An in situ Study of NiTi Powder Sintering Using Neutron Diffraction
Abstract
:1. Introduction
2. Experimental Section
3. Results
3.1. Microstructure

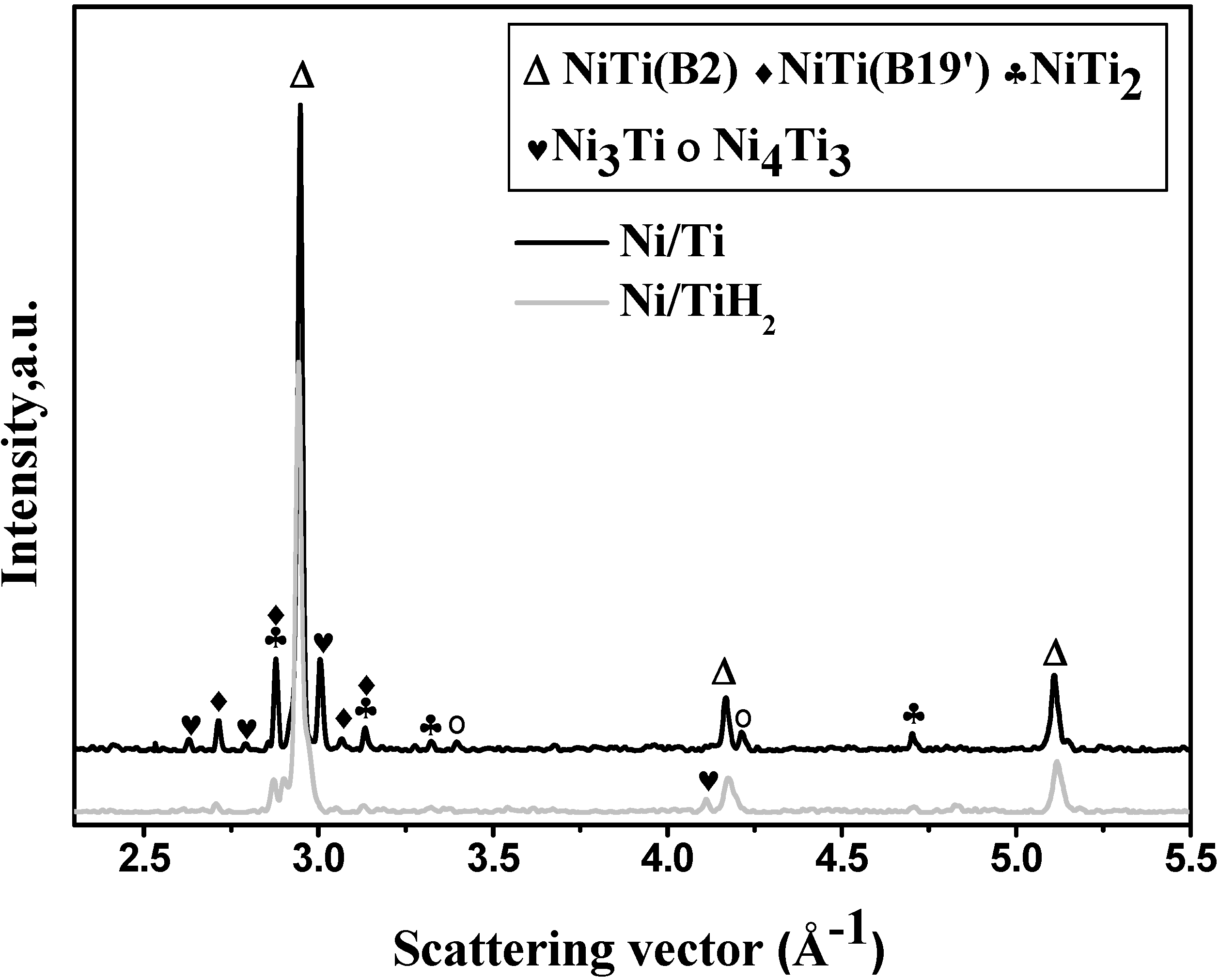

| Sample | Shrinkage/% | Density/g·cm−3 | Open porosity/% | Close-to-total porosity ratio/% | |
|---|---|---|---|---|---|
| axial | radial | ||||
