Investigation of the Undercoolability of Ni-Based Alloys Using High Temperature Thermal Analysis
Abstract
:1. Introduction
- •
- •
- Process parameters: The supercooling of a melt depends on the process parameters, especially the cooling rate. Usually, higher values of undercooling are obtained with high cooling rates.
- •
- Nucleation mechanism: Melts can be highly undercooled if homogeneous nucleation occurs. This can be achieved using containerless levitation or drop tube experiments [12]. For pure nickel, undercooling values of ΔT = 341–480 K [13,14] were obtained. In the industrial Bridgman process, undercooled zones are usually near the inner shell mold surface, and heterogeneous nucleation lowers the undercooling of the melt. In classical nucleation theory [15], the contact angle Θ correlates with the interface energy between the ceramic substrate and melt. A low contact angle means good wetting and low interface energy. As a consequence, low undercoolabilities for low contact angles are achieved [16].
- •
- Chemical reactions: To produce high-quality large SX gas turbine blades, it is essential to use shell mold systems with sufficiently high temperature properties. Usually, silica-sol-binder and different fractions of ceramic filler are used for the ceramic slurry of the front coating to ensure dimension accuracy, shell mold strength and a stable investment casting process. Contact angle measurements between different ceramics and superalloys show limited chemical reactions between the melt and ceramics containing SiO2 [17,18].
2. Experimental Section
2.1. Materials
| Name | Ni | Re | Co | Cr | Mo | Al | Ti | Ta | Total |
|---|---|---|---|---|---|---|---|---|---|
