Stress-Corrosion Interactions in Zr-Based Bulk Metallic Glasses
Abstract
:1. Introduction
2. Mechanical Properties at Room Temperature
Intrinsic Plasticity and Toughness of Metallic Glasses
3. Corrosion Aspects
3.1. Pitting at Local Surface Defects
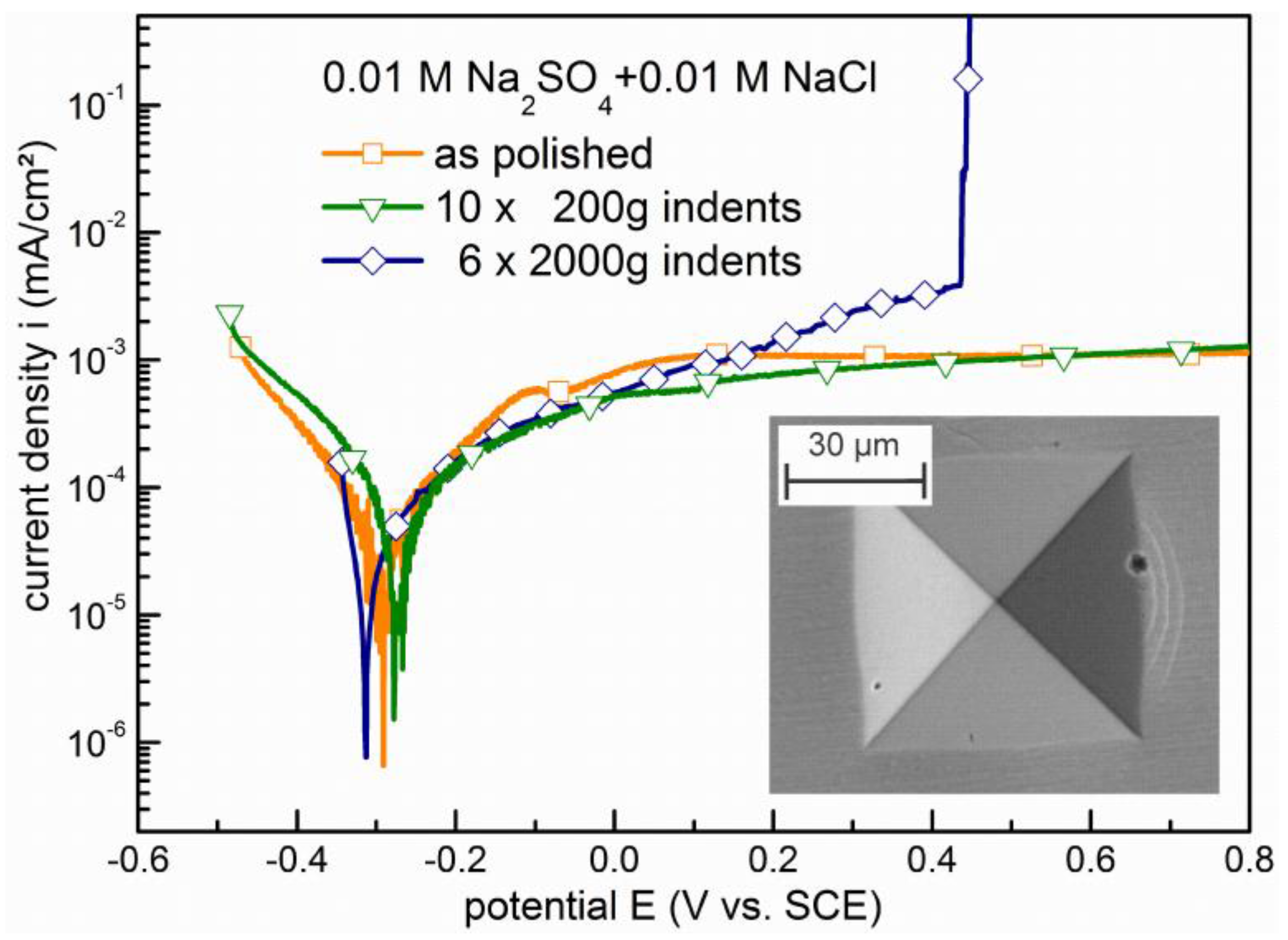

3.2. Pitting at Shear Steps and Shear Bands
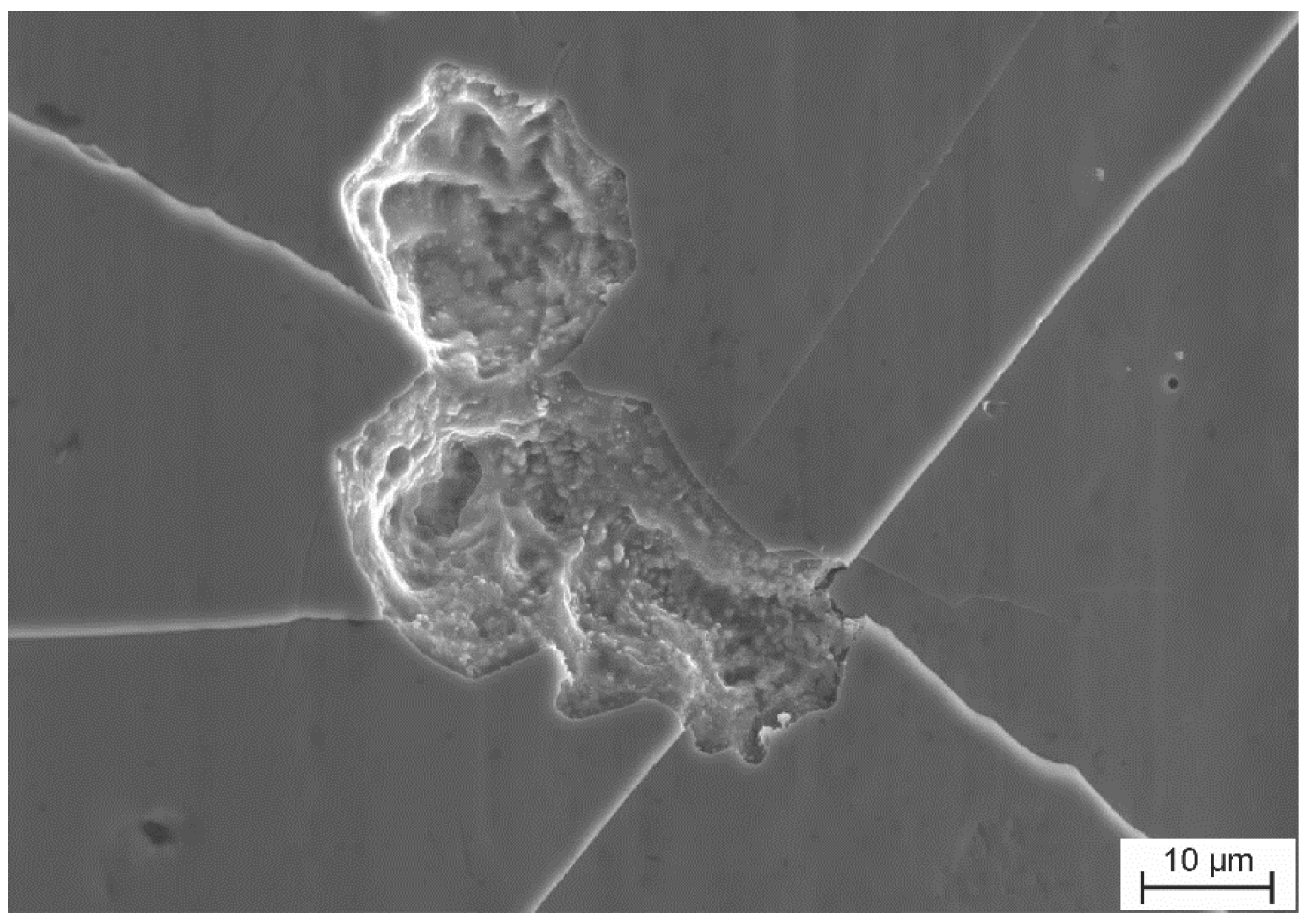
4. Environmentally Induced Cracking
4.1. Hydrogen Effects
4.2. Chloride-Induced Stress Corrosion Cracking
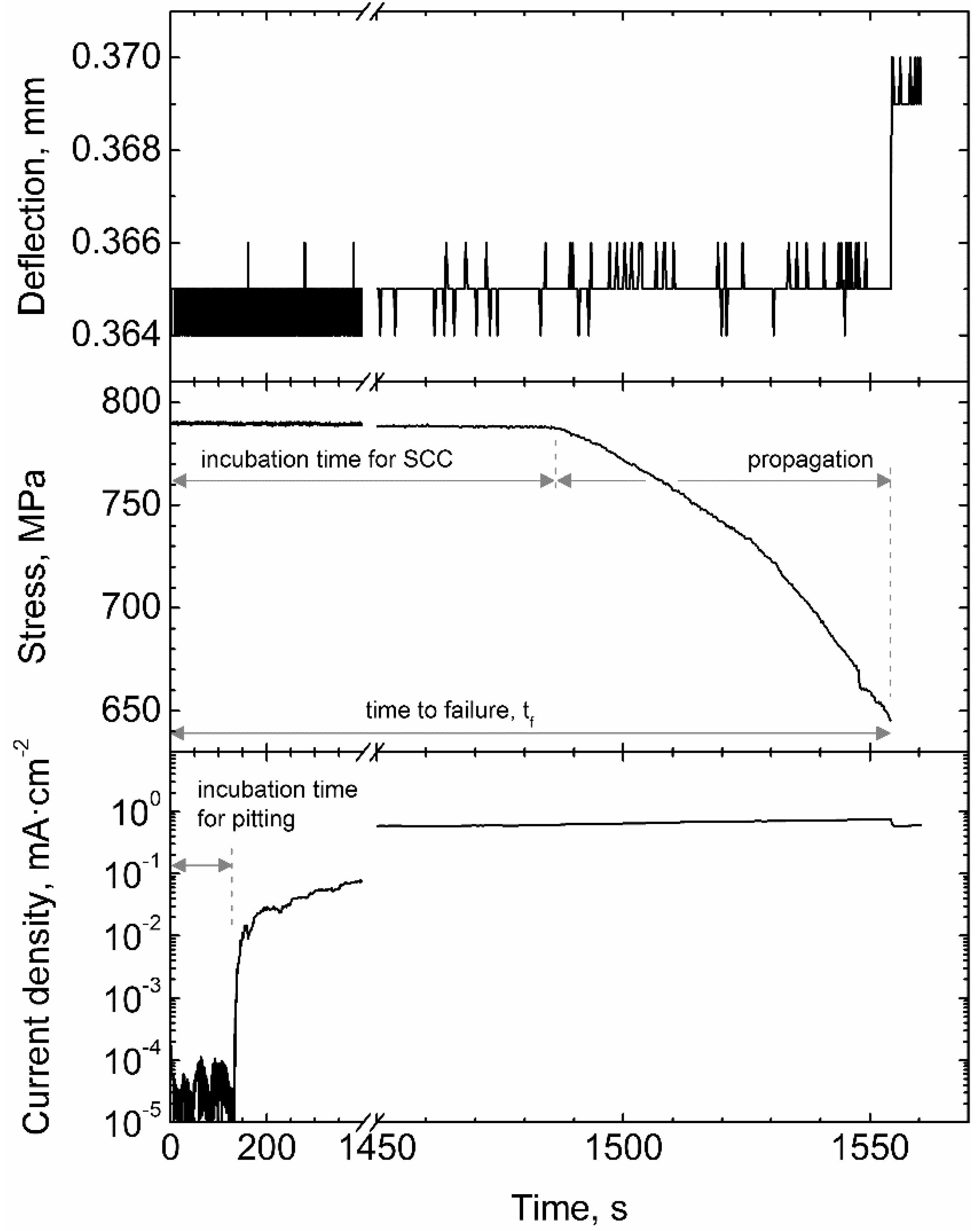

4.3. Corrosion Fatigue
5. Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Suryanarayana, C.; Inoue, A. Bulk Metallic Glasses; CRC Press: Boca Raton, FL, USA, 2011; p. 548. [Google Scholar]
- Wondraczek, L.; Mauro, J.C.; Eckert, J.; Kühn, U.; Horbach, J.; Deubener, J.; Rouxel, T. Towards ultrastrong glasses. Adv. Mater. 2011, 23, 4578–4586. [Google Scholar] [CrossRef] [PubMed]
- Trexler, M.M.; Thadhani, N.N. Mechanical properties of bulk metallic glasses. Prog. Mater. Sci. 2010, 55, 759–839. [Google Scholar] [CrossRef]
- Cheng, Y.Q.; Cao, A.J.; Sheng, H.W.; Ma, E. Local order influences initiation of plastic flow in metallic glass: Effects of alloy composition and sample cooling history. Acta Mater. 2008, 56, 5263–5275. [Google Scholar] [CrossRef]
- Eckert, J.; Das, J.; Pauly, S.; Duhamel, C. Processing routes, microstructure and mechanical properties of metallic glasses and their composites. Adv. Eng. Mater. 2007, 9, 443–453. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, W.H.; Greer, A.L. Making metallic glasses plastic by control of residual stress. Nat. Mater. 2006, 5, 857–860. [Google Scholar] [CrossRef] [PubMed]
- Song, K.K.; Pauly, S.; Zhang, Y.; Scudino, S.; Gargarella, P.; Surreddi, K.B.; Kühn, U.; Eckert, J. Significant tensile ductility induced by cold rolling in Cu47.5Zr47.5Al5 bulk metallic glass. Intermetallics 2011, 19, 1394–1398. [Google Scholar] [CrossRef]
- Schuh, C.A.; Hufnagel, T.C.; Ramamurty, U. Mechanical behavior of amorphous alloys. Acta Mater. 2007, 55, 4067–4109. [Google Scholar] [CrossRef]
