Production of Liquid Metal Spheres by Molding
Abstract
:1. Introduction
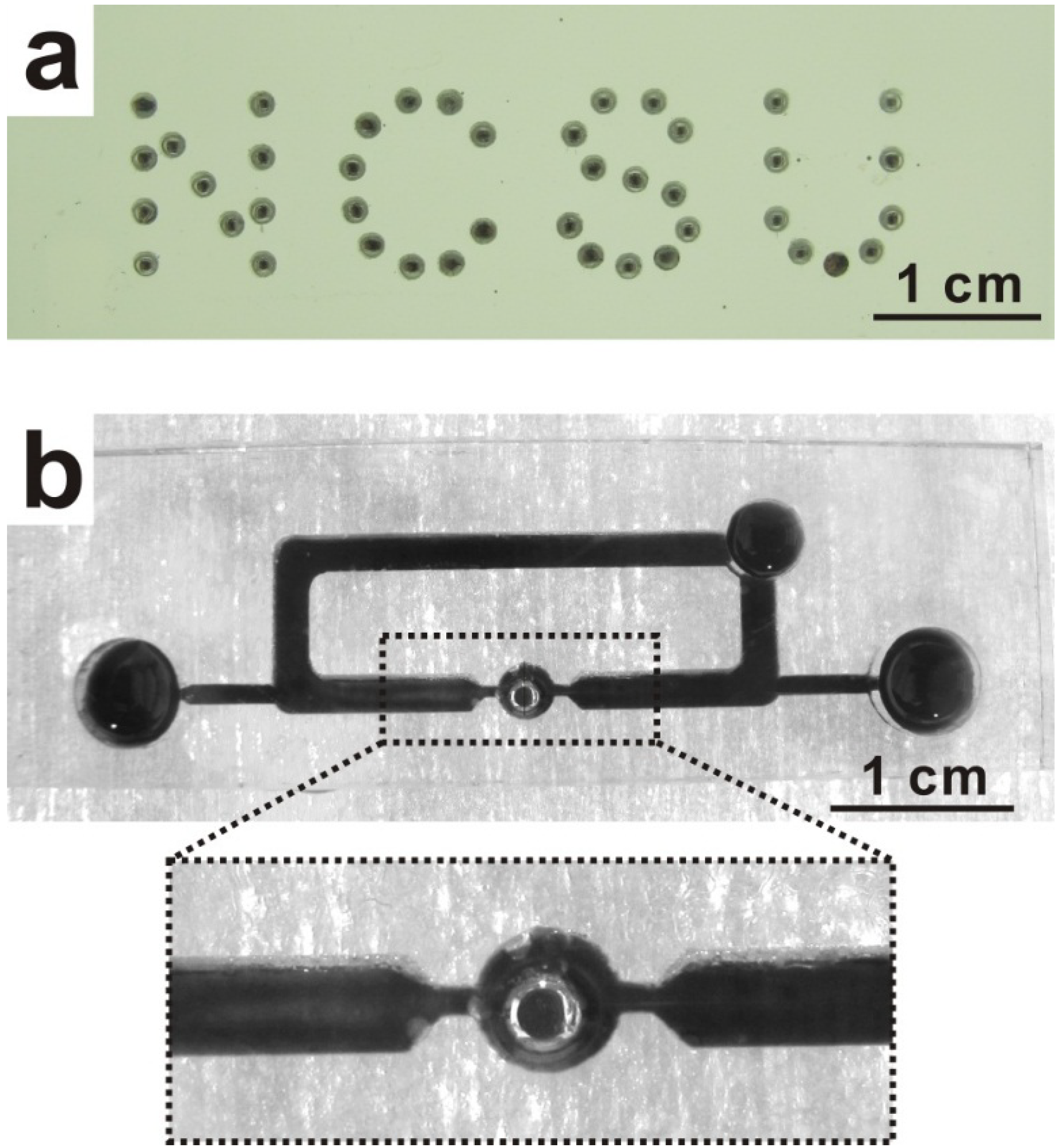
2. Experimental Design
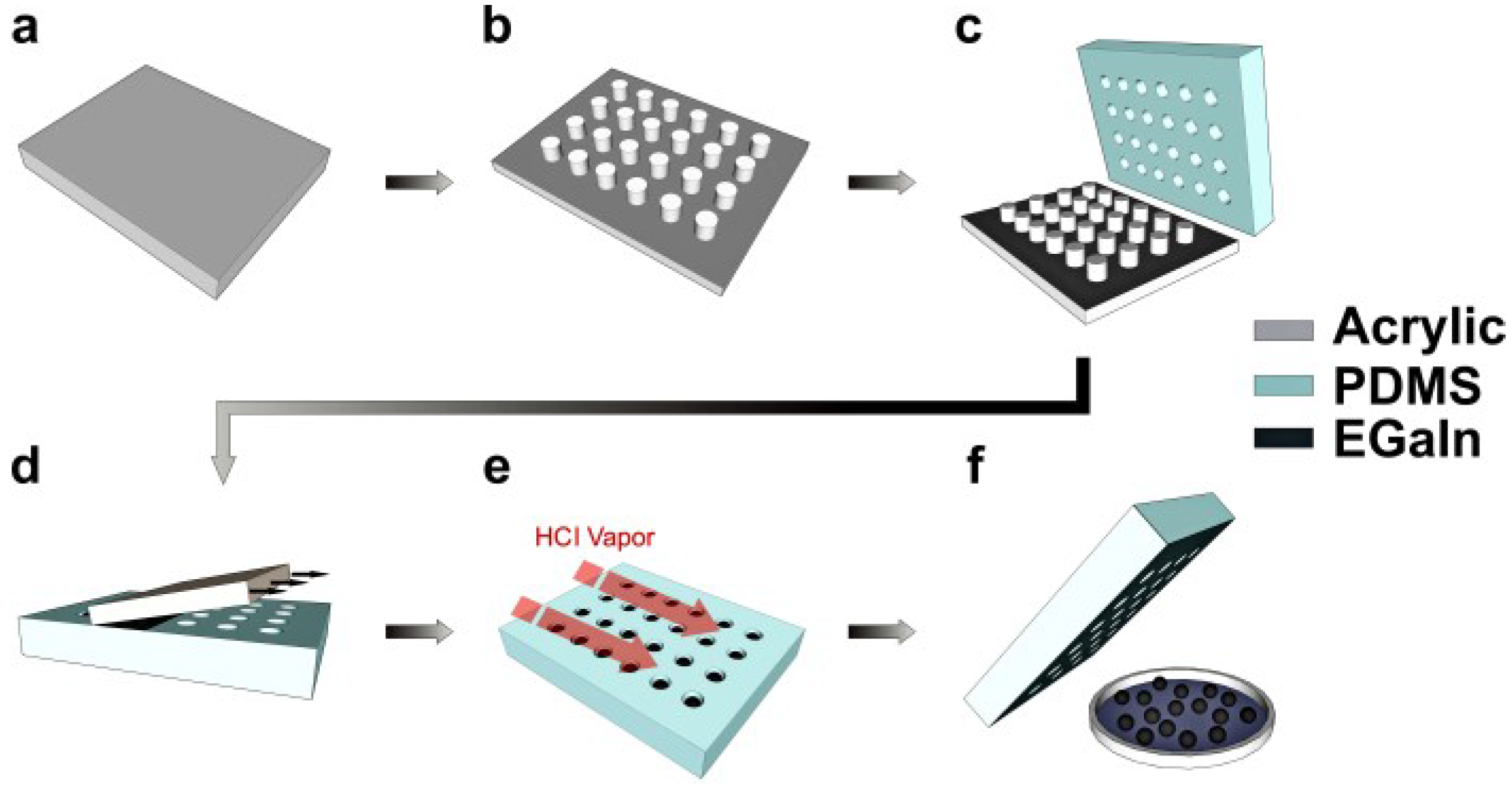
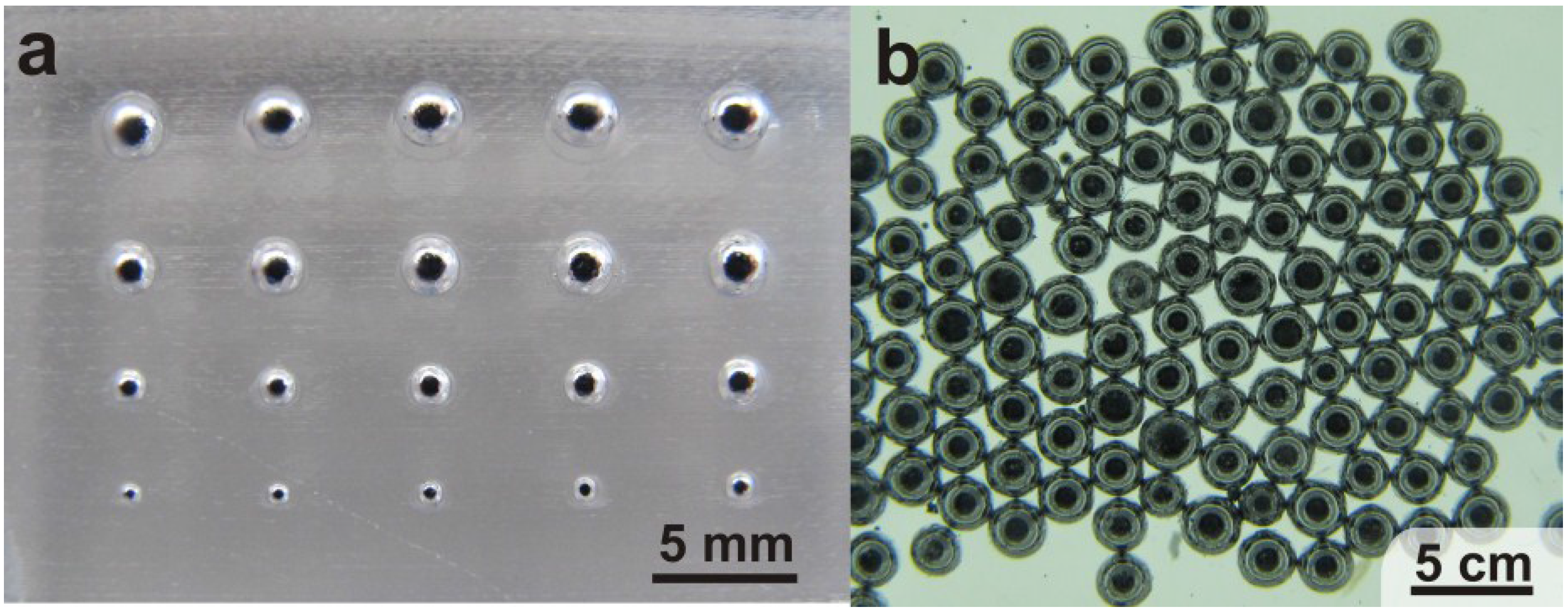
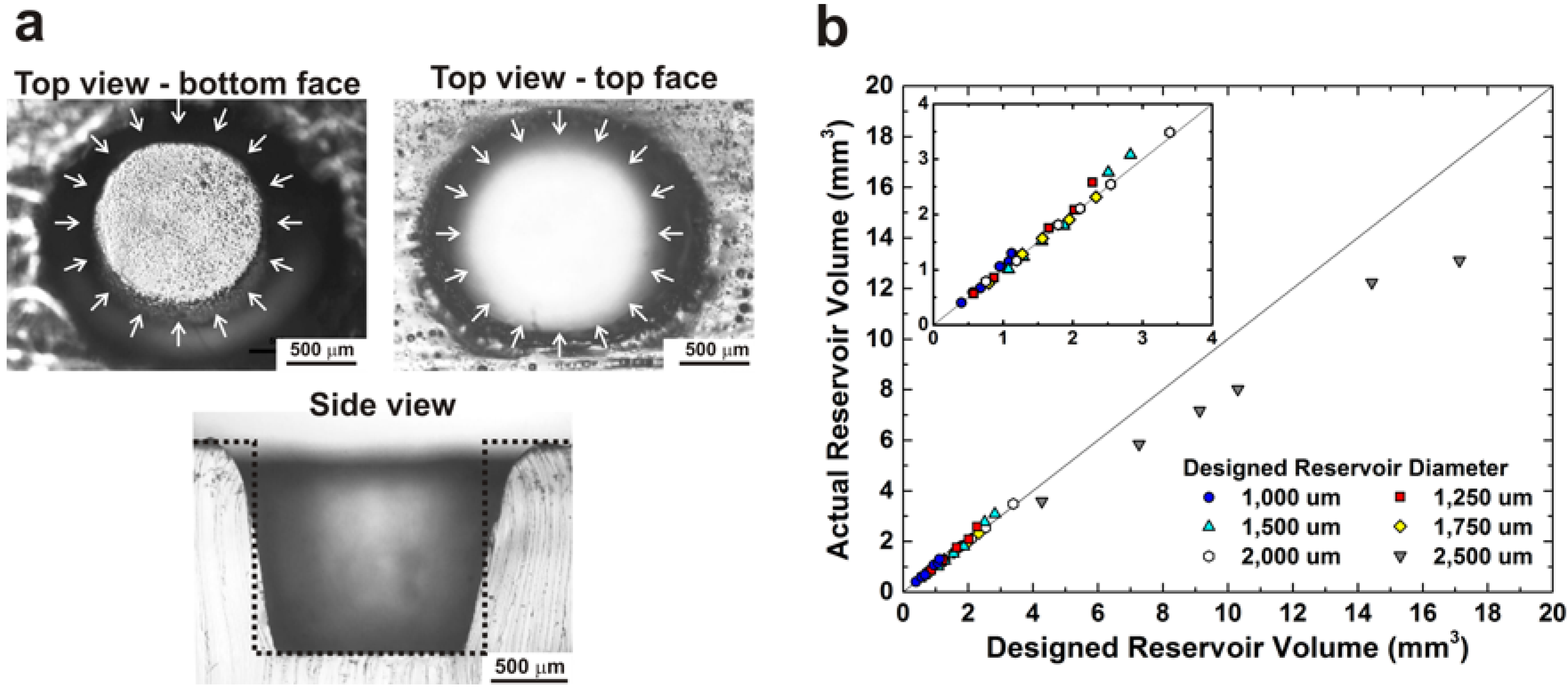
3. Results and Discussion
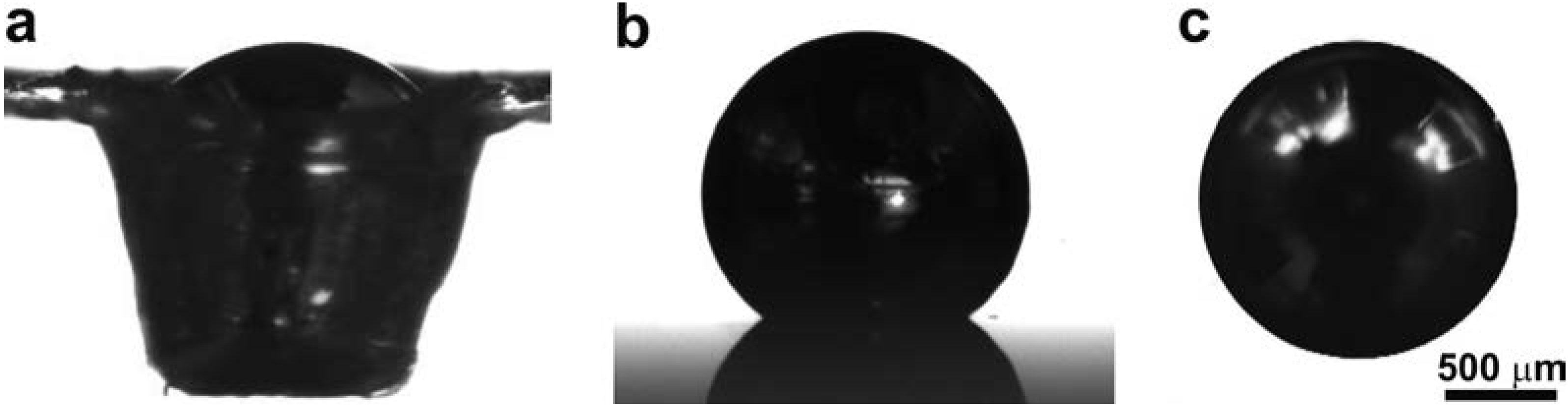
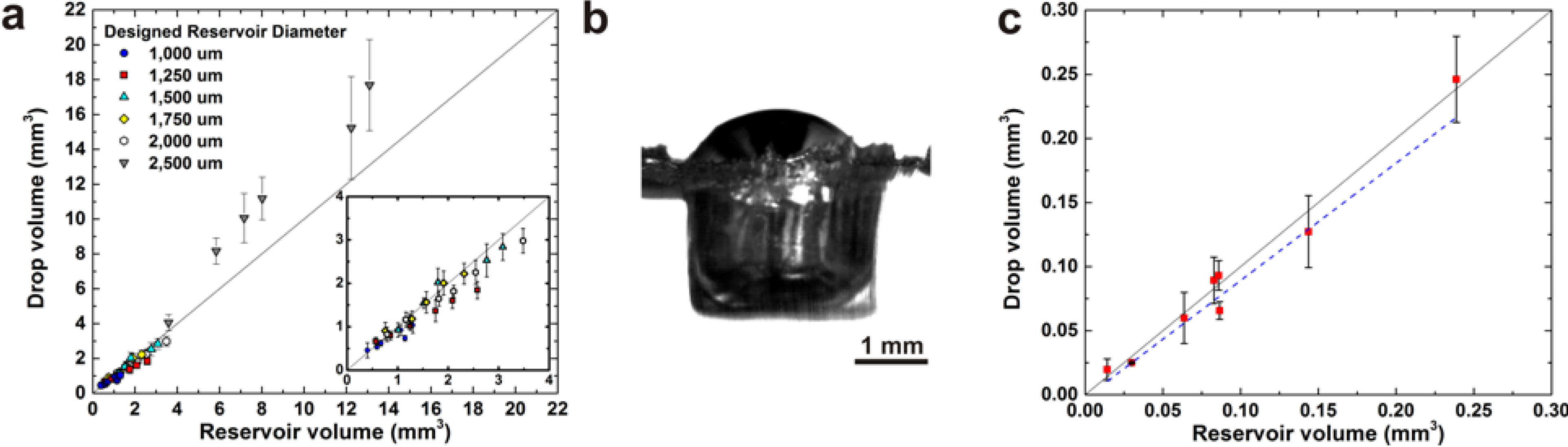
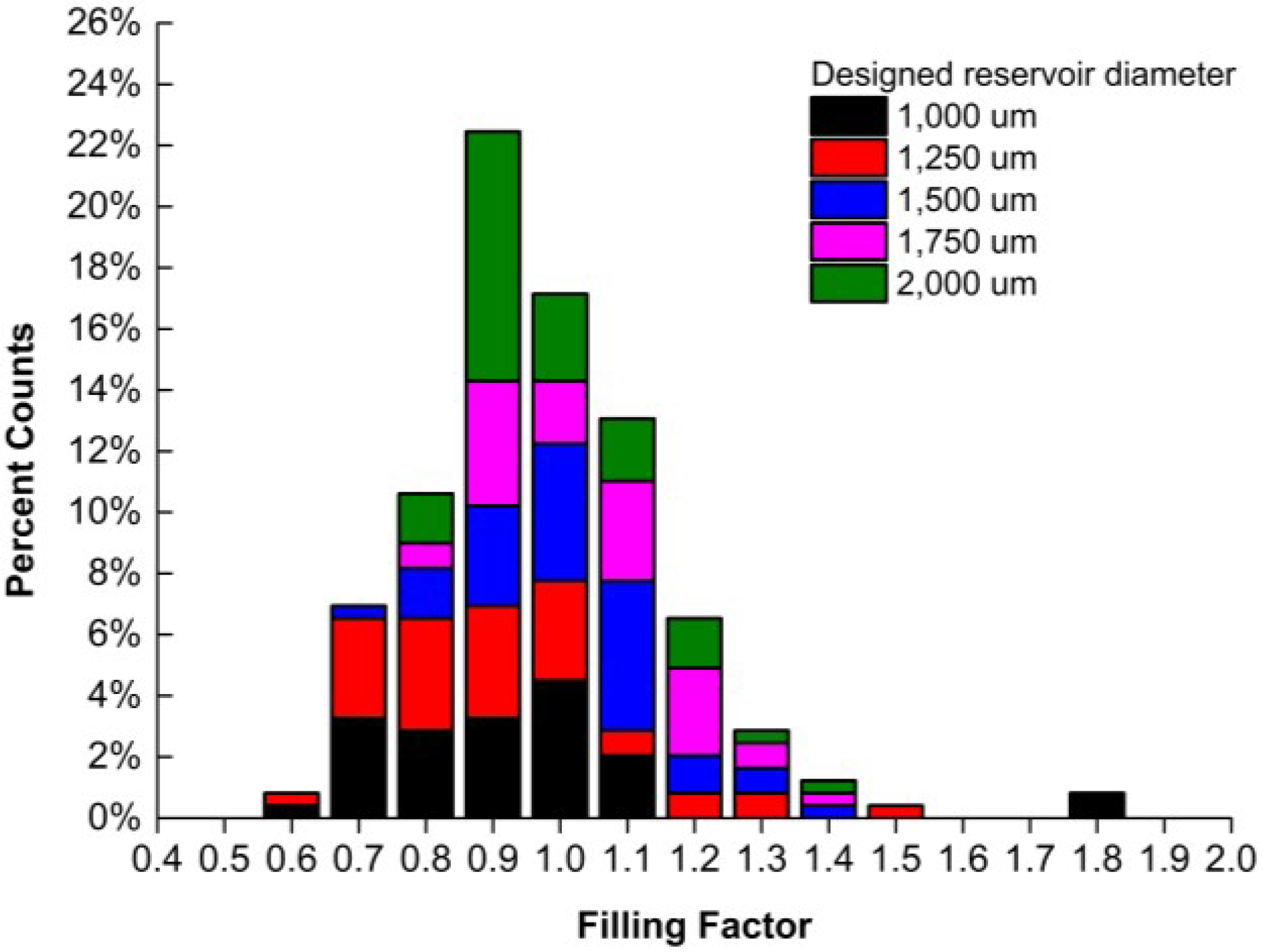
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- French, S.J.; Saunders, D.J.; Ingle, G.W. The system gallium-indium. J. Phys. Chem. 1937, 42, 265–274. [Google Scholar]
- Zrnic, D.; Swatik, D.S. On the resistivity and surface tension of the eutectic alloy of gallium and indium. J. Common Met. 1969, 18, 67–68. [Google Scholar]
- So, J.; Thelen, J.; Qusba, A.; Hayes, G.J.; Lazzi, G.; Dickey, M.D. Reversibly deformable and mechanically tunable fluidic antennas. Adv. Funct. Mater. 2009, 19, 3632–3637. [Google Scholar]
- Cheng, S.; Rydberg, A.; Hjort, K.; Wu, Z. Liquid metal stretchable unbalanced loop antenna. Appl. Phys. Lett. 2009, 94, 144103:1–144103:3. [Google Scholar]
- Kubo, M.; Li, X.; Kim, C.; Hashimoto, M.; Wiley, B.J.; Ham, D.; Whitesides, G.M. Stretchable Microfluidic Radiofrequency Antennas. Adv. Mater. 2010, 22, 2749–2752. [Google Scholar]
