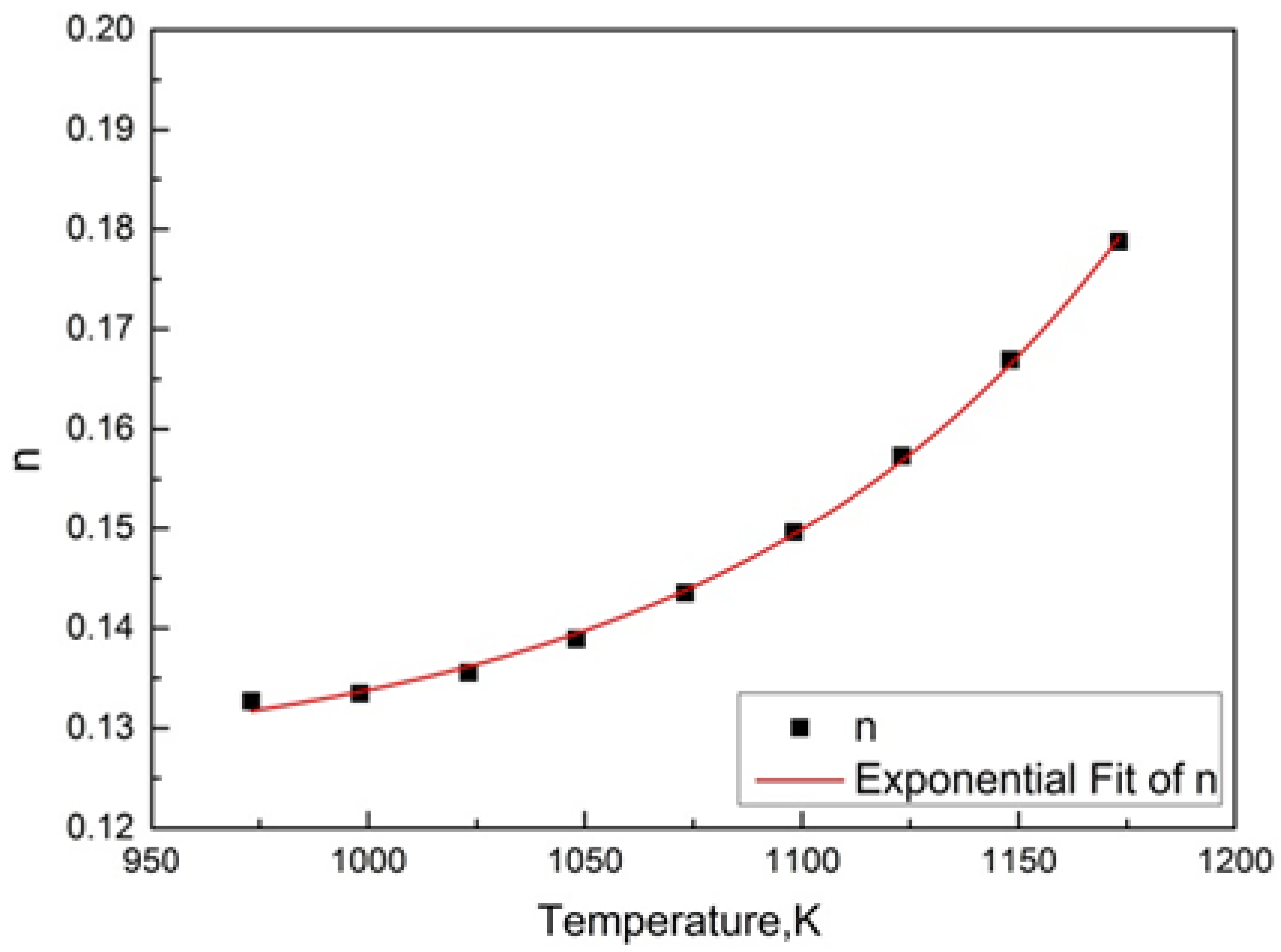
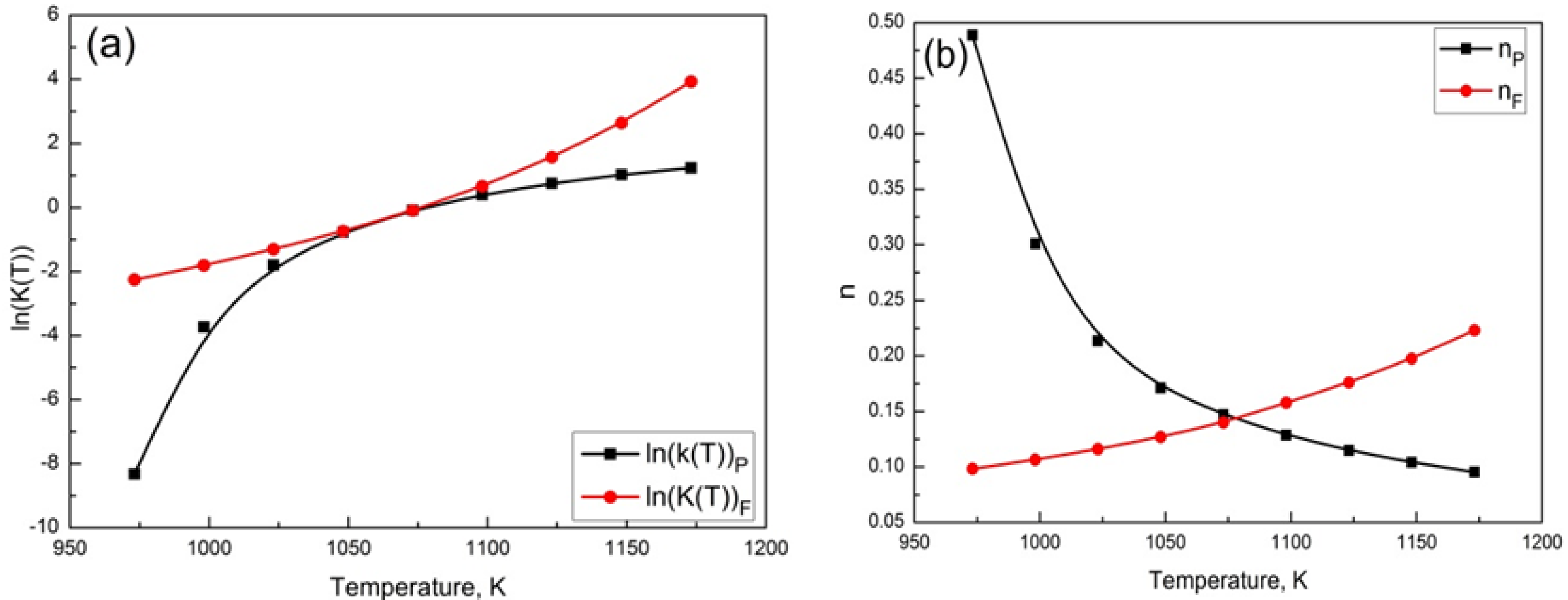
3.5. Determination of the Parameters k(T) and n in JMAK Equation Based on TTA Diagram
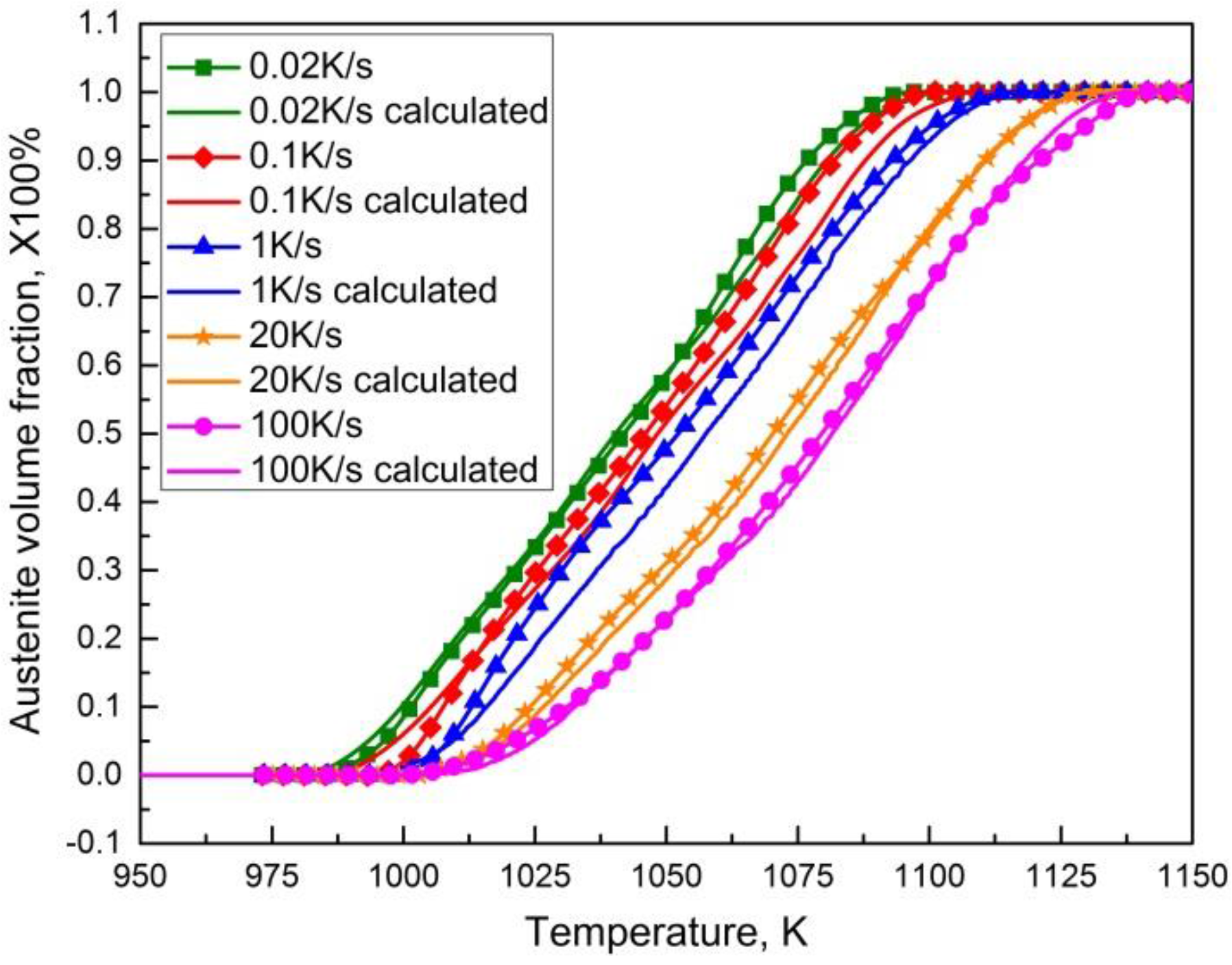