- Kruzic, J.J. Understanding the Problem of Fatigue in Bulk Metallic Glasses. Metall. Mater. Trans. A 2011, 42, 1516–1523. [Google Scholar] [CrossRef]
- Scully, J.R.; Gebert, A.; Payer, J. Corrosion and related mechanical properties of bulk metallic glasses. J. Mater. Res. 2007, 22, 302–313. [Google Scholar] [CrossRef]
- Bankmann, J.; Pundt, A.; Kirchheim, R. Hydrogen loading behaviour of multi-component amorphous alloys: Model and experiment. J. Alloy. Compd. 2003, 356–357, 566–569. [Google Scholar] [CrossRef]
- Archer, M.D.; Corke, C.C.; Harji, B.H. The Electrochemical Properties of Metallic Glasses. Electrochim. Acta 1987, 32, 13–26. [Google Scholar] [CrossRef]
- Scully, J.R.; Lucente, A. Corrosion of Amorphous Metals. In ASM Handbook Volume 13B, Corrosion: Materials; Cramer, S.D., Covino, B.S., Jr., Eds.; ASM International: Materials Park, OH, USA, 2005; Volume 13B, pp. 476–489. [Google Scholar]
- Hashimoto, K. Chemical properties. In Amorphous Metallic Alloys; Luborsky, F.E., Ed.; Butterworths: London, UK, 1983; pp. 471–486. [Google Scholar]
- Waseda, Y.; Aust, K.T. Corrosion behaviour of metallic glasses. J. Mater. Sci. 1981, 16, 2337–2359. [Google Scholar] [CrossRef]
- Wilde, G.; Rosner, H. Nanocrystallization in a shear band: An in situ investigation. Appl. Phys. Lett. 2011. [Google Scholar] [CrossRef]
- Wang, L.; Bei, H.; Gao, Y.F.; Lu, Z.P.; Nieh, T.G. Effect of residual stresses on the hardness of bulk metallic glasses. Acta Mater. 2011, 59, 2858–2864. [Google Scholar] [CrossRef]
- Zhao, J.X.; Wu, F.F.; Zhang, Z.F. Analysis on shear deformation mechanism of metallic glass under confined bending test. Mater. Sci. Eng. A 2010, 527, 6224–6229. [Google Scholar] [CrossRef]
- Scudino, S.; Jerliu, B.; Pauly, S.; Surreddi, K.B.; Kühn, U.; Eckert, J. Ductile bulk metallic glasses produced through designed heterogeneities. Scr. Mater. 2011, 65, 815–818. [Google Scholar] [CrossRef]