| CMSX-6 | bal. | - | 5.6 ± 0.2 | 10.5 ± 0.3 | 3.5 ± 0.2 | 4.6 ± 0.1 | 4.9 ± 0.1 | 2.2 ± 0.3 | 31.3 |
| Alloy1 | bal. | 1.2 ± 0.2 | 4.9 ± 0.2 | 9.1 ± 0.3 | 3.1 ± 0.2 | 4.0 ± 0.1 | 4.3 ± 0.1 | 1.9 ± 0.3 | 28.5 |
| Alloy2 | bal. | 2.3 ± 0.2 | 4.2 ± 0.2 | 7.8 ± 0.3 | 2.6 ± 0.2 | 3.4 ± 0.1 | 3.7 ± 0.1 | 1.6 ± 0.3 | 25.6 |
| Alloy3 | bal. | 3.5 ± 0.2 | 3.5 ± 0.2 | 6.4 ± 0.3 | 2.2 ± 0.2 | 2.8 ± 0.1 | 3.0 ± 0.1 | 1.4 ± 0.3 | 22.8 |
| Ni2Re | 97.7 | 2.3 ± 0.2 | - | - | - | - | - | - | - |
| Ni4Re | 95.4 | 4.6 ± 0.2 | - | - | - | - | - | - | - |
