Experimental and Numerical Studies on Self-Propagating High-Temperature Synthesis of Ta5Si3 Intermetallics
Abstract
:1. Introduction
2. Experimental and Numerical Methods
3. Results and Discussion
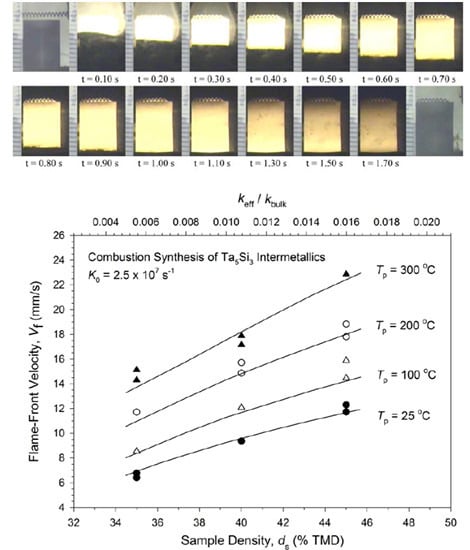
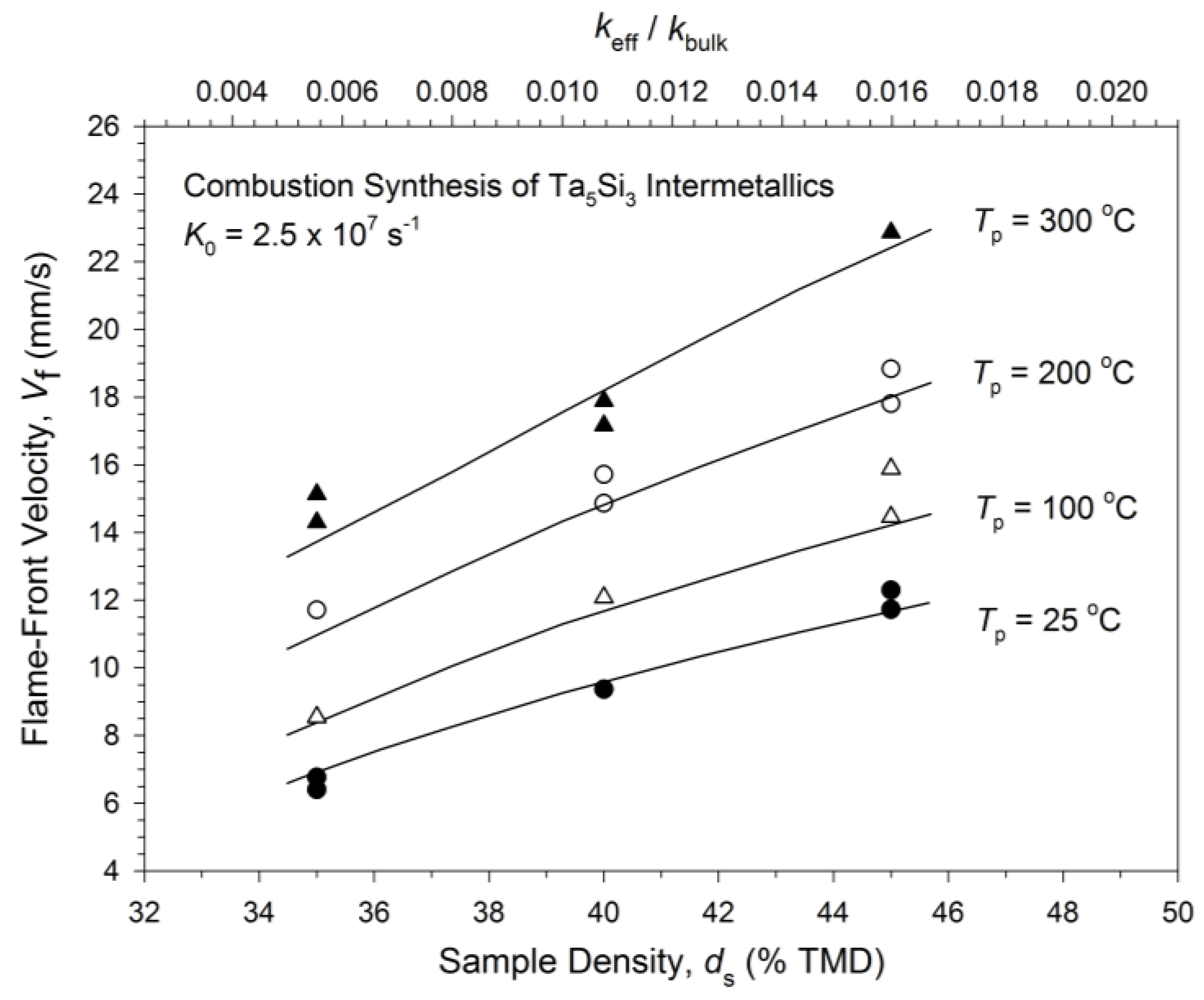
3.1. Experimental Measurement and Analysis
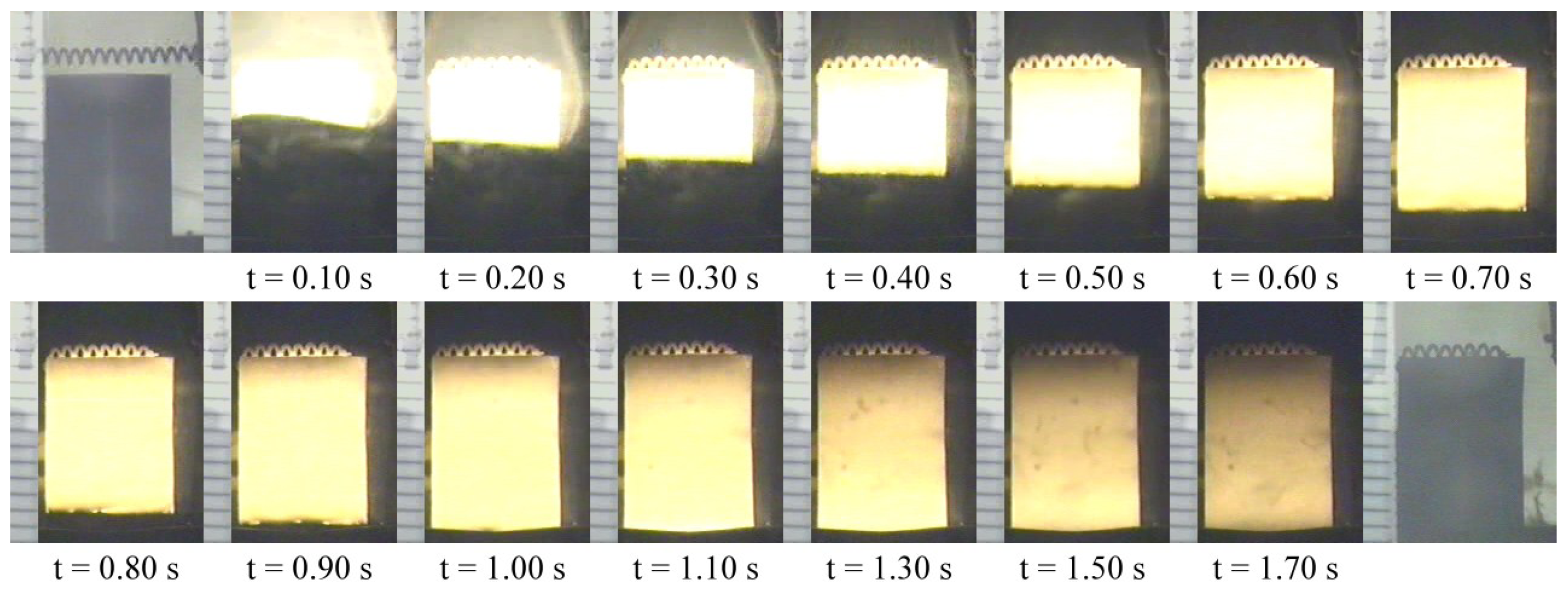

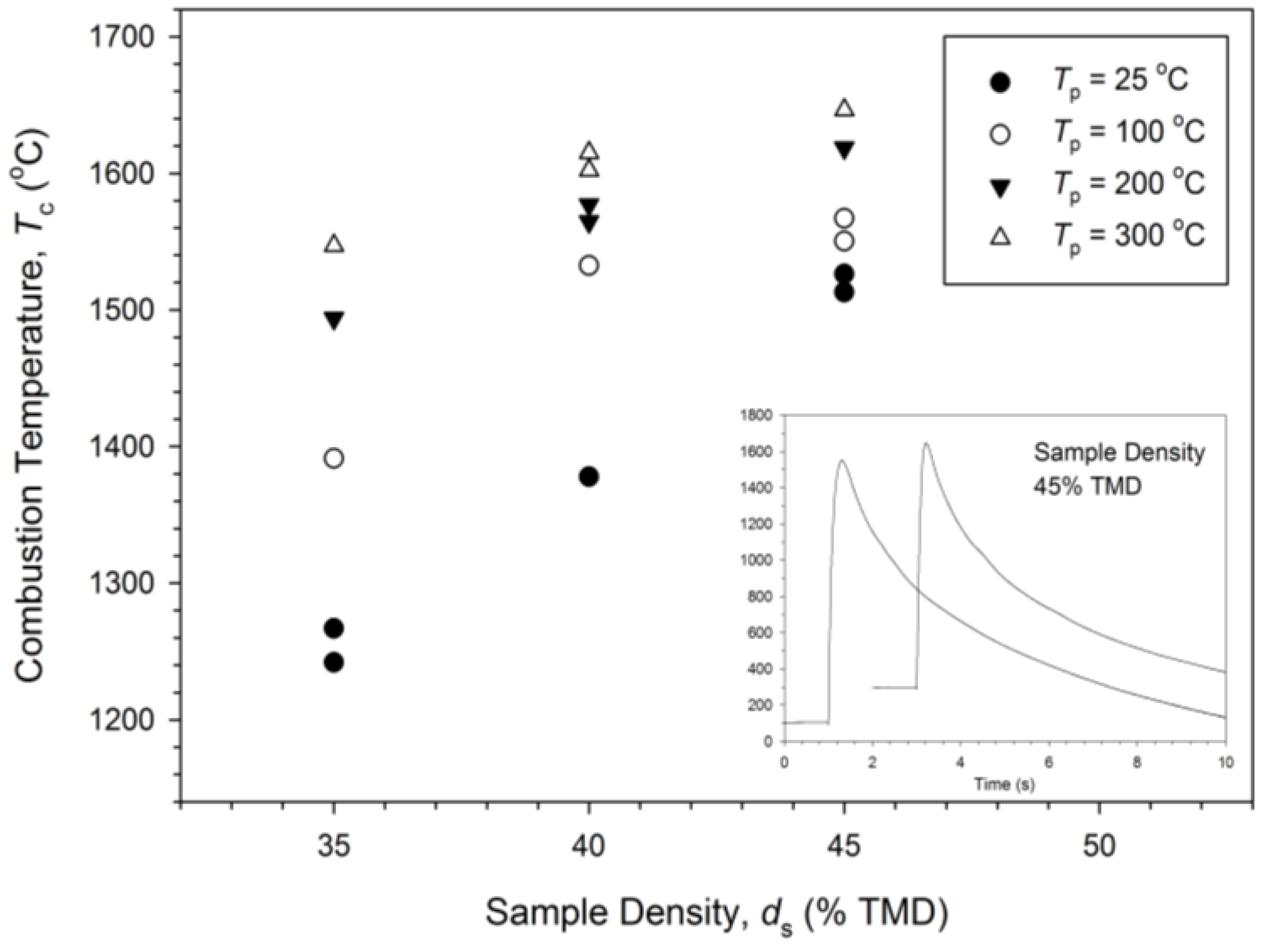

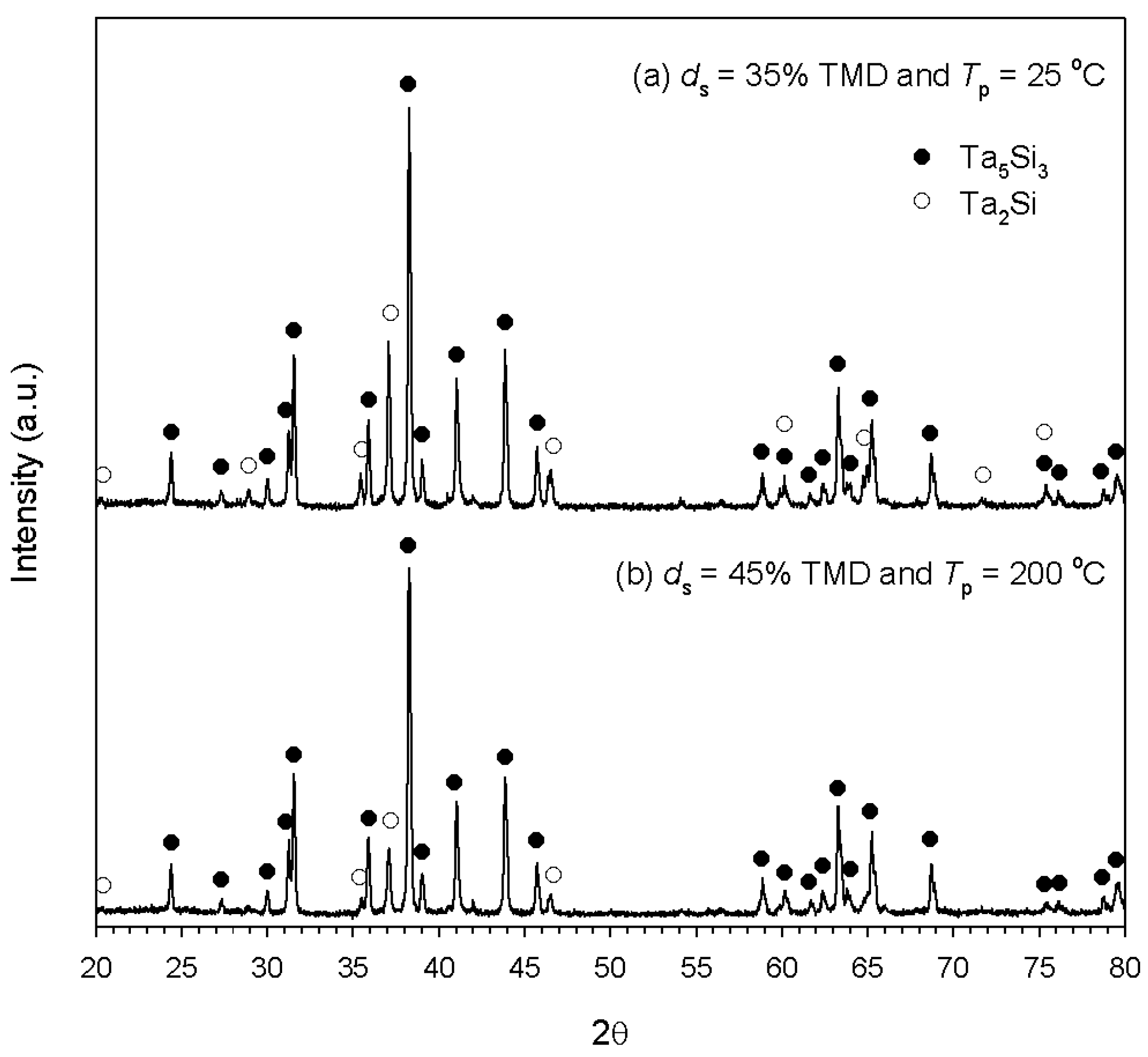
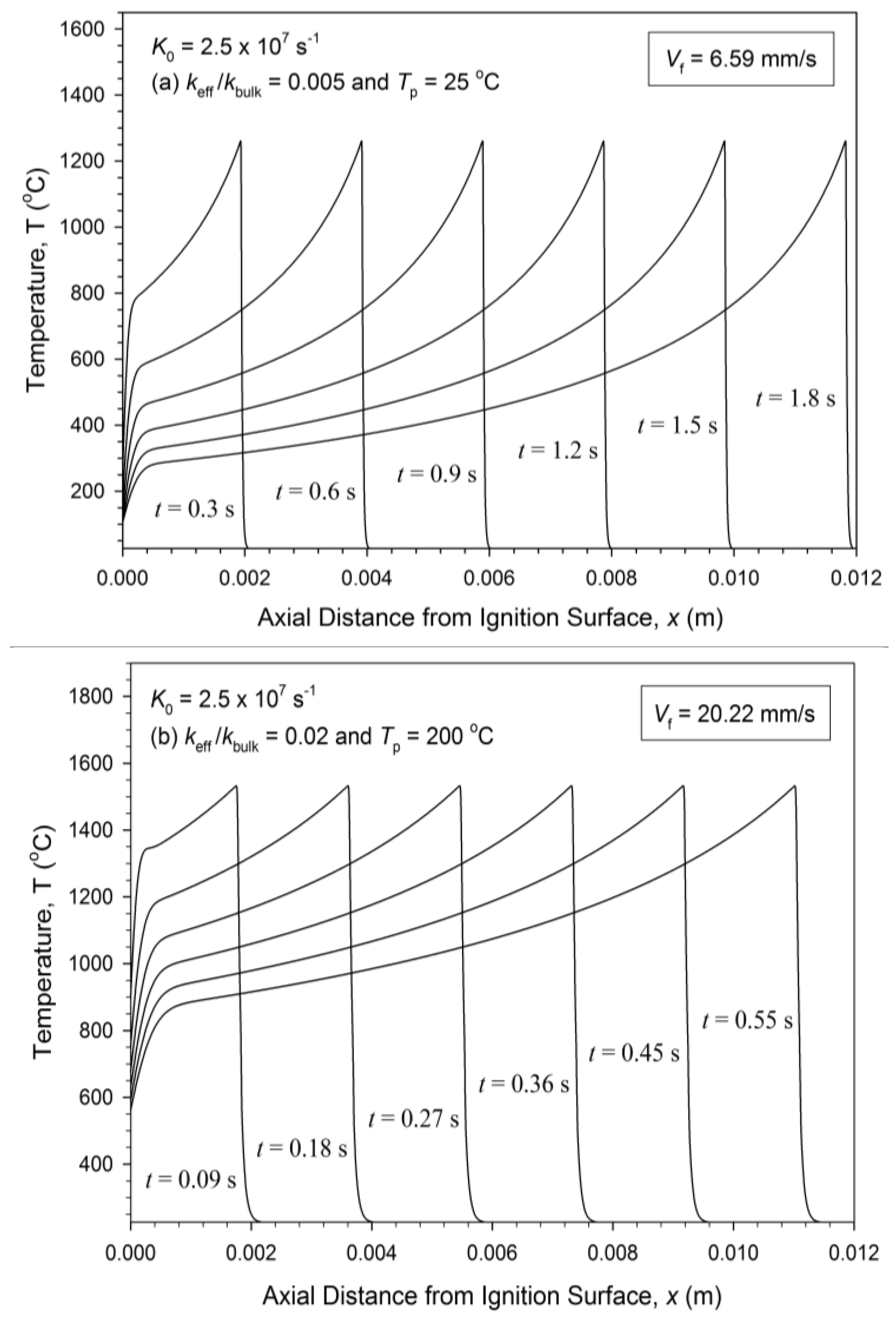
3.2. Numerical Simulation and Validation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Meschter, P.J.; Schwartz, D.S. Silicide-matrix materials for high temperature applications. JOM 1989, 41, 52–55. [Google Scholar] [CrossRef]
- Petrovic, J.J.; Vasudevan, A.K. Key developments in high temperature structural silicides. Mater. Sci. Eng. A 1999, 261, 1–5. [Google Scholar] [CrossRef]