- Newman, R.C. Stress-Corrosion Cracking Mechanisms. In Corrosion Mechanisms in Theory and Practice, 3rd ed.; Marcus, P., Ed.; CRC Press: Boca Raton, FL, USA, 2011; pp. 499–544. [Google Scholar]
- Clarke, D.R.; Faber, K.T. Fracture of ceramics and glasses. J. Phys. Chem. Solids 1987, 48, 1115–1157. [Google Scholar] [CrossRef]
- Lewandowski, J.J.; Wang, W.H.; Greer, A.L. Intrinsic plasticity or brittleness of metallic glasses. Philos. Mag. Lett. 2005, 85, 77–87. [Google Scholar] [CrossRef]
- Gebert, A.; Gostin, P.F.; Schultz, L. Effect of surface finishing of a Zr-based bulk metallic glass on its corrosion behaviour. Corros. Sci. 2010, 52, 1711–1720. [Google Scholar] [CrossRef]
- Huang, L.; Yokoyama, Y.; Wu, W.; Liaw, P.K.; Pang, S.J.; Inoue, A.; Zhang, T.; He, W. Ni-free Zr–Cu–Al–Nb–Pd bulk metallic glasses with different Zr/Cu ratios for biomedical applications. J. Biomed. Mater. Res. B 2012, 100B, 1472–1482. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.B.; Zhang, L.C.; Gebert, A.; Schultz, L. Pitting corrosion of Cu–Zr metallic glasses in hydrochloric acid solutions. J. Alloys Compd. 2008, 462, 60–67. [Google Scholar] [CrossRef]
- Pang, S.J.; Zhang, T.; Kimura, H.; Asami, K.; Inoue, A. Corrosion Behavior of Zr–(Nb–)Al–Ni–Cu Glassy Alloys. Mater. Trans. JIM 2000, 41, 1490–1494. [Google Scholar] [CrossRef]
- Pang, S.J.; Zhang, T.; Asami, K.; Inoue, A. Formation, corrosion behavior, and mechanical properties of bulk glassy Zr–Al–Co–Nb alloys. J. Mater. Res. 2003, 18, 1652–1658. [Google Scholar] [CrossRef]
- Raju, V.R.; Kühn, U.; Wolff, U.; Schneider, F.; Eckert, J.; Reiche, R.; Gebert, A. Corrosion behaviour of Zr-based bulk glass-forming alloys containing Nb or Ti. Mater. Lett. 2002, 57, 173–177. [Google Scholar] [CrossRef]
- Li, Y.H.; Zhang, W.; Dong, C.; Qiang, J.B.; Fukuhara, M.; Makino, A.; Inoue, A. Effects of Ni addition on the glass-forming ability, mechanical properties and corrosion resistance of Zr–Cu–Al bulk metallic glasses. Mater. Sci. Eng. A 2011, 528, 8551–8556. [Google Scholar] [CrossRef]