| Ni/Ti | 10.47 ± 1.23 | 6.49 ± 0.62 | 5.81 ± 0.11 | 1.0 ± 0.1 | 89.6 ± 3.4 |
| Ni/TiH2 | 5.93 ± 0.49 | 4.21 ± 0.37 | 4.47 ± 0.07 | 26.9 ± 2.9 | 12.2 ± 0.8 |
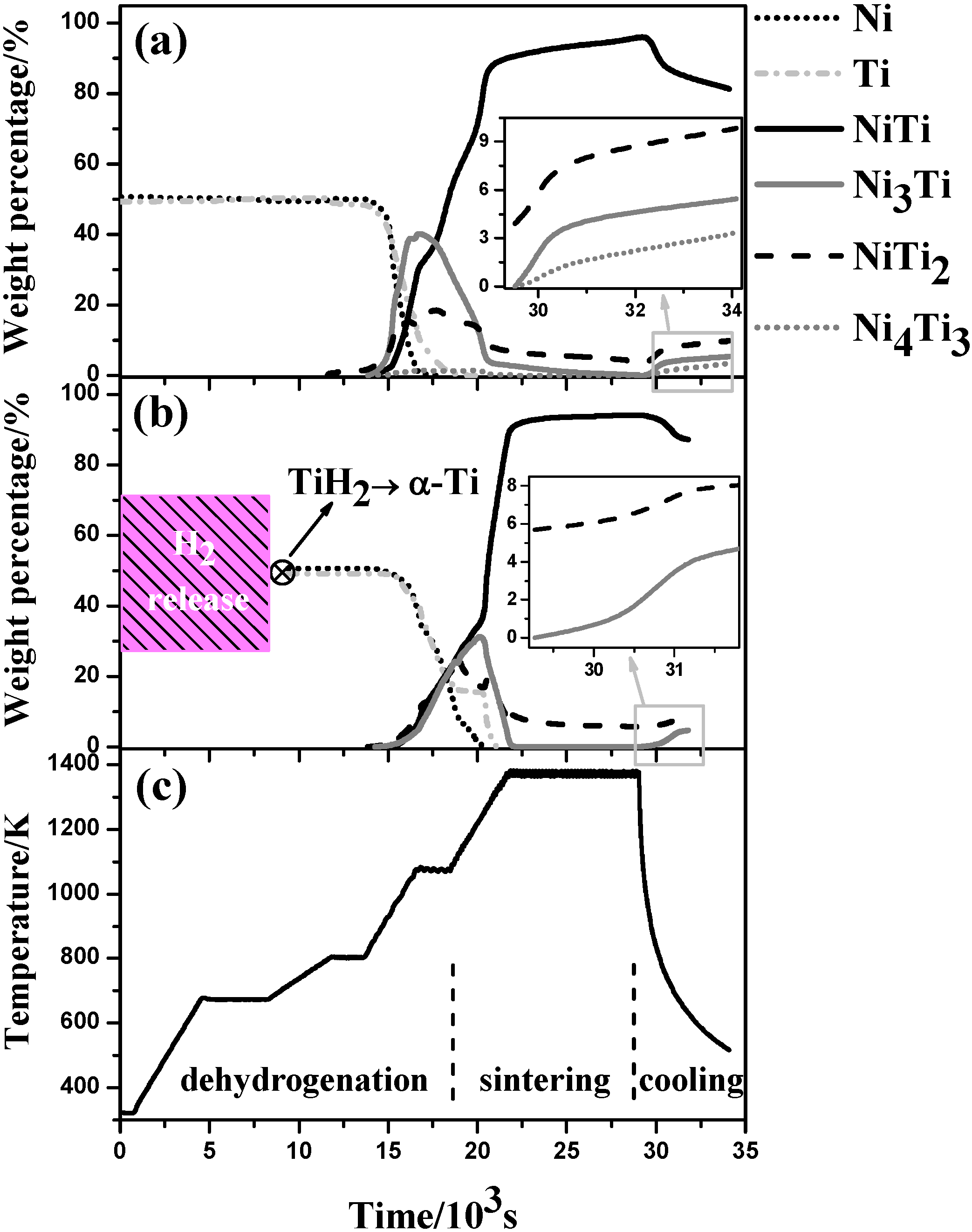
3.2. In situ Neutron Diffraction
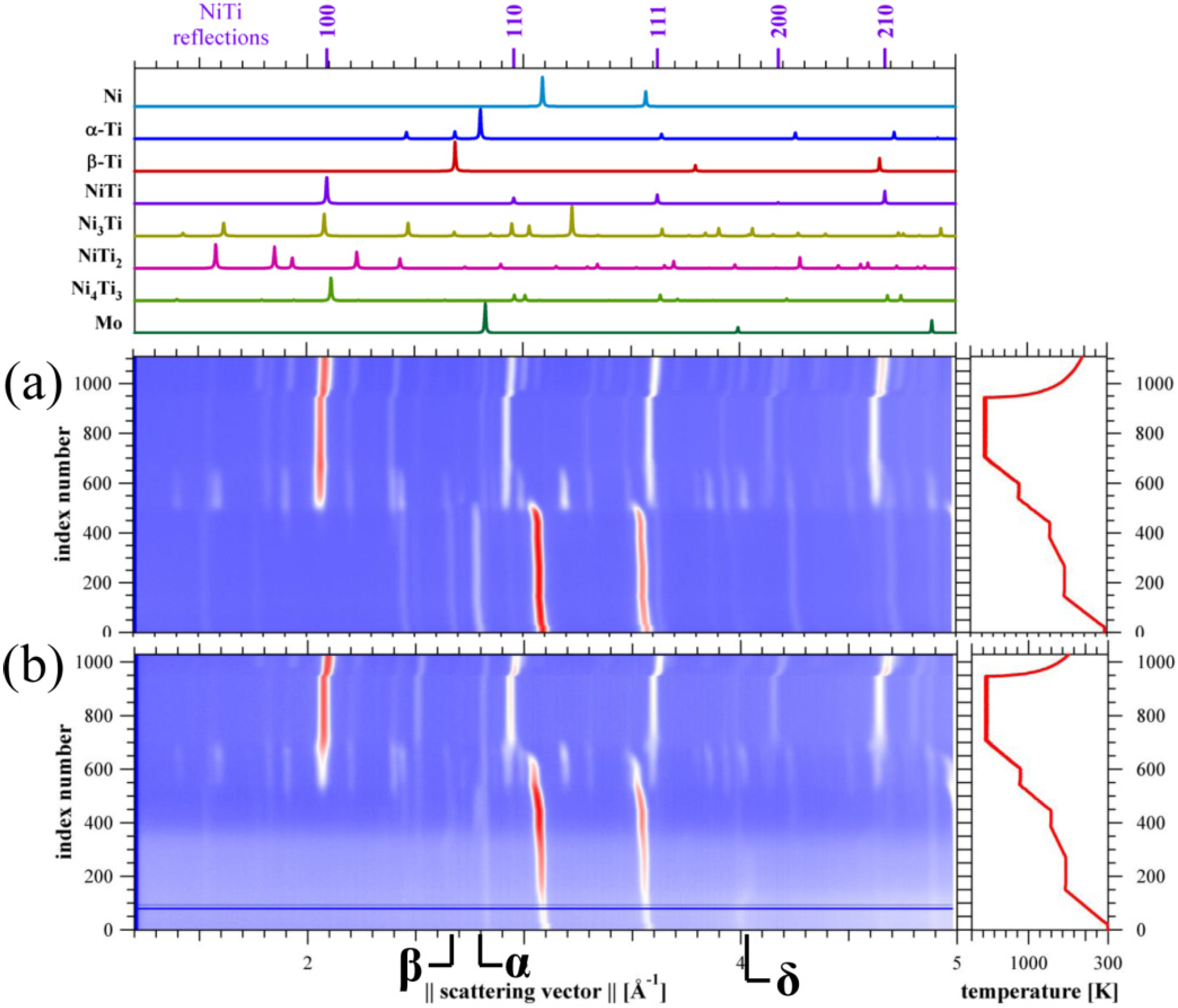
3.3. Pore-Size Distribution
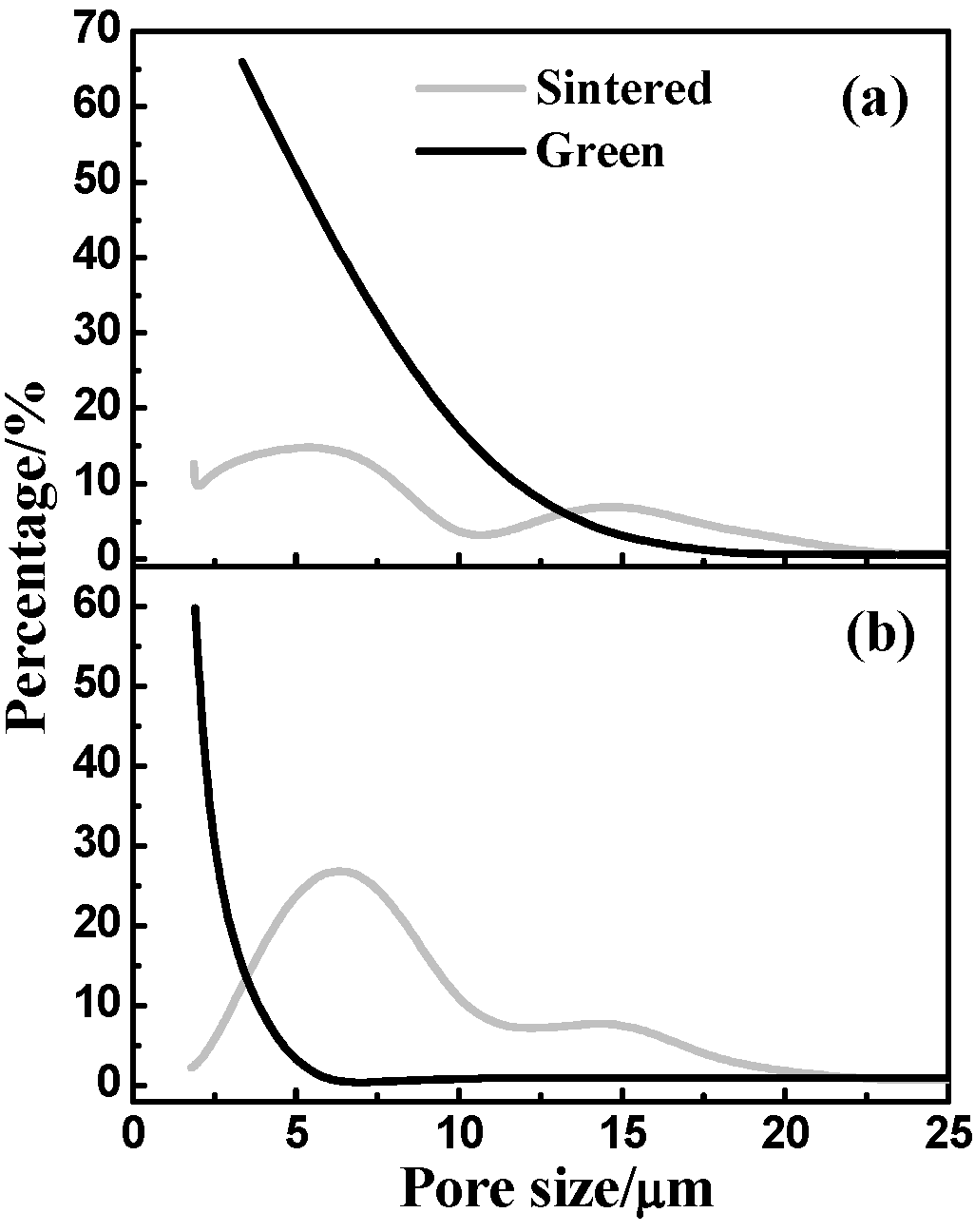