- Khan, M.R.; Hayes, G.J.; So, J.-H.; Lazzi, G.; Dickey, M.D. A frequency shifting liquid metal antenna with pressure responsiveness. Appl. Phys. Lett. 2011, 99, 013501:1–013501:3. [Google Scholar]
- Palleau, E.; Reece, S.; Desai, S.C.; Smith, M.E.; Dickey, M.D. Self-healing stretchable wires for reconfigurable circuit wiring and 3D microfluidics. Adv. Mater. 2013, 25, 1589–1592. [Google Scholar]
- Blaiszik, B.J.; Kramer, S.L.B.; Grady, M.E.; McIlroy, D.A.; Moore, J.S.; Sottos, N.R.; White, S.R. Autonomic restoration of electrical conductivity. Adv. Mater. 2012, 24, 398–401. [Google Scholar]
- Zhu, S.; So, J.-H.; Mays, R.L.; Desai, S.; Barnes, W.R.; Pourdeyhimi, B.; Dickey, M.D. Ultrastretchable fibers with metallic conductivity using a liquid metal alloy core. Adv. Fun. Mat. 2013, 23, 2308–2314. [Google Scholar]
- Kim, H.-J.; Son, C.; Ziaie, B. A multiaxial stretchable interconnect using liquid-alloy-filled elastomeric microchannels. Appl. Phys. Lett. 2008, 92, 011904:1–011904:3. [Google Scholar]
- Park, J.; Wang, S.; Li, M.; Ahn, C.; Hyun, J.K.; Kim, D.S.; Kim, D.K.; Rogers, J.A.; Huang, Y.; Jeon, S. Three-dimensional nanonetworks for giant stretchability in dielectrics and conductors. Nat. Commun. 2012, 3, 916. [Google Scholar]