- Gostin, P.F.; Eigel, D.; Grell, D.; Eckert, J.; Kerscher, E.; Gebert, A. Comparing the pitting corrosion behavior of prominent Zr-based bulk metallic glasses. J. Mater. Res. 2015, 30, 233–241. [Google Scholar] [CrossRef]
- Gebert, A.; Buchholz, K.; Leonhard, A.; Mummert, K.; Eckert, J.; Schultz, L. Investigations on the electrochemical behaviour of Zr-based bulk metallic glasses. Mater. Sci. Eng. A 1999, 267, 294–300. [Google Scholar] [CrossRef]
- Paillier, J.; Mickel, C.; Gostin, P.F.; Gebert, A. Characterization of corrosion phenomena of Zr–Ti–Cu–Al–Ni metallic glass by SEM and TEM. Mater. Charact. 2010, 61, 1000–1008. [Google Scholar] [CrossRef]
- Jones, R.H. Stress-Corrosion Cracking. In ASM Handbook Volume 13A, Corrosion: Fundamentals, Testing and Protection; Cramer, S.D., Covino, B.S., Jr., Eds.; ASM International: Materials Park, OH, USA, 2003; Volume 13A, pp. 346–366. [Google Scholar]
- Gebert, A.; Gostin, P.F.; Uhlemann, M.; Eckert, J.; Schultz, L. Interactions between mechanically generated defects and corrosion phenomena of Zr-based bulk metallic glasses. Acta Mater. 2012, 60, 2300–2309. [Google Scholar] [CrossRef]
- Mear, F.O.; Vaughan, G.; Yavari, A.R.; Greer, A.L. Residual-stress distribution in shot-peened metallic-glass plate. Philos. Mag. Lett. 2008, 88, 757–766. [Google Scholar] [CrossRef]
- Gebert, A.; Concustell, A.; Greer, A.L.; Schultz, L.; Eckert, J. Effect of shot-peening on the corrosion resistance of a Zr-based bulk metallic glass. Scr. Mater. 2010, 62, 635–638. [Google Scholar] [CrossRef]
- Pampillo, C.A.; Chen, H.S. Comprehensive Plastic-Deformation of a Bulk Metallic Glass. Mater. Sci. Eng. 1974, 13, 181–188. [Google Scholar] [CrossRef]
- Guo, H.; Wen, J.; Xiao, N.M.; Zhang, Z.F.; Sui, M.L. The more shearing, the thicker shear band and heat-affected zone in bulk metallic glass. J. Mater. Res. 2008, 23, 2133–2138. [Google Scholar] [CrossRef]
- Greer, A.L.; Cheng, Y.Q.; Ma, E. Shear bands in metallic glasses. Mat. Sci. Eng. R 2013, 74, 71–132. [Google Scholar] [CrossRef]