3.4. Mechanical Properties
3.4.1. Static Tensile Test
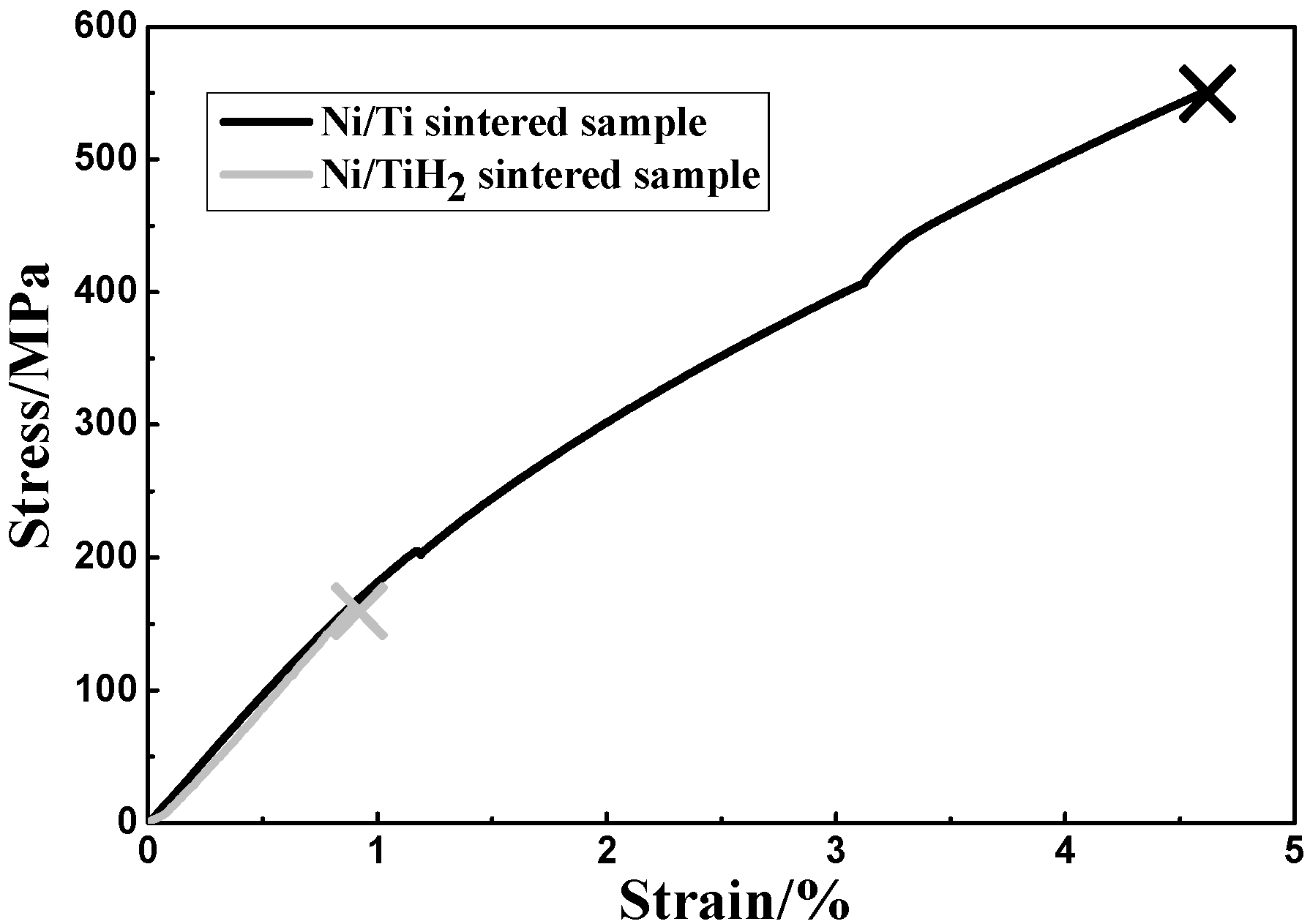
| Sample | Fracture tensile strength/MPa | Fracture strain/% | Young’s modulus/GPa |
|---|---|---|---|
| Ni/Ti | 549.4 ± 9.6 | 4.6 ± 0.2 | 18.9 ± 1.1 |
| Ni/TiH2 | 160.2 ± 7.3 | 0.9 ± 0.1 | 18.0 ± 0.9 |
3.4.2. Cyclic Compressive Test
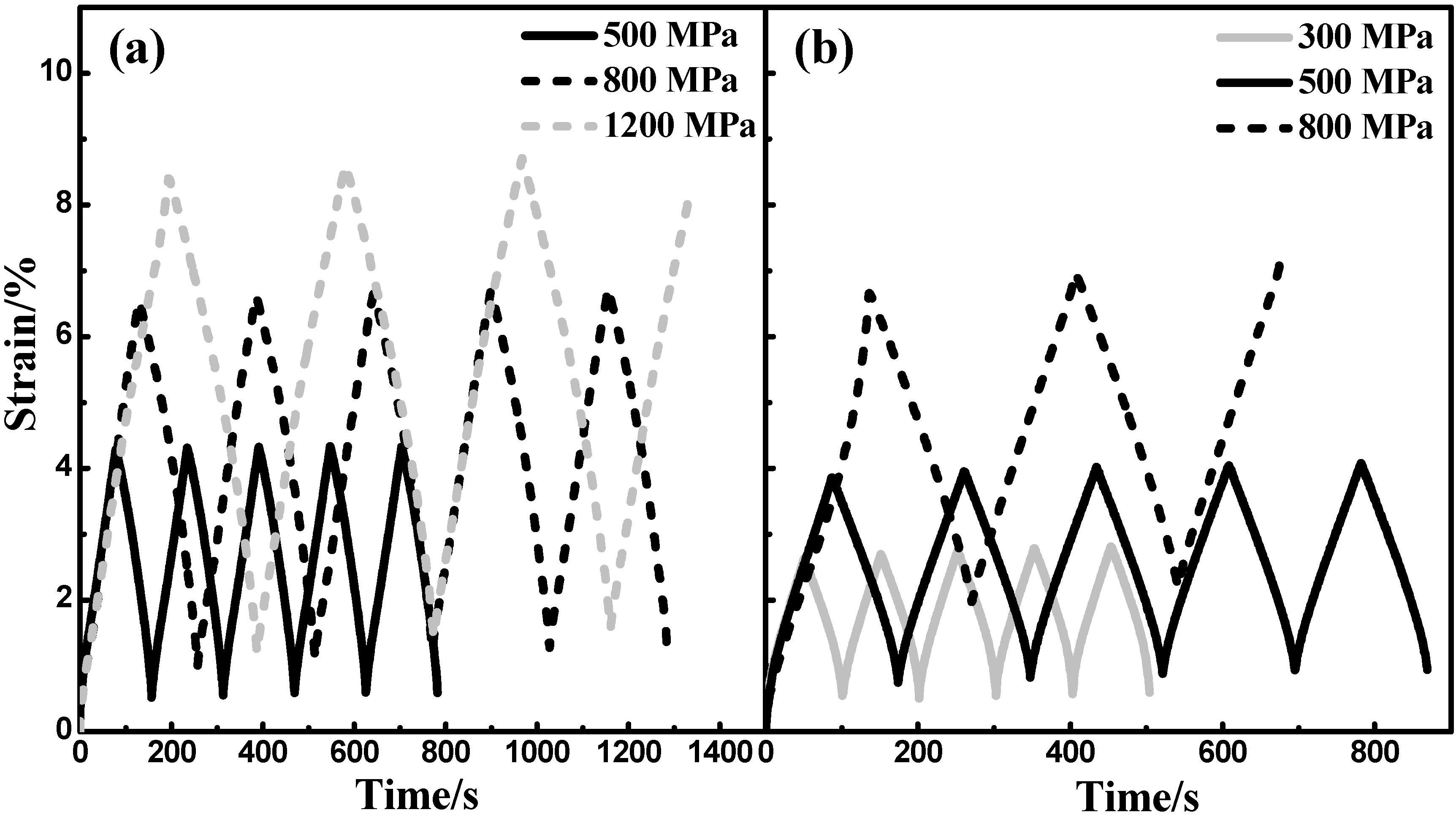
4. Discussion
4.1. Microstructural Evolution
4.1.1. Dehydrogenation process

4.1.2. Pore and Phase Evolution
4.2. Fracture, Superelasticity and Modulus
5. Summary
- (1)
- B2 NiTi phase is the dominant phase identified in both samples after being sintered at 1373 K holding for two hours together with the presence of some minor secondary phases.
- (2)
- Dehydrogenation from TiH2 leads to a lower density, a much higher porosity, a larger pore size but higher final chemical homogenization after sintering as compared with the Ni/Ti compact.
- (3)
- The use of TiH2 powder causes lower fracture strength and lower elastic modulus compared with the Ni/Ti sintered sample.