- Zhang, Y.; Zhao, Z.; Fracasso, D.; Chiechi, R.C. Bottom-up molecular tunneling junctions formed by self-assembly. Isr. J. Chem. 2014, 54, 513–533. [Google Scholar]
- So, J.-H.; Dickey, M.D. Inherently aligned microfluidic electrodes composed of liquid metal. Lab Chip 2011, 11, 905–911. [Google Scholar]
- Cheng, S.; Wu, Z. Microfluidic electronics. Lab. Chip 2012, 12, 2782–2791. [Google Scholar]
- Majidi, C.; Kramer, R.; Wood, R.J. A non-differential elastomer curvature sensor for softer-than-skin electronics. Smart Mater. Struct. 2011, 20, 105017. [Google Scholar]
- Park, Y.-L.; Tepayotl-Ramirez, D.; Wood, R.J.; Majidi, C. Influence of cross-sectional geometry on the sensitivity and hysteresis of liquid-phase electronic pressure sensors. Appl. Phys. Lett. 2012, 101, 191904. [Google Scholar]
- Dickey, M.D.; Chiechi, R.C.; Larsen, R.J.; Weiss, E.A.; Weitz, D.A.; Whitesides, G.M. Eutectic gallium-indium (EGaIn): A liquid metal alloy for the formation of stable structures in microchannels at room temperature. Adv. Funct. Mater. 2008, 18, 1097–1104. [Google Scholar]