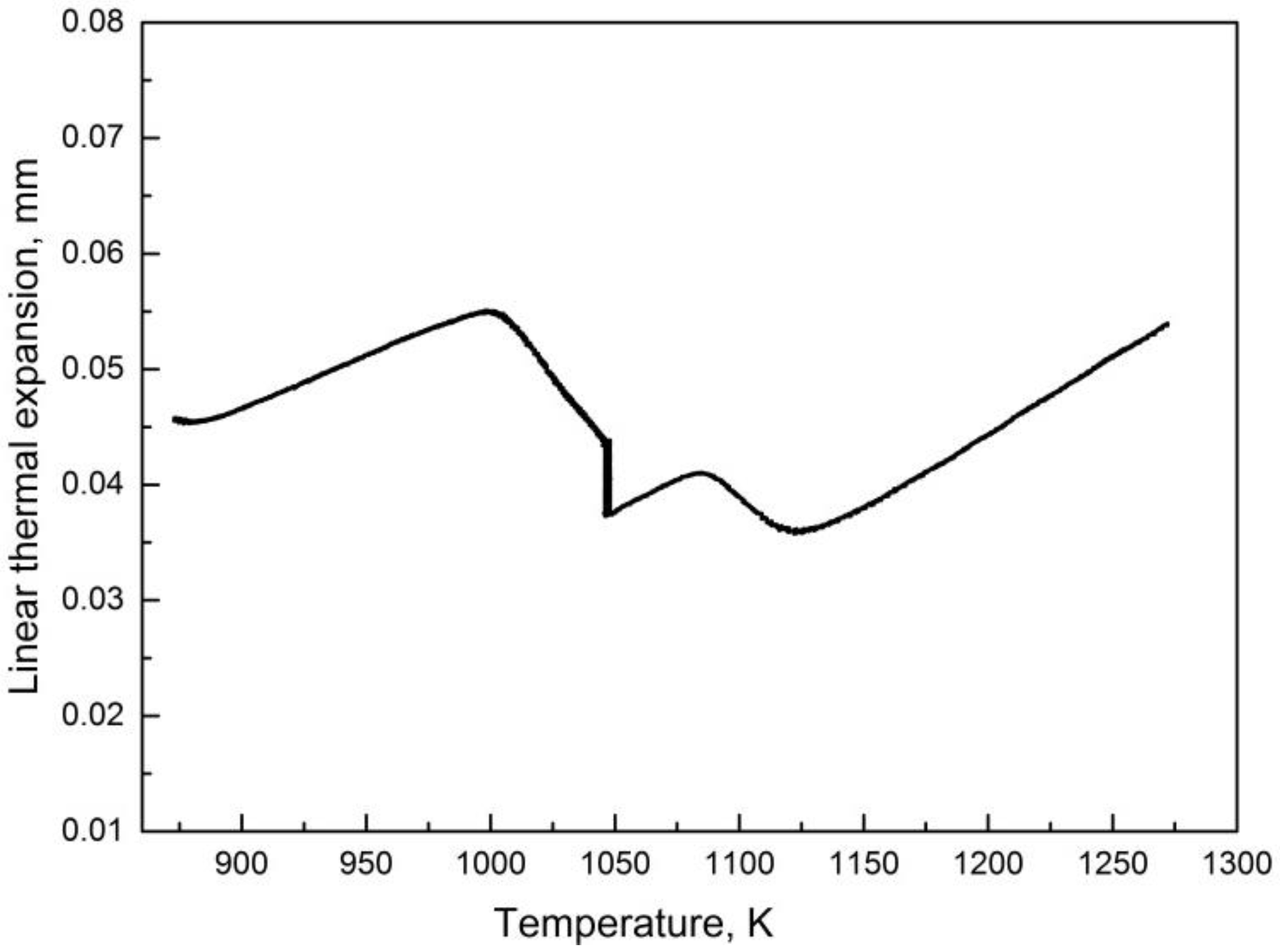
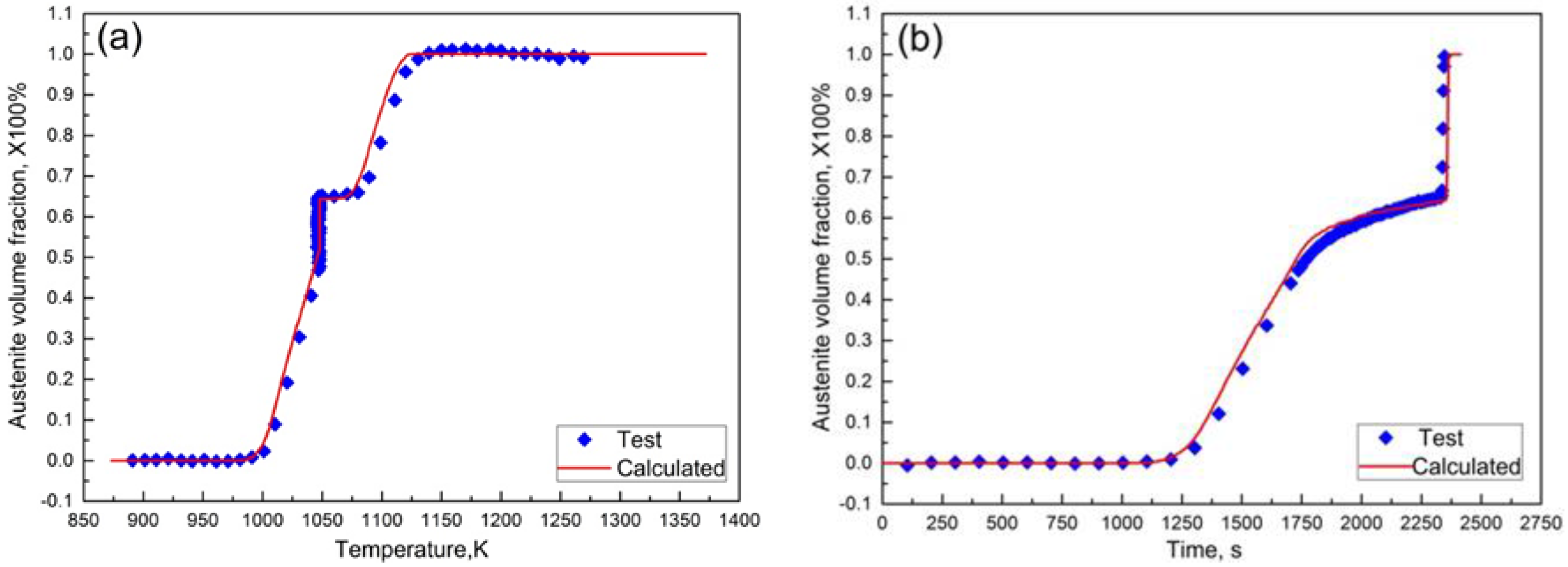
3.6. Prediction of Austenitization Process during Heating with Non-Constant Heating Rates
4. Conclusions
- (1)
- The model-free austenitization kinetics is built by the isoconversional method, and the effective activation energy Eα(α) is also determined. The curve of Eα(α) vs. α is characterized with a roughly “V” shape. The value of Eα(α) is in the range of 1760 to 2670 kJ/mol, and reach a minimum at α ≈ 0.35, consistent with what expected from the initial dual phase (ferrite and pearlite) microstructure of the investigated steel.