- Tillard, M. The mixed intermetallic silicide Nb5−xTaxSi3 (0 ≤ x ≤ 5). Crystal and electronic structure. J. Alloy. Compd. 2014, 584, 385–392. [Google Scholar] [CrossRef]
- Schlesinger, M.E. Thermodynamics of solid transition-metal silicides. Chem. Rev. 1990, 90, 607–628. [Google Scholar] [CrossRef]
- Stoloff, N.S. An overview of powder processing of silicides and their composites. Mater. Sci. Eng. A 1999, 261, 169–180. [Google Scholar] [CrossRef]
- Liu, G.; Li, J.; Chen, K. Combustion synthesis of refractory and hard materials: A review. Int. J. Refract. Met. Hard Mater. 2013, 39, 90–102. [Google Scholar] [CrossRef]
- Gromov, A.A.; Chukhlomina, L.N. Nitride Ceramics: Combustion Synthesis, Properties, and Applications; Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- Vicario, I.; Poulon-Quintin, A.; Lagos, M.A.; Silvain, J.F. Effect of material and process atmosphere in the preparation of Al–Ti–B grain refiner by SHS. Metals 2015, 5, 1387–1396. [Google Scholar] [CrossRef] [Green Version]
- Yeh, C.L.; Chen, W.H. Combustion synthesis of MoSi2 and MoSi2–Mo5Si3 composites. J. Alloy. Compd. 2007, 438, 165–170. [Google Scholar] [CrossRef]
- Yeh, C.L.; Chen, W.H.; Hsu, C.C. Formation of titanium silicides Ti5Si3 and TiSi2 by self-propagating combustion synthesis. J. Alloy. Compd. 2007, 432, 90–95. [Google Scholar] [CrossRef]
- Bertolino, N.; Anselmi-Tamburini, U.; Maglia, F.; Spinolo, G.; Munir, Z.A. Combustion synthesis of Zr–Si intermetallic compounds. J. Alloy. Compd. 1999, 288, 238–248. [Google Scholar] [CrossRef]
- Yeh, C.L.; Chen, W.H. Preparation of Nb5Si3 intermetallic and Nb5Si3/Nb composite by self-propagating high-temperature synthesis. J. Alloy. Compd. 2005, 402, 118–123. [Google Scholar] [CrossRef]
- Maglia, F.; Anselmi-Tamburini, U.; Milanese, C.; Bertolino, N.; Munir, Z.A. Field activated combustion synthesis of the silicides of vanadium. J. Alloy. Compd. 2001, 319, 108–118. [Google Scholar] [CrossRef]