- Ladd, C.; So, J.-H.; Muth, J.; Dickey, M.D. 3D Printing of free standing liquid metal microstructures. Adv. Mater. 2013, 25, 5081–5085. [Google Scholar]
- Krupenkin, T.; Taylor, J.A. Reverse electrowetting as a new approach to high-power energy harvesting. Nat. Commun. 2011, 2, 448. [Google Scholar]
- So, J.-H.; Koo, H.-J.; Dickey, M.D.; Velev, O.D. Ionic current rectification in soft-matter diodes with liquid-metal electrodes. Adv. Funct. Mater. 2012, 22, 625–631. [Google Scholar]
- Yun, K.-S.; Cho, I.-J.; Bu, J.-U.; Kim, C.-J.; Yoon, E. A surface-tension driven micropump for low-voltage and low-power operations. J. Microelectromech. Syst. 2002, 11, 454–461. [Google Scholar]
- Tang, S.-Y.; Khoshmanesh, K.; Sivan, V.; Petersen, P.; O’Mullane, A.P.; Abbott, D.; Mitchell, A.; Kalantar-zadeh, K. Liquid metal enabled pump. Proc. Natl. Acad. Sci. USA 2014, 111, 3304–3309. [Google Scholar]
- Kim, H.-J.; Zhang, M.; Ziaie, B. A biaxially stretchable interconnect with liquid alloy joints on flexible substrate. In TRANSDUCERS 2007. International Solid-State Sensors, Actuators and Microsystems Conference, 2007; IEEE: New York, NY, USA, 2007; pp. 1597–1600. [Google Scholar]