Acknowledgments
Conflicts of Interest
References
- Duering, T.W.; Pelton, A.R. Materials Properties Handbook: Titanium Alloys; ASM International, the Materials Information Society: Materials Park, OH, USA, 1994. [Google Scholar]
- Yamauchi, K.; Ohkata, I.; Tsuchiya, K.; Miyazaki, S. Shape Memory and Superelastic Alloys: Technologies and Applications; Woodhead Publishing: Cambridge, UK, 2011; p. 390. [Google Scholar]
- Elahinia, M.H.; Hashemi, M.; Tabesh, M.; Bhaduri, S.B. Manufacturing and processing of NiTi implants: A review. Prog. Mater. Sci. 2012, 57, 911–946. [Google Scholar] [CrossRef]
- Chen, G.; Cao, P.; Wen, G.; Edmonds, N.; Li, Y. Using an agar-based binder to produce porous NiTi alloys by metal injection moulding. Intermetallics 2013, 37, 92–99. [Google Scholar] [CrossRef]
- Whitney, M.; Corbin, S.F.; Gorbet, R.B. Investigation of the mechanisms of reactive sintering and combustion synthesis of NiTi using differential scanning calorimetry and microstructural analysis. Acta Mater. 2008, 56, 559–570. [Google Scholar] [CrossRef]
- Sadrnezhaad, S.K.; Hosseini, S.A. Fabrication of porous NiTi-shape memory alloy objects by partially hydrided titanium powder for biomedical applications. Mater. Des. 2009, 30, 4483–4487. [Google Scholar] [CrossRef]
- Tosun, G.; Ozler, L.; Kaya, M.; Orhan, N. A study on microstructure and porosity of NiTi alloy implants produced by SHS. J. Alloys Compd. 2009, 487, 605–611. [Google Scholar] [CrossRef]
- Whitney, M.; Corbin, S.F.; Gorbet, R.B. Investigation of the influence of Ni powder size on microstructural evolution and the thermal explosion combustion synthesis of NiTi. Intermetallics 2009, 17, 894–906. [Google Scholar] [CrossRef]
- Liu, X.; Wu, S.; Yeung, K.W.K.; Xu, Z.S.; Chung, C.Y.; Chu, P. Superelastic porous NiTi with adjustable porosities synthesized by powder metallurgical method. J. Mater. Eng. Perform. 2012, 21, 2553–2558. [Google Scholar] [CrossRef]
- Chen, G.; Cao, P.; Edmonds, N. Porous NiTi alloys produced by press-and-sinter from Ni/Ti and Ni/TiH2 mixtures. Mater. Sci. Eng. A 2013, 582, 117–125. [Google Scholar] [CrossRef]
- Li, B.-Y.; Rong, L.-J.; Li, Y.-Y. Stress–strain behavior of porous Ni-Ti shape memory intermetallics synthesized from powder sintering. Intermetallics 2000, 8, 643–646. [Google Scholar] [CrossRef]
- Li, B.-Y.; Rong, L.-J.; Li, Y.-Y. The influence of addition of TiH2 in elemental powder sintering porous Ni-Ti alloys. Mater. Sci. Eng. A 2000, 281, 169–175. [Google Scholar] [CrossRef]
- Bertheville, B.; Neudenberger, M.; Bidaux, J.E. Powder sintering and shape-memory behaviour of NiTi compacts synthesized from Ni and TiH2. Mater. Sci. Eng. A 2004, 384, 143–150. [Google Scholar] [CrossRef]
- Robertson, I.M.; Schaffer, G.B. Swelling during sintering of titanium alloys based on titanium hydride powder. Powder Metall. 2010, 53, 27–33. [Google Scholar] [CrossRef]
- Wu, S.; Liu, X.; Yeung, K.W.K.; Hu, T.; Xu, Z.; Chung, J.C.Y.; Chu, P.K. Hydrogen release from titanium hydride in foaming of orthopedic NiTi scaffolds. Acta Biomater. 2011, 7, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wen, G.A.; Cao, P.; Edmonds, N.; Li, Y.M. Processing and characterisation of porous NiTi alloy produced by metal injection moulding. Powder Injection Moulding Int. 2012, 6, 83–88. [Google Scholar]
- Chen, G.; Cao, P. NiTi powder sintering from TiH2 powder: An in situ investigation. Metall. Mater. Trans. A 2013, 1–4. [Google Scholar]
- Wang, H.; Fang, Z.Z.; Sun, P. A critical review of mechanical properties of powder metallurgy titanium. Int. J. Powder Metall. 2010, 46, 45–57. [Google Scholar]
- Wang, H.T.; Lefler, M.; Fang, Z.Z.; Lei, T.; Fang, S.M.; Zhang, J.M.; Zhao, Q. Titanium and titanium alloy via sintering of TiH2. Key Eng. Mater. 2010, 436, 157–163. [Google Scholar] [CrossRef]
- Chen, G.; Liss, K.-D.; Cao, P. In situ observation and neutron diffraction of NiTi powder sintering. Acta Mater. 2014, 67, 32–44. [Google Scholar] [CrossRef]
- Chen, G. Powder Metallurgical Titanium Alloys (TiNi and Ti-6Al-4V): Injection Moulding, Press-and-Sinter, and Hot Pressing. Ph.D. Thesis, The University of Auckland, Auckland, New Zealand, 2014. [Google Scholar]