- Gutman, E.M. Mechanochemistry of Materials; Cambridge International Science Publishing: Cambridge, UK, 1998. [Google Scholar]
- Nie, X.P.; Cao, Q.P.; Wu, Z.F.; Ma, Y.; Wang, X.D.; Ding, S.Q.; Jiang, J.Z. The pitting corrosion behavior of shear bands in a Zr-based bulk metallic glass. Scr. Mater. 2012, 67, 376–379. [Google Scholar] [CrossRef]
- Wang, Y.M.; Zhang, C.; Liu, Y.; Chan, K.C.; Liu, L. Why does pitting preferentially occur on shear bands in bulk metallic glasses? Intermetallics 2013, 42, 107–111. [Google Scholar] [CrossRef]
- Grell, D.; Gostin, P.F.; Eckert, J.; Gebert, A.; Kerscher, E. In situ electrochemical analysis during deformation of a Zr-based bulk metallic glass: A sensitive tool revealing early shear banding. Adv. Eng. Mater. 2015. accepted. [Google Scholar]
- Suh, D.; Dauskardt, R.H. Hydrogen effects on the mechanical and fracture behavior of a Zr–Ti–Ni–Cu–Be bulk metallic glass. Scr. Mater. 2000, 42, 233–240. [Google Scholar] [CrossRef]
- Suh, D.; Dauskardt, R.H. The effects of hydrogen on deformation and fracture of a Zr–Ti–Ni–Cu–Be bulk metallic glass. Mater. Sci. Eng. A 2001, 319, 480–483. [Google Scholar] [CrossRef]
- Suh, D.; Dauskardt, R.H. Effects of pre-charged hydrogen on the mechanical and thermal behavior of Zr–Ti–Ni–Cu–Be bulk metallic glass alloys. Mater. Trans. 2001, 42, 638–641. [Google Scholar] [CrossRef]
- Daewoong, S.; Asoka-Kumar, P.; Dauskardt, R.H. The effects of hydrogen on viscoelastic relaxation in Zr–Ti–Ni–Cu–Be bulk metallic glasses: Implications for hydrogen embrittlement. Acta Mater. 2002, 50, 537–551. [Google Scholar]
- Shan, G.B.; Wang, Y.W.; Chu, W.Y.; Li, J.X.; Hui, X.D. Hydrogen damage and delayed fracture in bulk metallic glass. Corros. Sci. 2005, 47, 2731–2739. [Google Scholar] [CrossRef]
- Shan, G.B.; Wei, B.C.; Li, J.X.; Qiao, L.J.; Chu, W.Y. Effects of hydrogen on shear bands and cracking in Zr base bulk amorphous alloy. Acta Metall. Sin. 2006, 42, 689–693. [Google Scholar]