- Sivan, V.; Tang, S.-Y.; O’Mullane, A.P.; Petersen, P.; Eshtiaghi, N.; Kalantar-zadeh, K.; Mitchell, A. Liquid metal marbles. Adv. Funct. Mater. 2013, 23, 144–152. [Google Scholar]
- Sen, P.; Kim, C.-J. A fast liquid-metal droplet microswitch using ewod-driven contact-line sliding. J. Microelectromech. Syst. 2009, 18, 174–185. [Google Scholar]
- Simon, J.; Saffer, S.; Kim, J.Y. A liquid-filled microrelay with a moving mercury microdrop. J. Microelectromech. Syst. 1997, 6, 208–216. [Google Scholar]
- Hayes, D.J.; Wallace, D.B.; Cox, W.R. MicroJet printing of solder and polymers for multi-chip modules and chip-scale packages. In Proceedings-SPIE the International Society for Optical Engineering; SPIE: Bellingham WA, USA, 1999; pp. 242–247. [Google Scholar]
- Tong, H.-M.; Lai, Y.-S.; Wong, C.P. Advanced Flip Chip Packaging, 2013 edition; Springer: New York, NY, USA; London, UK, 2013. [Google Scholar]
- Van der Graaf, S.; Schroën, C.G.P.H.; Boom, R.M. Preparation of double emulsions by membrane emulsification—A review. J. Membr. Sci. 2005, 251, 7–15. [Google Scholar]
- Vladisavljević, G.T.; Williams, R.A. Recent developments in manufacturing emulsions and particulate products using membranes. Adv. Colloid Interface Sci. 2005, 113, 1–20. [Google Scholar] [Green Version]