- Azevedo, C.R.F.; Rodrigues, D.; Beneduce Neto, F. Ti-Al-V powder metallurgy (PM) via the hydrogenation–dehydrogenation (HDH) process. J. Alloys Compd. 2003, 353, 217–227. [Google Scholar] [CrossRef]
- Robertson, I.M.; Schaffer, G.B. Comparison of sintering of titanium and titanium hydride powders. Powder Metall. 2010, 53, 12–19. [Google Scholar] [CrossRef]
- Ivasishin, O.M.; Eylon, D.; Bondarchuk, V.I.; Savvakin, D.G. Diffusion during powder metallurgy synthesis of titanium alloys. Defect Diffus. Forum 2008, 277, 177–185. [Google Scholar] [CrossRef]
- Zhang, J.M.; Yi, J.H.; Gan, G.Y.; Yan, J.K.; Du, J.H.; Liu, Y.C. Research on dehydrogenation and sintering process of titanium hydride for manufacture titanium and titanium alloy. Adv. Mater. Res. 2013, 616–618, 1823–1829. [Google Scholar]
- Ivasishin, O.M.; Savvakin, D.G.; Froes, F.; Mokson, V.C.; Bondareva, K.A. Synthesis of alloy Ti-6Al-4V with low residual porosity by a powder metallurgy method. Powder Metall. Metal Ceram. 2002, 41, 382–390. [Google Scholar] [CrossRef]
- Bhosle, V.; Baburaj, E.G.; Miranova, M.; Salama, K. Dehydrogenation of nanocrystalline TiH2 and consequent consolidation to form dense Ti. Metall. Mater. Trans. A 2003, 34, 2793–2799. [Google Scholar] [CrossRef]
- Li, B.Y.; Rong, L.J.; Li, Y.Y. Porous NiTi alloy prepared from elemental powder sintering. J. Mater. Res. 1998, 13, 2847–2851. [Google Scholar] [CrossRef]
- Li, B.-Y.; Rong, L.-J.; Li, Y.-Y.; Gjunter, V.E. An investigation of the synthesis of Ti-50 at. Pct Ni alloys through combustion synthesis and conventional powder sintering. Metall. Mater. Trans. A 2000, 31, 1867–1871. [Google Scholar] [CrossRef]
- Bhosle, V.; Baburaj, E.G.; Miranova, M.; Salama, K. Dehydrogenation of TiH2. Mater. Sci. Eng. A 2003, 356, 190–199. [Google Scholar] [CrossRef]
- Sandim, H.R.Z.; Morante, B.V.; Suzuki, P.A. Kinetics of thermal decomposition of titanium hydride powder using in situ high-temperature X-ray diffraction (HTXRD). Mater. Res. 2005, 8, 293–297. [Google Scholar] [CrossRef]
- Liu, H.; He, P.; Feng, J.C.; Cao, J. Kinetic study on nonisothermal dehydrogenation of TiH2 powders. Int. J. Hydrog. Energy 2009, 34, 3018–3025. [Google Scholar] [CrossRef]
- Jiménez, C.; Garcia-Moreno, F.; Pfretzschner, B.; Klaus, M.; Wollgarten, M.; Zizak, I.; Schumacher, G.; Tovar, M.; Banhart, J. Decomposition of TiH2 studied in situ by synchrotron X-ray and neutron diffraction. Acta Mater. 2011, 59, 6318–6330. [Google Scholar] [CrossRef]
- Farhana, H.N.; Wang, Y.; Noor, M.M.; Chan, S.I. Static X-ray scans on the titanium hydride (TiH2) powder during dehydrogenation. Adv. Mater. Res. 2013, 795, 124–127. [Google Scholar] [CrossRef]
- Jiménez, C.; Garcia-Moreno, F.; Pfretzschner, B.; Kamm, P.H.; Neu, T.R.; Klaus, M.; Genzel, C.; Hilger, A.; Manke, I.; Banhart, J. Metal foaming studied in situ by energy dispersive X-ray diffraction of synchrotron radiation, X-ray radioscopy, and optical expandometry. Adv. Eng. Mater. 2013, 15, 141–148. [Google Scholar] [CrossRef]
- Chen, G.; Liss, K.-D.; Cao, P. In situ observation of phase transformation of powder sintering from Ni/TiH2 using neutron diffraction. In TMS 2014 Supplemental Proceedings; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 967–973. [Google Scholar]
- Studer, A.J.; Hagen, M.E.; Noakes, T.J. Wombat: The high-intensity powder diffractometer at the opal reactor. Phys. B Condens. Matter 2006, 385–386, 1013–1015. [Google Scholar] [CrossRef]
- Yu, J.; Hu, X.; Huang, Y. A modification of the bubble-point method to determine the pore-mouth size distribution of porous materials. Sep. Purif. Technol. 2010, 70, 314–319. [Google Scholar] [CrossRef]
- Viswanathan, B.; Murthy, S.S.; Sastri, M.V.C. Metal Hydrides: Fundamentals and Applications, 1st ed.; Springer: Berlin, Germany, 1999; p. 189. [Google Scholar]
- Duwez, P.; Taylor, J.L. The structure of intermediate phases in alloys of titanium with iron, cobalt, and nickel. Trans. AIME 1950, 188, 1173–1176. [Google Scholar]