| Ni9Re | 90.9 | 9.2 ± 0.2 | - | - | - | - | - | - | - |
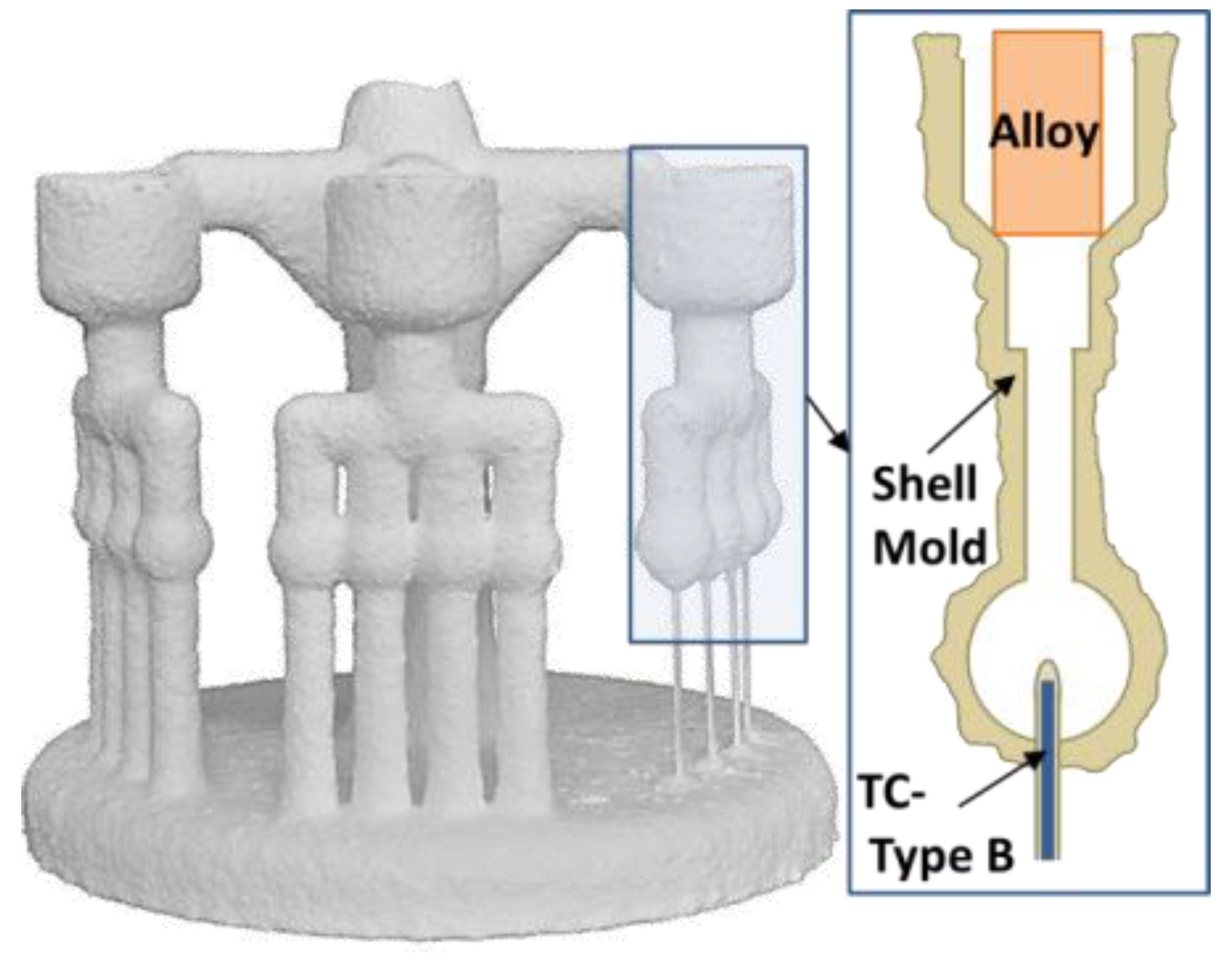
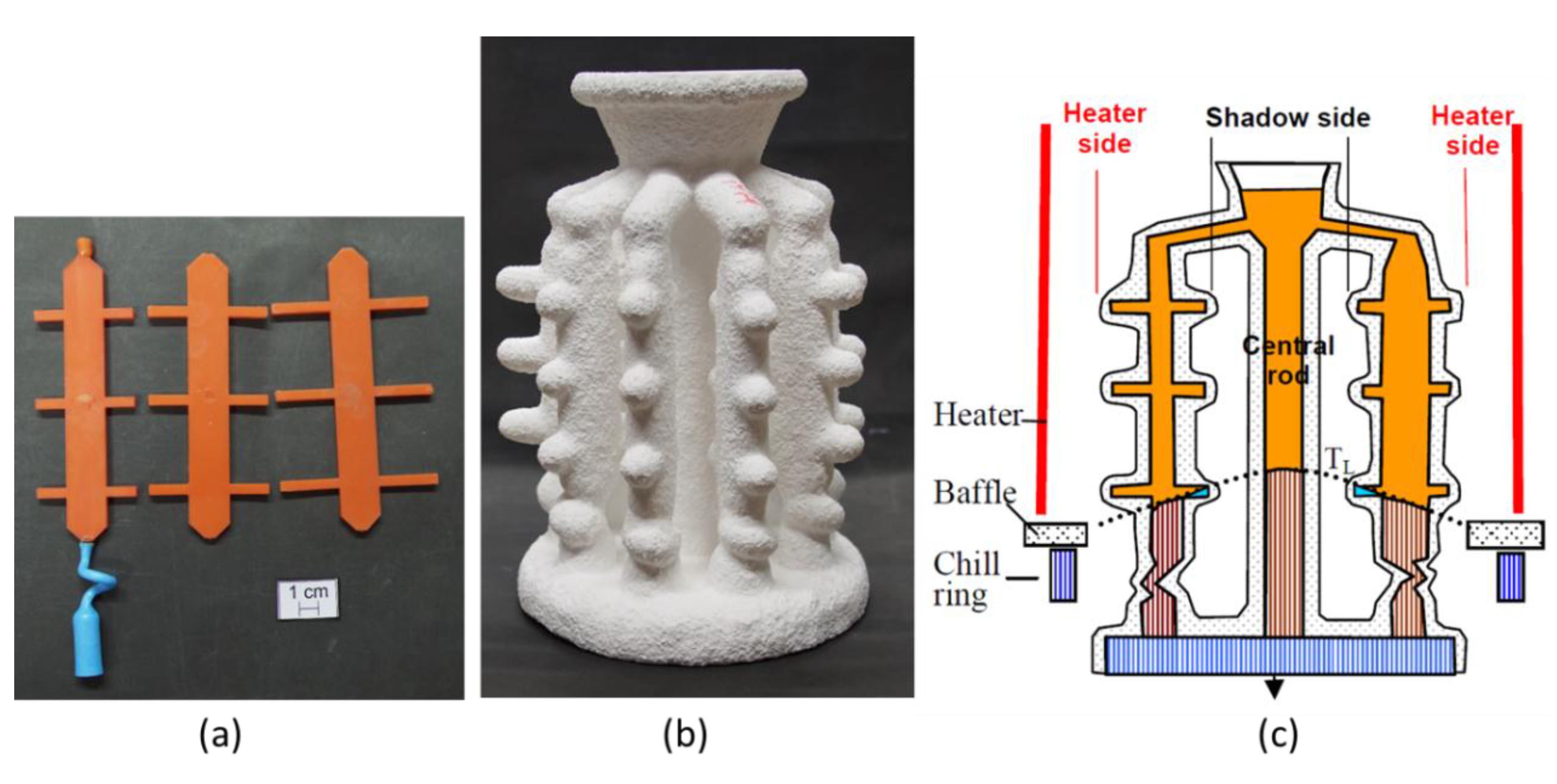
2.2. Shell Mold Design and Manufacture
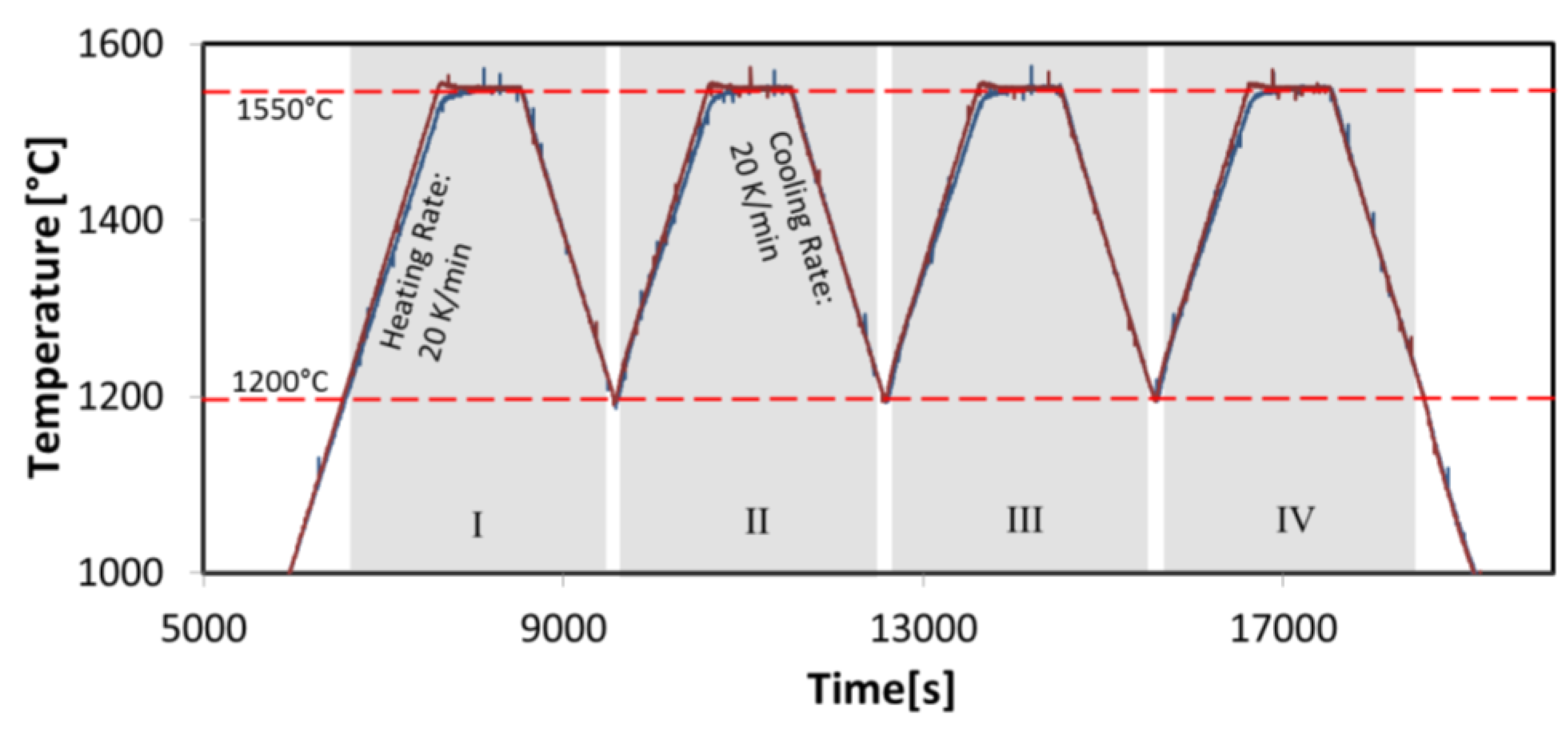
2.3. Casting Set-Up and Temperature Measurements
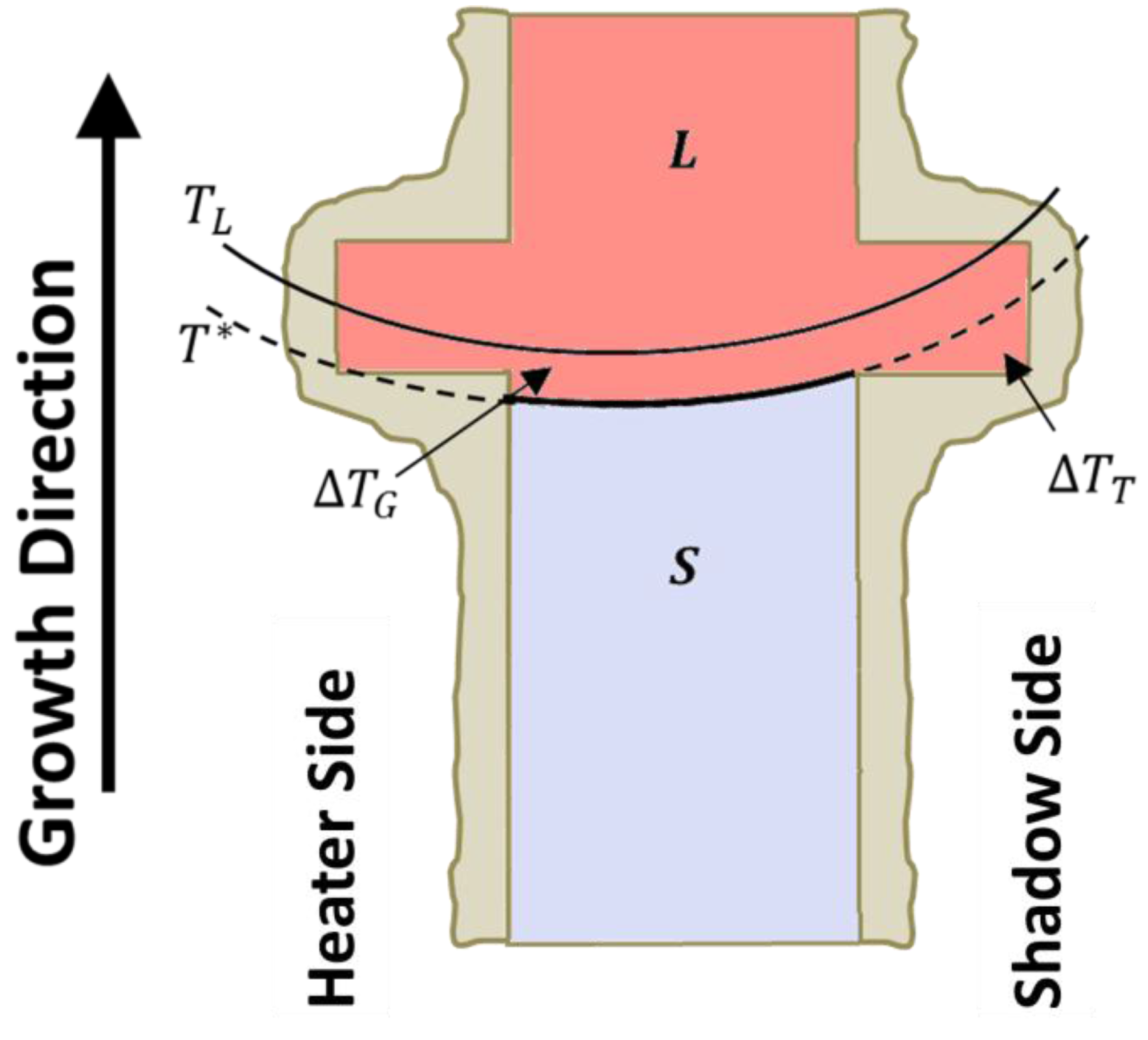
2.4. Directional Solidification
| Alloy | Shell Mold Temperature | Pouring Temperature of the Melt | Withdrawal Velocity |
|---|---|---|---|
| CMSX-6 | 1430 °C | 1430 °C | v = 3.0 mm/min |
| Alloy1 | 1480 °C | 1480 °C | v = 3.0 mm/min |
| Alloy3 | 1500 °C | 1500 °C | v = 3.0 mm/min |