- (2)
- Further, the TTA diagram of SA508 Gr.3 steel has been obtained using the model-free austenitization kinetics and the isoconversional method, providing an effective way for the accurate determination of a TTA diagram, which is usually difficult to obtain from experiments.
- (3)
- Under non-constant heating rate conditions, a satisfactory agreement between the curve of the phase transformation fraction using numerical prediction with the established model-free austenitization kinetics and that of the calculation from validation dilatometric curve, demonstrates that the isoconversional method can be used to characterize the austenitization kinetics during the heating process.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kohout, J. An alternative to the JMAK equation for a better description of phase transformation kinetics. J. Mater. Sci. 2008, 43, 1334–1339. [Google Scholar] [CrossRef]
- Roosz, A.; Garcia, Z.; Fuchs, E.G. Isothermal formation of austenite in eutectoid plain carbon steel. Acta Mater. 1983, 31, 509–517. [Google Scholar] [CrossRef]
- De Andrés, C.G.; Caballero, F.G.; Capdevila, C.; Bhadeshia, H.K.D.H. Modelling of kinetics and dilatometric behavior of non-isothermal pearlite-to-austenite transformation in an eutectoid steel. Scr. Mater. 1998, 39, 791–796. [Google Scholar] [CrossRef]
- Caballero, F.G.; Capdevila, C.; de Andrés, C.G. Kinetics and dilatometric behaviour of non-isothermal ferrite-austenite transformation. Mater. Sci. Technol. 2001, 17, 1114–1118. [Google Scholar] [CrossRef] [Green Version]
- Caballero, F.G.; Capdevila, C.; de Andrés, C.G. Modelling of kinetics of austenite formation in steels with different initial microstructures. ISIJ Int. 2001, 41, 1093–1102. [Google Scholar] [CrossRef] [Green Version]
- Gaude-Fugarolas, D.; Bhadeshia, H. A model for austenitisation of hypoeutectoid steels. J. Mater. Sci. 2003, 38, 1195–1201. [Google Scholar] [CrossRef]
- Surm, H.; Kessler, O.; Hoffmann, F.; Zoch, H.W. Modelling of austenitising with non-constant heating rate in hypereutectoid steels. Int. J. Microstruct. Mater. Prop. 2008, 3, 35–48. [Google Scholar] [CrossRef]
- Tszeng, T.C.; Shi, G. A global optimization technique to identify overall transformation kinetics using dilatometry data—Applications to austenitization of steels. Mater. Sci. Eng. A 2004, 380, 123–136. [Google Scholar] [CrossRef]
- Tszeng, T.C.; Shi, G.; Purohit, S. Cementite and carbide dissolution in steels during austenitization at high heating rates. In Proceedings of the Modeling Control and Optimization in Ferrous and Nonferrous Industry Symposium, Chicago, IL, USA, 9–12 November 2003; pp. 379–379.