- Kobayashi, I.; Uemura, K.; Nakajima, M. Formulation of monodisperse emulsions using submicron-channel arrays. Colloids Surf. Physicochem. Eng. Asp. 2007, 296, 285–289. [Google Scholar]
- Teh, S.-Y.; Lin, R.; Hung, L.-H.; Lee, A.P. Droplet microfluidics. Lab Chip 2008, 8, 198–220. [Google Scholar]
- Bon, S.A.F.; Mookhoek, S.D.; Colver, P.J.; Fischer, H.R.; van der Zwaag, S. Route to stable non-spherical emulsion droplets. Eur. Polym. J. 2007, 43, 4839–4842. [Google Scholar]
- Maa, Y.-F.; Hsu, C.C. Performance of sonication and microfluidization for liquid–liquid emulsification. Pharm. Dev. Technol. 1999, 4, 233–240. [Google Scholar]
- Yu, Y.; Wang, Q.; Yi, L.; Liu, J. Channelless fabrication for large-scale preparation of room temperature liquid metal droplets. Adv. Eng. Mater. 2014, 16, 255–262. [Google Scholar]
- Kobayashi, I.; Nakajima, M.; Mukataka, S. Preparation characteristics of oil-in-water emulsions using differently charged surfactants in straight-through microchannel emulsification. Colloids Surf. Physicochem. Eng. Asp. 2003, 229, 33–41. [Google Scholar]