- Poole, D.M.; Hume-Rothery, W. The equilibrium diagram of the system nickel-titanium. J. Inst. Metals 1954, 83, 473–480. [Google Scholar]
- Gupta, S.P.; Mukherjee, K.; Johnson, A.A. Diffusion controlled solid state transformation in the near-equiatomic Ti-Ni alloys. Mater. Sci. Eng. 1973, 11, 283–297. [Google Scholar] [CrossRef]
- Liss, K.-D.; Bartels, A.; Schreyer, A.; Clemens, H. High-energy X-rays: A tool for advanced bulk investigations in materials science and physics. Textures Microstruct. 2003, 35, 219–252. [Google Scholar] [CrossRef]
- Predel, B. H-Ti (Hydrogen-Titanium). In Ga-Gd-Hf-Zr; Madelung, O., Ed.; Springer: Berlin/Heidelberg, Germany, 1996; Volume 5f, pp. 1–2. [Google Scholar]
- Greiner, C.; Oppenheimer, S.M.; Dunand, D.C. High strength, low stiffness, porous NiTi with superelastic properties. Acta Biomater. 2005, 1, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Mandrino, D.; Paulin, I.; Škapin, S.D. Scanning electron microscopy, X-ray diffraction and thermal analysis study of the TiH2 foaming agent. Mater. Charact. 2012, 72, 87–93. [Google Scholar] [CrossRef]
- Paulin, I.; Donik, Č.; Mandrino, D.; Vončina, M.; Jenko, M. Surface characterization of titanium hydride powder. Vacuum 2012, 86, 608–613. [Google Scholar] [CrossRef]
- Okamoto, H. H-Ti (Hydrogen-Titanium). J. Phase Equilib. Diffus. 2011, 32, 174–175. [Google Scholar] [CrossRef]
- Fukai, Y. The Metal-Hydrogen System, Basic Bulk Properties, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2005; p. 497. [Google Scholar]
- Igharo, M.; Wood, I.V. Compaction and sintering phenomena in titanium-nickel shape memory alloys. Powder Metall. 1985, 28, 131–139. [Google Scholar] [CrossRef]
- Biswas, A. Porous NiTi by thermal explosion mode of SHS: Processing, mechanism and generation of single phase microstructure. Acta Mater. 2005, 53, 1415–1425. [Google Scholar] [CrossRef]
- Otsuka, K.; Ren, X. Physical metallurgy of Ti-Ni-based shape memory alloys. Prog. Mater. Sci. 2005, 50, 511–678. [Google Scholar] [CrossRef]
- Laeng, J.; Xiu, Z.; Xu, X.; Sun, X.; Ru, H.; Liu, Y. Phase formation of Ni–Ti via solid state reaction. Phys. Scr. 2007, 2007, 250. [Google Scholar] [CrossRef]
- Bansiddhi, A.; Dunand, D.C. Shape-memory NiTi foams produced by replication of NaCl space-holders. Acta Biomater. 2008, 4, 1996–2007. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yuan, B.; Gao, Y.; Chung, C.Y.; Zhu, M. High-porosity NiTi superelastic alloys fabricated by low-pressure sintering using titanium hydride as pore-forming agent. J. Mater. Sci. 2009, 44, 875–881. [Google Scholar] [CrossRef]
- Wen, C.E.; Xiong, J.Y.; Li, Y.C.; Hodgson, P.D. Porous shape memory alloy scaffolds for biomedical applications: A review. Phys. Scr. 2010, 2010, 014070. [Google Scholar] [CrossRef]
- German, R.M. Powder Metallurgy Science; Metal Powder Industries Federation: Princeton, NJ, USA, 1998. [Google Scholar]
- German, R.; Suri, P.; Park, S. Review: Liquid phase sintering. J. Mater. Sci. 2009, 44, 1–39. [Google Scholar] [CrossRef]
- Ashby, M.F.; Evans, A.; Fleck, N.A.; Gibson, L.J.; Hutchinson, J.W.; Wadley, H. Metal Foams: A Design Guide; Butterworth-Heinemann: Boston, MA, USA, 2000. [Google Scholar]
- Anderson, T.L. Fracture Mechanics Fundamentals and Applications, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Nemat-Nasser, S.; Guo, W.-G. Superelastic and cyclic response of NiTi SMA at various strain rates and temperatures. Mech. Mater. 2006, 38, 463–474. [Google Scholar] [CrossRef]
- Gibson, L.J.; Ashby, M.F. Cellular Solids: Structure and Properties, 2nd ed.; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.; Liss, K.-D.; Cao, P. An in situ Study of NiTi Powder Sintering Using Neutron Diffraction. Metals 2015, 5, 530-546. https://doi.org/10.3390/met5020530
Chen G, Liss K-D, Cao P. An in situ Study of NiTi Powder Sintering Using Neutron Diffraction. Metals. 2015; 5(2):530-546. https://doi.org/10.3390/met5020530
Chicago/Turabian StyleChen, Gang, Klaus-Dieter Liss, and Peng Cao. 2015. "An in situ Study of NiTi Powder Sintering Using Neutron Diffraction" Metals 5, no. 2: 530-546. https://doi.org/10.3390/met5020530