- Jayalakshmi, S.; Kim, K.B.; Fleury, E. Effect of hydrogenation on the structural, thermal and mechanical properties of Zr50Ni27Nb18Co5 amorphous alloy. J. Alloy. Compd. 2006, 417, 195–202. [Google Scholar] [CrossRef]
- Shan, G.B.; Li, J.X.; Yang, Y.Z.; Qiao, L.J.; Chu, W.Y. Hydrogen-enhanced plastic deformation during indentation for bulk metallic glass of Zr65Al7.5Ni10Cu17.5. Mater. Lett. 2007, 61, 1625–1628. [Google Scholar] [CrossRef]
- Dong, F.Y.; Su, Y.Q.; Luo, L.S.; Wang, L.; Wang, S.J.; Guo, L.J.; Fu, H.Z. Enhanced plasticity in Zr-based bulk metallic glasses by hydrogen. Int. J. Hydrog. Energy 2012, 37, 14697–14701. [Google Scholar] [CrossRef]
- Ritchie, R.O.; Schroeder, V.; Gilbert, C.J. Fracture, fatigue and environmentally-assisted failure of a Zr-based bulk amorphous metal. Intermetallics 2000, 8, 469–475. [Google Scholar] [CrossRef]
- Schroeder, V.; Gilbert, C.J.; Ritchie, R.O. A comparison of the mechanisms of fatigue-crack propagation behavior in a Zr-based bulk amorphous metal in air and an aqueous chloride solution. Mater. Sci. Eng. A 2001, 317, 145–152. [Google Scholar] [CrossRef]
- Schroeder, V.; Gilbert, C.J.; Ritchie, R.O. Effect of aqueous environment on fatigue-crack propagation behavior in a Zr-based bulk amorphous metal. Scr. Mater. 1999, 40, 1057–1061. [Google Scholar] [CrossRef]
- Schroeder, V.; Ritchie, R.O. Stress-corrosion fatigue-crack growth in a Zr-based bulk amorphous metal. Acta Mater. 2006, 54, 1785–1794. [Google Scholar] [CrossRef]
- Morrison, M.L.; Buchanan, R.A.; Liaw, P.K.; Green, B.A.; Wang, G.Y.; Liu, C.T.; Horton, J.A. Corrosion-fatigue studies of the Zr-based Vitreloy 105 bulk metallic glass. Mater. Sci. Eng. A 2007, 467, 198–206. [Google Scholar] [CrossRef]
- Nakai, Y.; Yoshioka, Y. Stress Corrosion and Corrosion Fatigue Crack Growth of Zr-Based Bulk Metallic Glass in Aqueous Solutions. Metall. Mater. Trans. A 2010, 41A, 1792–1798. [Google Scholar] [CrossRef]