3. Results and Discussion
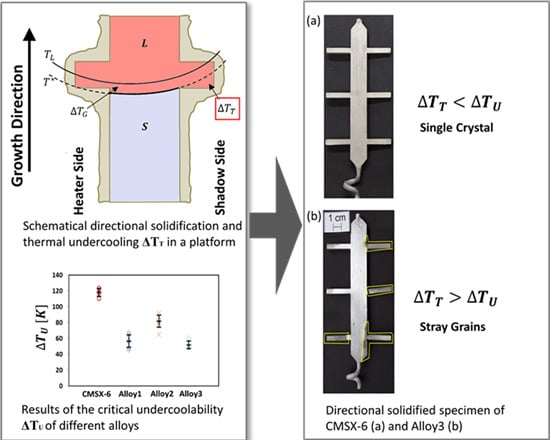
3.1. Visual Inspection
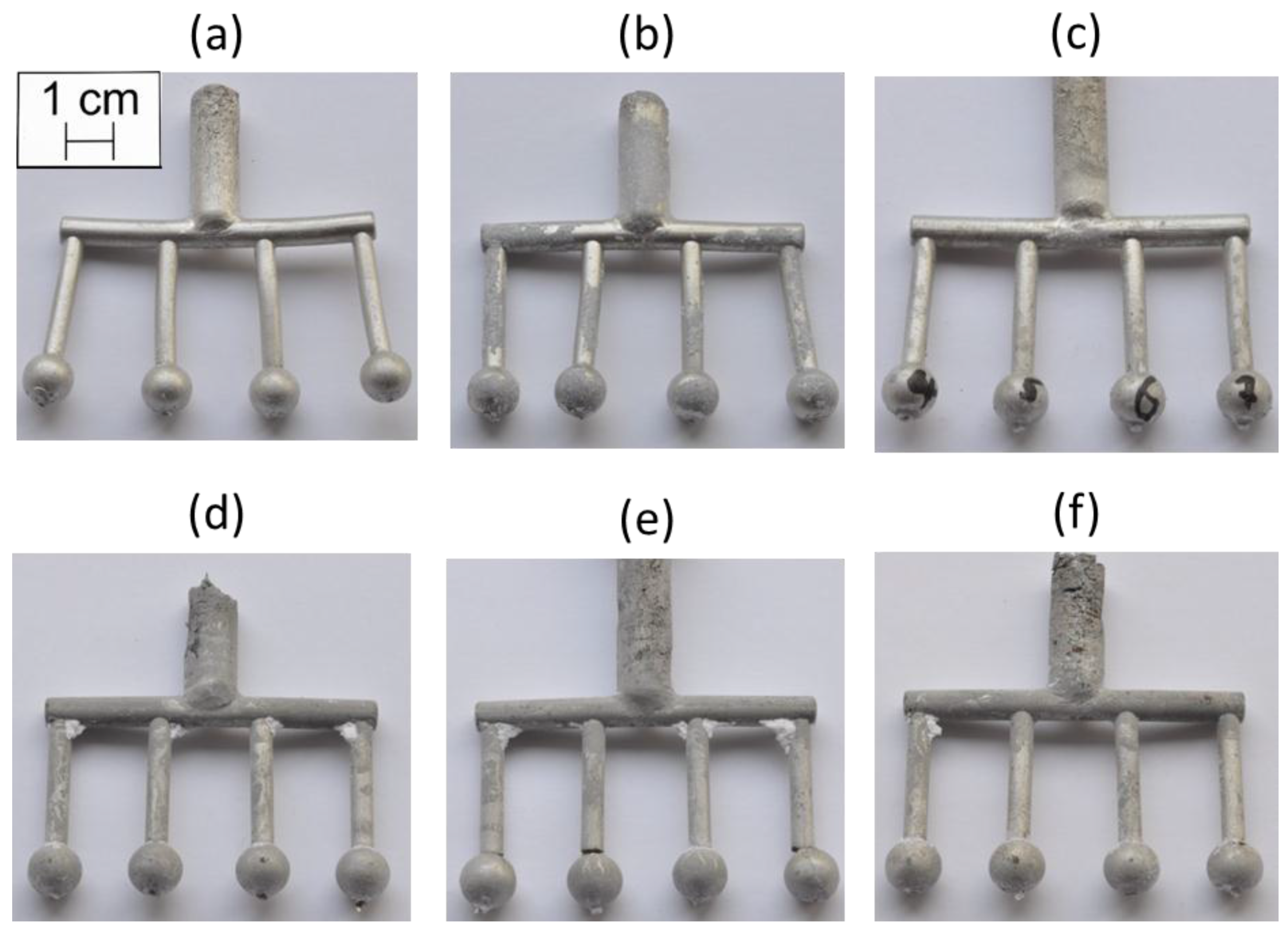
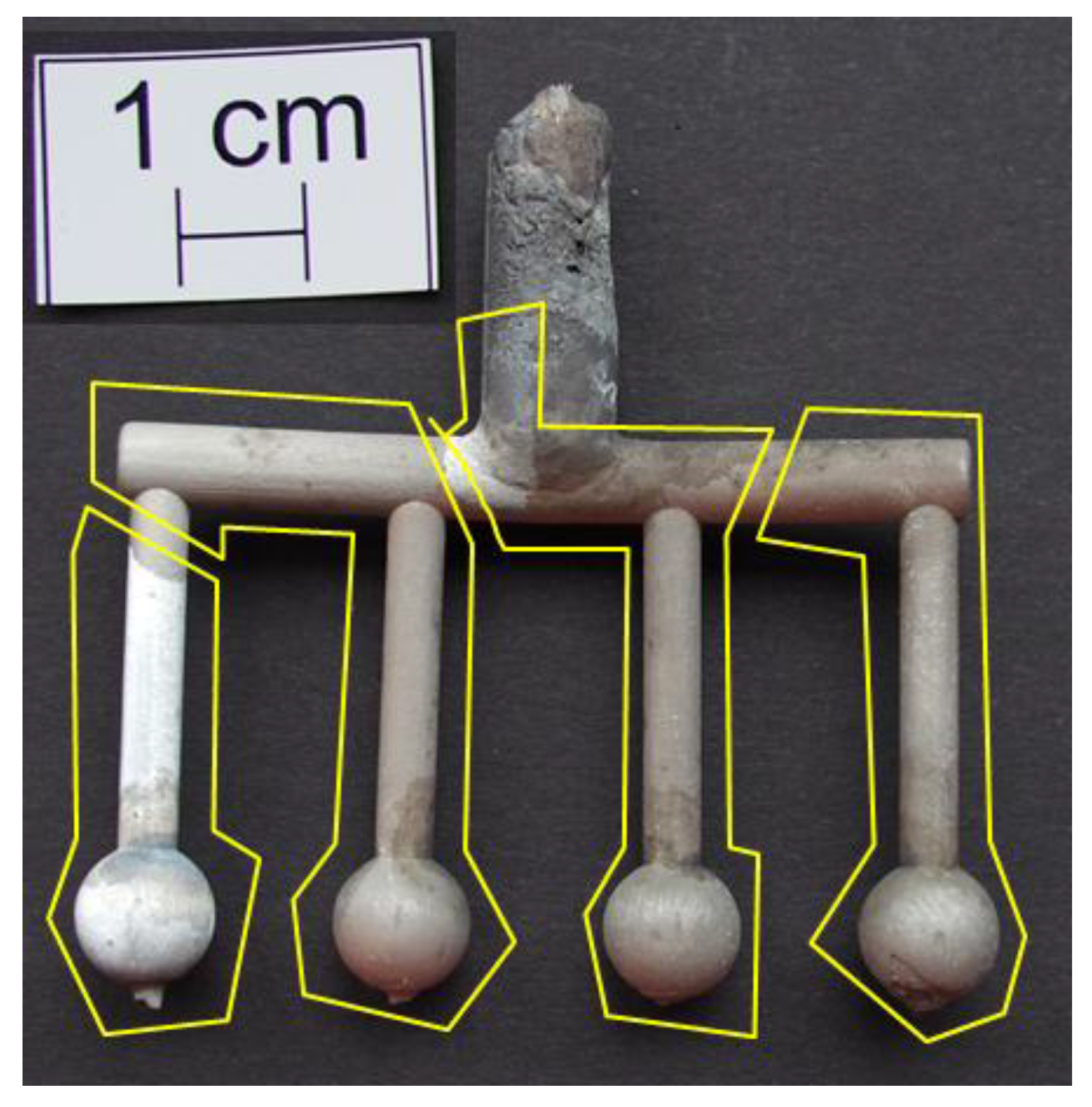
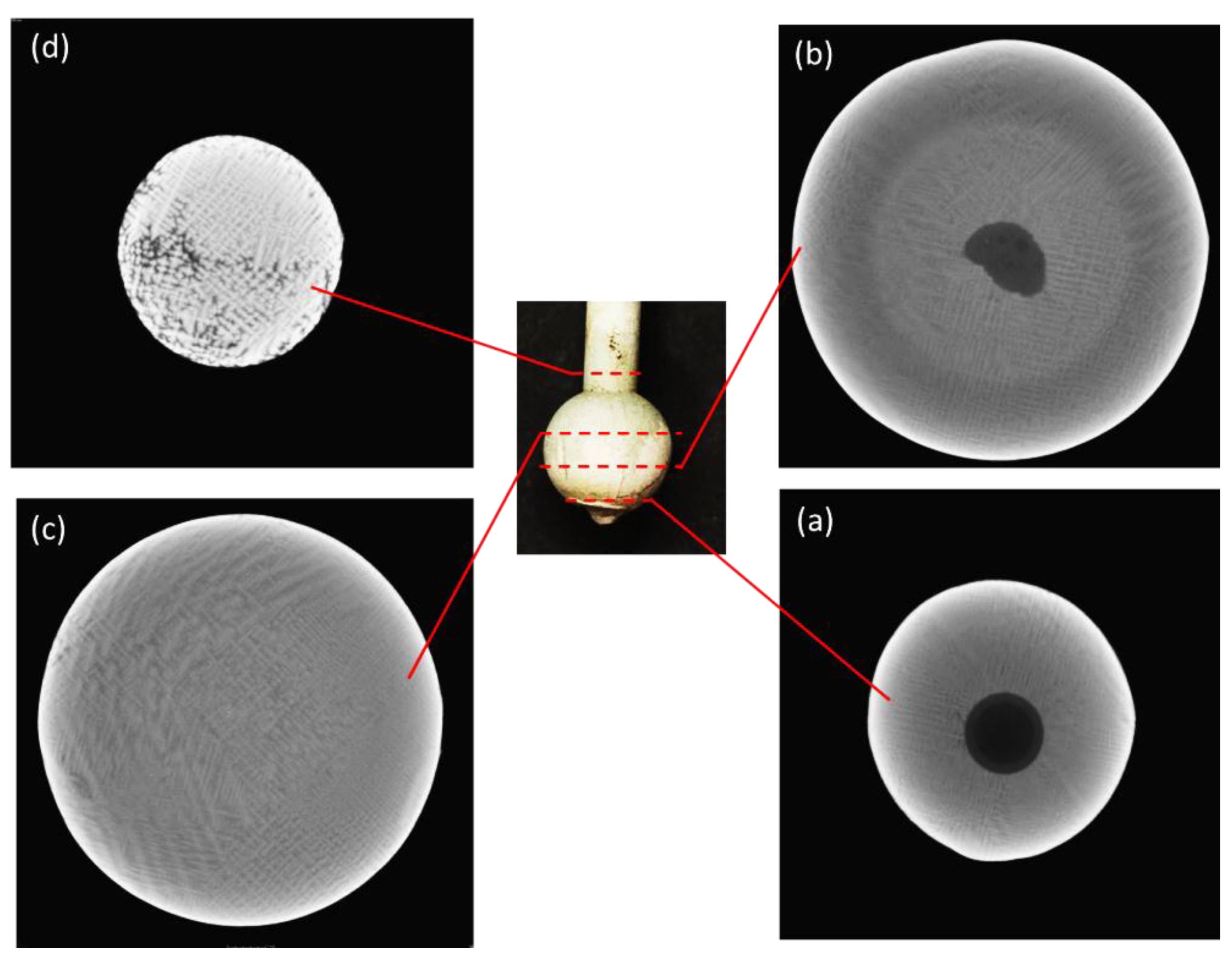
3.2. Temperature Measurement
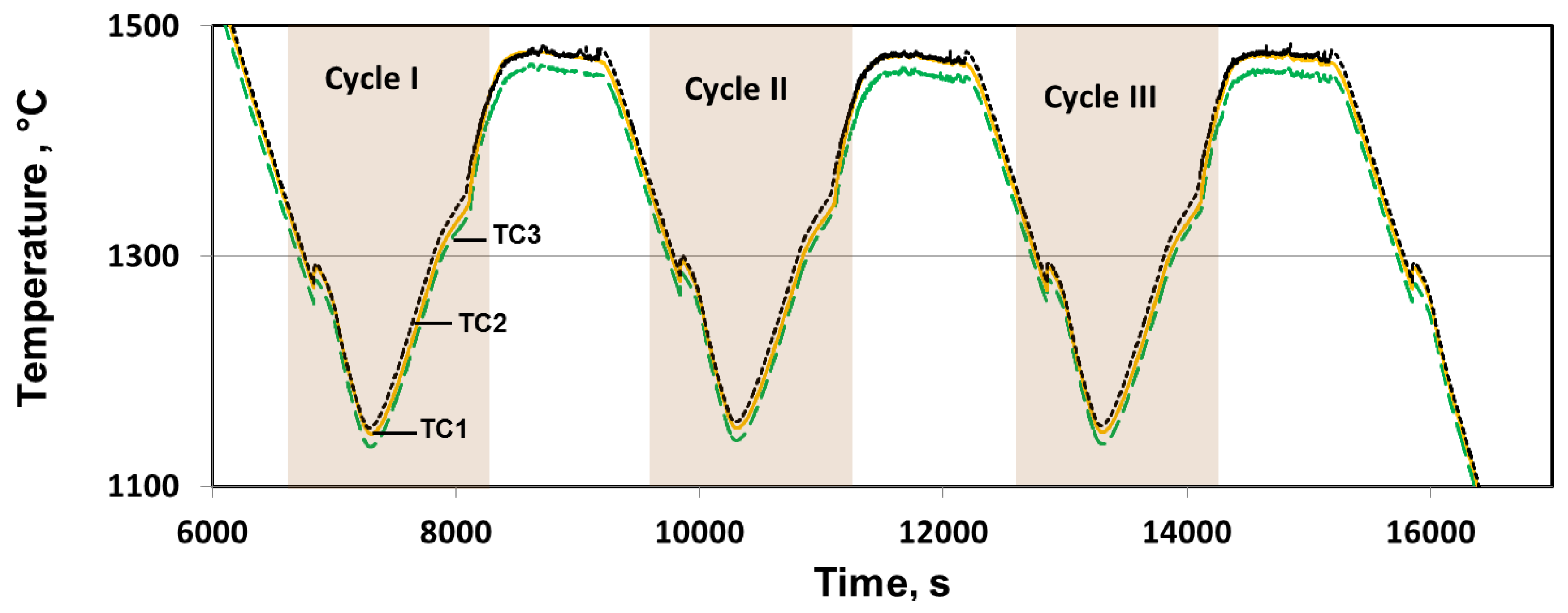
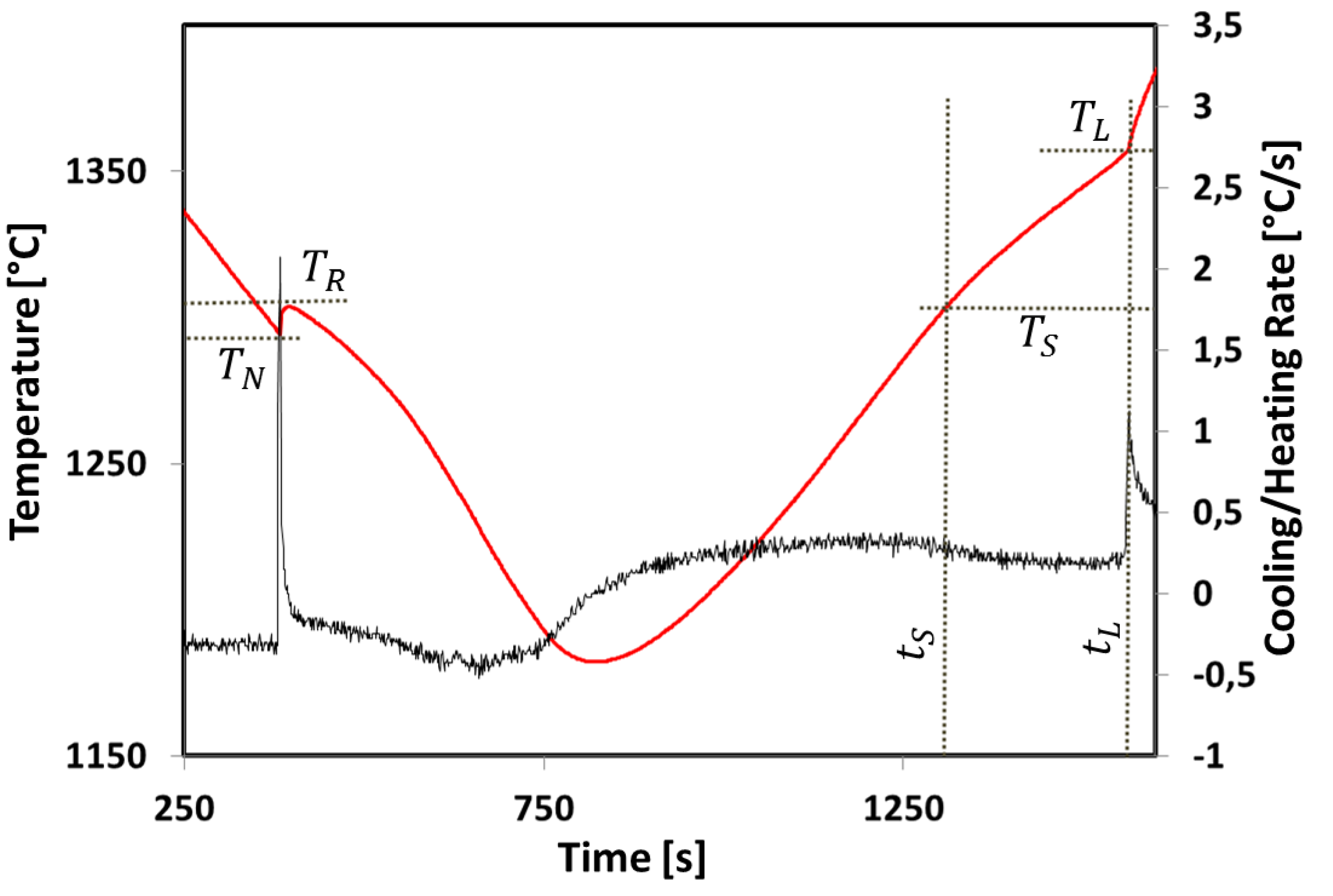
| Name | TS (°C) | TL (°C) | Solidification Interval (°C) | Experimental TS (°C) | Experimental TL (°C) | Melting Interval (°C) |
|---|---|---|---|---|---|---|
| Alloy1 | 1324.1 | 1366.4 | 42.4 | 1296 | 1360 | 64 |