- Maglia, F.; Anselmi-Tamburini, U.; Bertolino, N.; Milanese, C.; Munir, Z.A. Field-activated combustion synthesis of Ta–Si intermetallic compounds. J. Mater. Res. 2001, 16, 534–544. [Google Scholar] [CrossRef]
- Gras, C.; Gaffet, E.; Bernard, F. Combustion wave structure during the MoSi2 synthesis by mechanically-activated self-propagating high-temperature synthesis (MASHS): In situ time-resolved investigations. Intermetallics 2006, 14, 521–529. [Google Scholar] [CrossRef]
- Maglia, F.; Milanese, C.; Anselmi-Tamburini, U.; Doppiu, S.; Cocco, G.; Munir, Z.A. Combustion synthesis of mechanically activated powders in the Ta–Si system. J. Alloy. Compd. 2004, 385, 269–275. [Google Scholar] [CrossRef]
- Li, H.P. The numerical simulation of the heterogeneous composition effect on the combustion synthesis of TiB2 compound. Acta Mater. 2003, 51, 3213–3224. [Google Scholar] [CrossRef]
- Li, H.P. An investigation of the ignition manner effects on combustion synthesis. Mater. Chem. Phys. 2003, 80, 758–767. [Google Scholar] [CrossRef]
- Yeh, C.L.; Hwang, P.W.; Chen, W.K.; Li, J.Y. Modeling evaluation of Arrhenius factor and thermal conductivity for combustion synthesis of transition metal aluminides. Intermetallics 2013, 39, 20–24. [Google Scholar] [CrossRef]
- Gennari, S.; Maglia, F.; Anselmi-Tamburini, U.; Spinolo, G. SHS (Self-sustained high-temperature synthesis) of intermetallic compounds: Effect of process parameters by computer simulation. Intermetallics 2003, 11, 1355–1359. [Google Scholar] [CrossRef]
- Gennari, S.; Anselmi-Tamburini, U.; Maglia, F.; Spinolo, G.; Munir, Z.A. A new approach of the modeling of SHS reactions: Combustion synthesis of transition metal aluminides. Acta Mater. 2006, 54, 2343–2351. [Google Scholar] [CrossRef]
- Yeh, C.L.; Lin, J.Z. Combustion synthesis of Cr–Al and Cr–Si intermetallics with Al2O3 additions from Cr2O3–Al and Cr2O3–Al–Si reaction systems. Intermetallics 2013, 33, 126–133. [Google Scholar] [CrossRef]
- Miura, S.; Terada, Y.; Suzuki, T.; Liu, C.T.; Mishima, Y. Thermal conductivity of Ni–Al powder compacts for reaction synthesis. Intermetallics 2000, 8, 151–155. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeh, C.-L.; Chou, C.-C.; Hwang, P.-W. Experimental and Numerical Studies on Self-Propagating High-Temperature Synthesis of Ta5Si3 Intermetallics. Metals 2015, 5, 1580-1590. https://doi.org/10.3390/met5031580
Yeh C-L, Chou C-C, Hwang P-W. Experimental and Numerical Studies on Self-Propagating High-Temperature Synthesis of Ta5Si3 Intermetallics. Metals. 2015; 5(3):1580-1590. https://doi.org/10.3390/met5031580
Chicago/Turabian StyleYeh, Chun-Liang, Chi-Chian Chou, and Po-Wen Hwang. 2015. "Experimental and Numerical Studies on Self-Propagating High-Temperature Synthesis of Ta5Si3 Intermetallics" Metals 5, no. 3: 1580-1590. https://doi.org/10.3390/met5031580