- Wiest, A.; Wang, G.Y.; Huang, L.; Roberts, S.; Demetriou, M.D.; Liaw, P.K.; Johnson, W.L. Corrosion and corrosion fatigue of Vitreloy glasses containing low fractions of late transition metals. Scr. Mater. 2010, 62, 540–543. [Google Scholar] [CrossRef]
- Huang, L.; Wang, G.Y.; Qiao, D.C.; Liaw, P.K.; Pang, S.J.; Wang, J.F.; Zhang, T. Corrosion-fatigue study of a Zr-based bulk-metallic glass in a physiologically relevant environment. J. Alloy. Compd. 2010, 504, S159–S162. [Google Scholar] [CrossRef]
- Gostin, P.F.; Eigel, D.; Grell, D.; Uhlemann, M.; Kerscher, E.; Eckert, J.; Gebert, A. Stress corrosion cracking of a Zr-based bulk metallic glass. Mater. Sci. Eng. A 2015, 639, 681–690. [Google Scholar] [CrossRef]
- Craig, B. Introduction to Environmentally Induced Cracking. In ASM Handbook; Cramer, S.D., Covino, B.S.J., Eds.; ASM International: Materials Park, OH, USA, 2003; Volume 13A, p. 345. [Google Scholar]
- Mattern, N.; Gebert, A. Hydrogenation of Zr60Ti2Cu20Al10Ni8 bulk metallic glass. Appl. Phys. Lett. 2003, 83, 1134–1135. [Google Scholar] [CrossRef]
- Mizubayashi, H.; Shibasaki, M.; Murayama, S. Local strain around hydrogen in amorphous Cu50Zr50 and Cu50Ti50. Acta Mater. 1999, 47, 3331–3338. [Google Scholar] [CrossRef]
- Hasegawa, M.; Kotani, K.; Yamaura, S.; Kato, H.; Kodama, I.; Inoue, A. Hydrogen-induced internal friction of Zr-based bulk glassy alloys in a rod shape above 90 K. J. Alloys Compd. 2004, 365, 221–227. [Google Scholar] [CrossRef]
- Rodmacq, B.; Maret, M.; Laugier, J.; Billard, L.; Chamberod, A. Hydrogen-Induced Phase-Separation in Amorphous Cu0.5Ti0.5 Alloys. 1. Room-Temperature Experiments. Phys. Rev. B 1988, 38, 1105–1115. [Google Scholar] [CrossRef]
- Ismail, N.; Gebert, A.; Uhlemann, M.; Eckert, J.; Schultz, L. Effect of hydrogen on Zr65Cu17.5Al7.5Ni10 metallic glass. J. Alloys Compd. 2001, 314, 170–176. [Google Scholar] [CrossRef]
- Ismail, N.; Uhlemann, M.; Gebert, A.; Eckert, J. Hydrogenation and its effect on the crystallisation behaviour of Zr55Cu30Al10Ni5 metallic glass. J. Alloys Compd. 2000, 298, 146–152. [Google Scholar] [CrossRef]