| Alloy2 | 1353.9 | 1389.0 | 35.1 | 1315 | 1372 | 57 |
| Alloy3 | 1380.0 | 1412 | 32 | 1350 | 1400 | 50 |
| Ni2Re[20] | 1460 | 1465 | 5 | 1428 | 1453 | 25 |
| Ni4Re[20] | 1470 | 1490 | 20 | 1435 | 1469 | 34 |
| Ni9Re[20] | 1490 | 1530 | 40 | 1456 | 1505 | 49 |
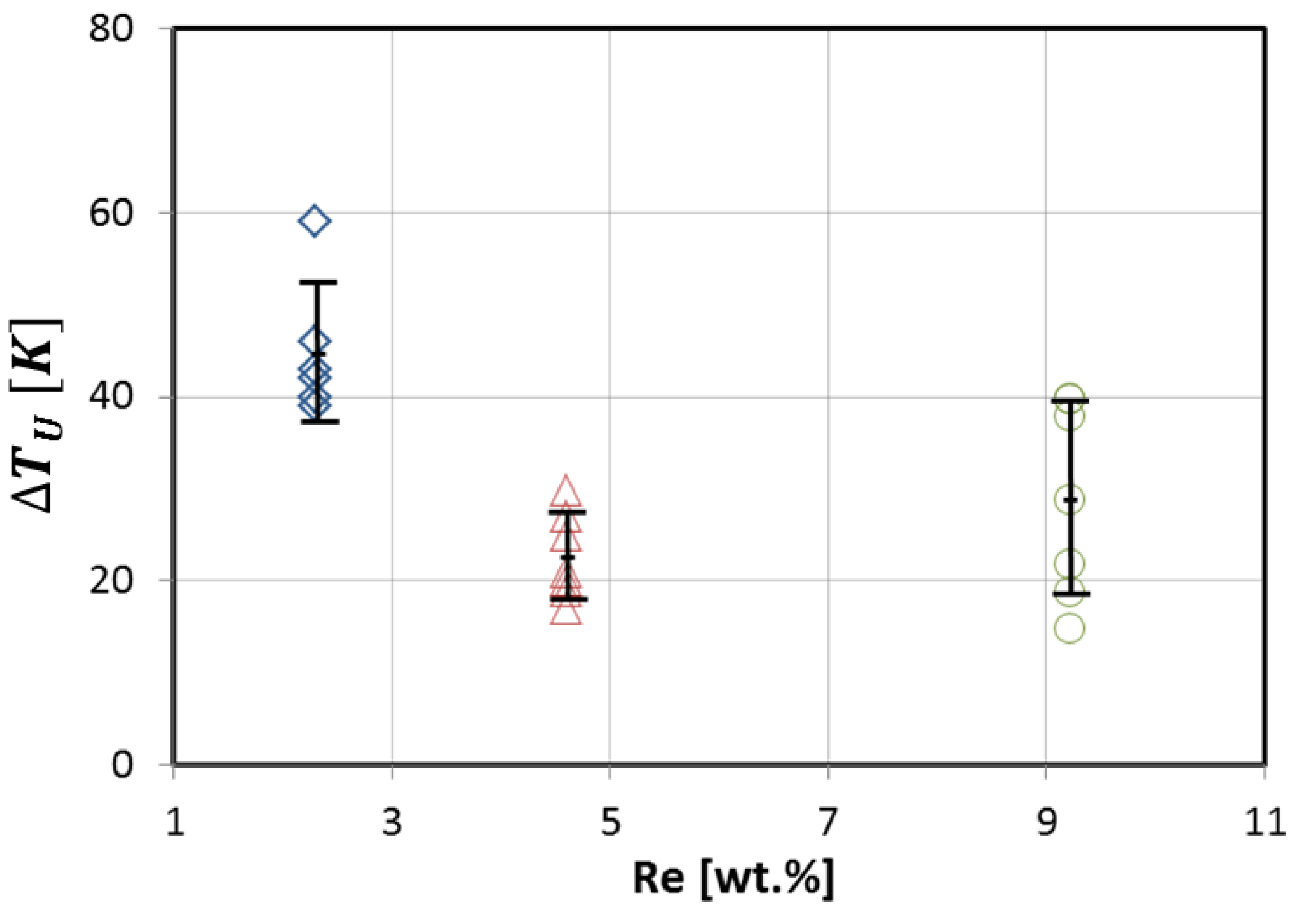

3.3. Experimental Results of the SX Castings
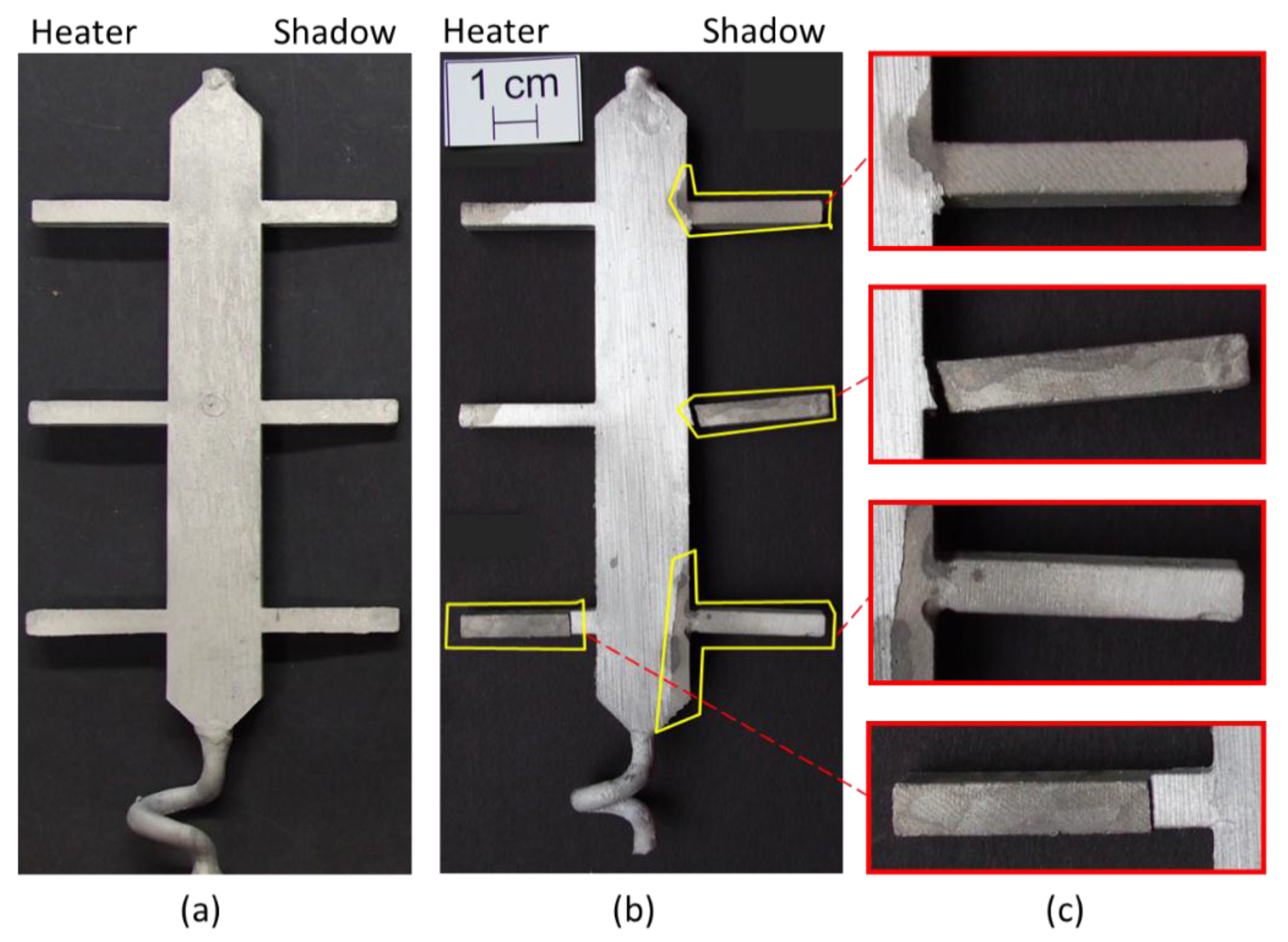
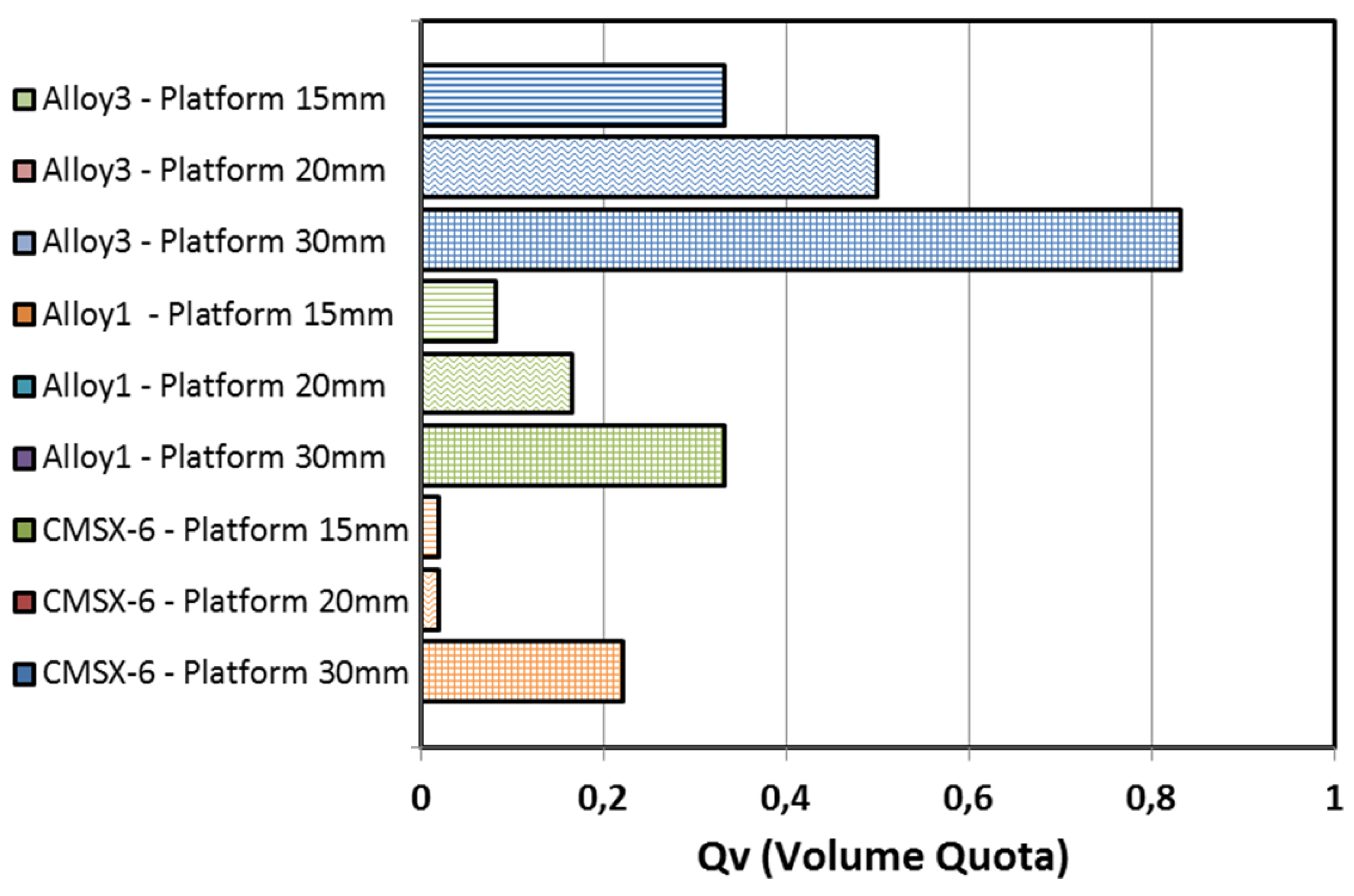
- •
- Alloying elements of the multicomponent alloys limit the atomic movement in the melt which leads to a hindrance in heterogeneous nucleation.