- Sugiura, S.; Nakajima, M.; Yamamoto, K.; Iwamoto, S.; Oda, T.; Satake, M.; Seki, M. Preparation characteristics of water-in-oil-in-water multiple emulsions using microchannel emulsification. J. Colloid Interface Sci. 2004, 270, 221–228. [Google Scholar]
- Zhao, C.-X.; Middelberg, A.P.J. Two-phase microfluidic flows. Chem. Eng. Sci. 2011, 66, 1394–1411. [Google Scholar]
- Christopher, G.F.; Anna, S.L. Microfluidic methods for generating continuous droplet streams. J. Phys. Appl. Phys. 2007, 40, R319. [Google Scholar]
- Gu, H.; Duits, M.H.G.; Mugele, F. Droplets formation and merging in two-phase flow microfluidics. Int. J. Mol. Sci. 2011, 12, 2572–2597. [Google Scholar]
- Cramer, C.; Fischer, P.; Windhab, E.J. Drop formation in a co-flowing ambient fluid. Chem. Eng. Sci. 2004, 59, 3045–3058. [Google Scholar]
- Nisisako, T.; Torii, T.; Takahashi, T.; Takizawa, Y. Synthesis of monodisperse bicolored janus particles with electrical anisotropy using a microfluidic co-flow system. Adv. Mater. 2006, 18, 1152–1156. [Google Scholar]
- Thorsen, T.; Roberts, R.W.; Arnold, F.H.; Quake, S.R. Dynamic pattern formation in a vesicle-generating microfluidic device. Phys. Rev. Lett. 2001, 86, 4163–4166. [Google Scholar]