- Yoo, B.G.; Oh, J.H.; Kim, Y.J.; Jang, J.I. Effect of hydrogen on subsurface deformation during indentation of a bulk metallic glass. Intermetallics 2010, 18, 1872–1875. [Google Scholar] [CrossRef]
- Wang, Y.W.; Chu, W.Y.; Li, J.X.; Hui, X.D.; Wang, Y.; Gao, K.W.; Su, Y.J.; Qiao, L.J. In situ observation of hydrogen-enhanced localized plastic flow in Zr-based bulk metallic glass. Mater. Lett. 2004, 58, 2393–2396. [Google Scholar] [CrossRef]
- Kawashima, A.; Yokoyama, Y.; Inoue, A. Zr-based bulk glassy alloy with improved resistance to stress corrosion cracking in sodium chloride solutions. Corros. Sci. 2010, 52, 2950–2957. [Google Scholar] [CrossRef]
- Philo, S.L.; Heinrich, J.; Gallino, I.; Busch, R.; Kruzic, J.J. Fatigue crack growth behavior of a Zr58.5Cu15.6Ni12.8Al10.3Nb2.8 bulk metallic glass-forming alloy. Scr. Mater. 2011, 64, 359–362. [Google Scholar] [CrossRef]
- Launey, M.E.; Busch, R.; Kruzic, J.J. Effects of free volume changes and residual stresses on the fatigue and fracture behavior of a Zr–Ti–Ni–Cu–Be bulk metallic glass. Acta Mater. 2008, 56, 500–510. [Google Scholar] [CrossRef]
- Philo, S.L.; Kruzic, J.J. Fatigue crack growth behavior of a Zr–Ti–Cu–Ni–Be bulk metallic glass: Role of ambient air environment. Scr. Mater. 2010, 62, 473–476. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gostin, P.F.; Eigel, D.; Grell, D.; Uhlemann, M.; Kerscher, E.; Eckert, J.; Gebert, A. Stress-Corrosion Interactions in Zr-Based Bulk Metallic Glasses. Metals 2015, 5, 1262-1278. https://doi.org/10.3390/met5031262
Gostin PF, Eigel D, Grell D, Uhlemann M, Kerscher E, Eckert J, Gebert A. Stress-Corrosion Interactions in Zr-Based Bulk Metallic Glasses. Metals. 2015; 5(3):1262-1278. https://doi.org/10.3390/met5031262
Chicago/Turabian StyleGostin, Petre Flaviu, Dimitri Eigel, Daniel Grell, Margitta Uhlemann, Eberhard Kerscher, Jürgen Eckert, and Annett Gebert. 2015. "Stress-Corrosion Interactions in Zr-Based Bulk Metallic Glasses" Metals 5, no. 3: 1262-1278. https://doi.org/10.3390/met5031262