- •
- Superalloys have highly controlled melt treatments which include the removal of oxides, nitides and other impurites. During alloying CMSX-6 with the binary Ni9Re alloy, a chemical componet besides Nickel or Rhenium was inserted and lowered the critical undercoolability ΔTU considerably.
- •
- Rhenium is an alloy element in Ni-based alloys that decreases the critical undercoolability ΔTU.
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pollock, T.M.; Tin, S. Nickel-based superalloys for advanced turbine engines: Chemistry, microstructure and properties. J. Propul. Power 2006, 22, 361–374. [Google Scholar] [CrossRef]
- Caron, P.; Khan, T. Evolution of Ni-based superalloys for single crystal gas turbine blade applications. Aerosp. Sci. Technol. 1999, 3, 513–523. [Google Scholar] [CrossRef]
- Blavette, D.; Caron, P.; Khan, T. An atom probe investigation of the role of rhenium additions in improving creep resistance of Ni-base superalloys. Scr. Metall. 1986, 20, 1395–1400. [Google Scholar] [CrossRef]
- Giamei, A.; Anton, D. Rhenium additions to a Ni-base superalloy: Effects on microstructure. Metall. Trans. A 1985, 16, 1997–2005. [Google Scholar] [CrossRef]
- Hobbs, R.; Tin, S.; Rae, C. A castability model based on elemental solid-liquid partitioning in advanced nickel-base single-crystal superalloys. Metall. Mater. Trans. A 2005, 36, 2761–2773. [Google Scholar] [CrossRef]
- Ter Vehn, M.M.; Dedecke, D.; Paul, U.; Sahm, P. Undercooling related casting defects in single crystal turbine blades. Superalloys 1996. [Google Scholar] [CrossRef]
- Ma, D.; Wu, Q.; Hollad, S.; Bührig-Polaczek, A. Investigation on the asymmetry of thermal condition and grain defect formation in the customary directional solidification process. IOP Conf. Ser. Mater. Sci. Eng. 2012. [Google Scholar] [CrossRef]
- Eckler, K.; Norman, A.; Gärtner, F.; Greer, A.; Herlach, D. Microstructures of dilute Ni-C alloys obtained from undercooled droplets. J. Cryst. Growth 1997, 173, 528–540. [Google Scholar] [CrossRef]
- Li, J.F.; Liu, Y.C.; Lu, Y.L.; Yang, G.C.; Zhou, Y.H. Structural evolution of undercooled Ni-Cu alloys. J. Cryst. Growth 1998, 192, 462–470. [Google Scholar] [CrossRef]
- Li, J.F.; Jie, W.Q.; Yang, G.C.; Zhou, Y.H. Solidification structure formation in undercooled Fe-Ni alloy. Acta Mater. 2002, 50, 1797–1807. [Google Scholar] [CrossRef]
- Assadi, H.; Barth, M.; Greer, A.L.; Herlach, D.M. Kinetics of solidification of intermetallic compounds in the Ni-Al system. Acta Mater. 1998, 46, 491–500. [Google Scholar] [CrossRef]
- Herlach, D.M. Containerless undercooling and solidification of pure metals. Annu. Rev. Mater. Sci. 1991, 21, 23–44. [Google Scholar] [CrossRef]
- Willnecker, R.; Herlach, D.; Feuerbacher, B. Containerless undercooling of bulk Fe-Ni melts. Appl. Phys. Lett. 1986, 49, 1339–1341. [Google Scholar] [CrossRef]
- Gomersall, D.; Shiraishi, S.; Ward, R. Undercooling phenomena in liquid metal droplets. J. Aust. Inst. Met. 1965, 10, 220–222. [Google Scholar]
- Turnbull, D. Kinetics of solidification of supercooled liquid mercury droplets. J. Chem. Phys. 1952, 20, 411–424. [Google Scholar] [CrossRef]
- Liu, F.; Yang, G. Solidification of superalloy in a SiO2-ZrO2-B2O3 coating mould. J. Non-Cryst. Solids 2001, 290, 105–114. [Google Scholar] [CrossRef]
- Virieux, X.; Desmaison, J.; Labbe, J.; Gabriel, A. Interaction between two Ni-base alloys and oxide ceramics: SiO2, ZrO2, HfO2, Al2O3. Mater. Sci. Forum 1997, 251–254, 925–934. [Google Scholar] [CrossRef]
- Valenza, F.; Muolo, M.; Passerone, A. Wetting and interactions of Ni-and Co-based superalloys with different ceramic materials. J. Mater. Sci. 2010, 45, 2071–2079. [Google Scholar] [CrossRef]
- Ma, D.; Wu, Q.; Bührig-Polaczek, A. Undercoolability of superalloys and solidification defects in single crystal components. Adv. Mater. Res. 2011, 278, 417–422. [Google Scholar] [CrossRef]
- Okamoto, H. Ni-Re (nickel-rhenium). J. Ph. Equilib. Diffus. 2012, 33, 346. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogner, S.; Ivanova, E.; Müller, M.; Wang, F.; Ma, D.; Bührig-Polaczek, A. Investigation of the Undercoolability of Ni-Based Alloys Using High Temperature Thermal Analysis. Metals 2015, 5, 1971-1983. https://doi.org/10.3390/met5041971
Bogner S, Ivanova E, Müller M, Wang F, Ma D, Bührig-Polaczek A. Investigation of the Undercoolability of Ni-Based Alloys Using High Temperature Thermal Analysis. Metals. 2015; 5(4):1971-1983. https://doi.org/10.3390/met5041971
Chicago/Turabian StyleBogner, Samuel, Elvira Ivanova, Marcel Müller, Fu Wang, Dexin Ma, and Andreas Bührig-Polaczek. 2015. "Investigation of the Undercoolability of Ni-Based Alloys Using High Temperature Thermal Analysis" Metals 5, no. 4: 1971-1983. https://doi.org/10.3390/met5041971