- Savran, V.I.; Offerman, S.E.; Sietsma, J. Austenite Nucleation and Growth Observed on the Level of Individual Grains by Three-Dimensional X-Ray Diffraction Microscopy. Metall. Mater. Trans. A 2010, 41, 583–591. [Google Scholar] [CrossRef]
- Esin, V.A.; Denand, B.; Bihan, L.Q.; Dehmas, M.; Teixeira, J.; Geandier, G.; Denis, S.; Sourmail, T.; Aeby-Gautier, E. In situ synchrotron X-ray diffraction and dilatometric sthdy of austenite formation in a multi-component steel: Influence of initial microstructure and heating rate. Acta Mater. 2014, 80, 118–131. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Perez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Flynn, J.H.; Wall, L.A. General Treatment of the Thermogravimetry of Polymers. J. Res. Natl. Bur. Stand. 1966, 70, 487–523. [Google Scholar] [CrossRef]
- Ozawa, T. A new method of quantitative differential thermal analysis. Bull. Chem. Soc. Jpn. 1966, 39, 2071–2085. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Sunose, T.; Akahira, T. Method of determining activation deterioration constant of electrical insulating materials. Res. Rep. Chiba Inst. Technol. 1971, 16, 22–31. [Google Scholar]
- Vyazovkin, S.; Dollimore, D. Linear and Nonlinear Procedures in Isoconversional Computations of the Activation Energy of Nonisothermal Reactions in Solids. J. Chem. Inf. Comput. Sci. 1996, 36, 42–45. [Google Scholar] [CrossRef]
- Friedman, H.L. New methods for evaluating kinetic parameters from thermal analysis data. J. Polym. Sci. B Polym. Lett. 1969, 7, 41–46. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J. Polym. Sci. C Polym. Symp. 1964, 6, 183–195. [Google Scholar] [CrossRef]
- Luiggi, N.; Betancourt, M. On the non-isothermal precipitation of the β′ and β phases in Al-12.6 mass% Mg alloys using dilatometric techniques. J. Therm. Anal. Calorim. 2003, 74, 883–894. [Google Scholar] [CrossRef]
- Adorno, A.T.; Carvalho, T.M.; Magdalena, A.G.; dos Santos, C.M.A.; Silva, R.A.G. Activation energy for the reverse eutectoid reaction in hypo-eutectoid Cu–Al alloys. Thermochim. Acta 2012, 531, 35–41. [Google Scholar] [CrossRef]
- Silva, R.A.G.; Gama, S.; Paganotti, A.; Adorno, A.T.; Carvalho, T.M.; Santos, C.M.A. Effect of Ag addition on phase transitions of the Cu-22.26 at. %Al-9.93 at. %Mn alloy. Thermochim. Acta 2013, 554, 71–75. [Google Scholar] [CrossRef]
- Vyazovkin, S. Computational aspects of kinetic analysis. Part C. The ICTAC Kinetics Project—The light at the end of the tunnel? Thermochim. Acta 2000, 355, 155–163. [Google Scholar] [CrossRef]
- Brown, M.E.; Dollimore, D.; Galwey, A.K. Reactions in the Solid State, Comprehensive Chemical Kinetics; Elsevier: Amsterdam, The Netherlands, 1980. [Google Scholar]
- Brown, M.E. Introduction to Thermal Analysis: Techniques and Applications; Springer Science & Business Media: Lodon, UK, 2001; p. 264. [Google Scholar]
- Brown, M.E.; Maciejewski, M.; Vyazovkin, S.; Nomen, R.; Sempere, J.; Burnham, A.; Opfermann, J.; Strey, R.; Anderson, H.L.; Kemmler, A.; et al. Computational aspects of kinetic analysis Part A: The ICTAC kinetics project-data, methods and results. Thermochim. Acta 2000, 355, 125–143. [Google Scholar] [CrossRef]
- Marcilla, A.; Garcia-Quesada, J.C.; Ruiz-Femenia, R. Additional considerations to the paper entitled: “Computational aspects of kinetic analysis. Part B: The ICTAC Kinetics Project—The decomposition kinetics of calcium carbonate revisited, or some tips on survival in the kinetic minefield”. Thermochim. Acta 2006, 445, 92–96. [Google Scholar] [CrossRef]
- Chen, R.; Gu, J.; Han, L.; Pan, J. Austenization kinetics of 30Cr2Ni4MoV steel. Trans. Mater. Heat Treat. 2013, 34, 170–174. [Google Scholar]
- Chiba, A. Isothermal transformation kinetics from ferrite to austenite in an Fe-8% Cr alloy. Trans. Jpn. Inst. Met. 1984, 25, 523–530. [Google Scholar] [CrossRef]
- Burnham, A.K.; Dinh, L.N. A comparison of isoconversional and model-fitting approaches to kinetic parameter estimation and application predictions. J. Therm. Anal. Calorim. 2007, 89, 479–490. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, X.; Han, L.; Gu, J. Study on Austenitization Kinetics of SA508 Gr.3 Steel Based on Isoconversional Method. Metals 2016, 6, 8. https://doi.org/10.3390/met6010008
Luo X, Han L, Gu J. Study on Austenitization Kinetics of SA508 Gr.3 Steel Based on Isoconversional Method. Metals. 2016; 6(1):8. https://doi.org/10.3390/met6010008
Chicago/Turabian StyleLuo, Xiaomeng, Lizhan Han, and Jianfeng Gu. 2016. "Study on Austenitization Kinetics of SA508 Gr.3 Steel Based on Isoconversional Method" Metals 6, no. 1: 8. https://doi.org/10.3390/met6010008