- Yang, C.-H.; Huang, K.-S.; Lin, P.-W.; Lin, Y.-C. Using a cross-flow microfluidic chip and external crosslinking reaction for monodisperse TPP-chitosan microparticles. Sens. Actuators B Chem. 2007, 124, 510–516. [Google Scholar]
- Fidalgo, L.M.; Whyte, G.; Bratton, D.; Kaminski, C.F.; Abell, C.; Huck, W.T.S. From microdroplets to microfluidics: Selective emulsion separation in microfluidic devices. Angew. Chem. Int. Ed. 2008, 47, 2042–2045. [Google Scholar]
- Anna, S.L.; Bontoux, N.; Stone, H.A. Formation of dispersions using “flow focusing” in microchannels. Appl. Phys. Lett. 2003, 82, 364–366. [Google Scholar]
- Friedman, H.; Reich, S.; Popovitz-Biro, R.; von Huth, P.; Halevy, I.; Koltypin, Y.; Gedanken, A.; Porat, Z. Micro- and nano-spheres of low melting point metals and alloys, formed by ultrasonic cavitation. Ultrason. Sonochem. 2013, 20, 432–444. [Google Scholar]
- Han, Z.H.; Yang, B.; Qi, Y.; Cumings, J. Synthesis of low-melting-point metallic nanoparticles with an ultrasonic nanoemulsion method. Ultrasonics 2011, 51, 485–488. [Google Scholar]
- Hohman, J.N.; Kim, M.; Wadsworth, G.A.; Bednar, H.R.; Jiang, J.; LeThai, M.A.; Weiss, P.S. Directing substrate morphology via self-assembly: Ligand-mediated scission of gallium–indium microspheres to the nanoscale. Nano Lett. 2011, 11, 5104–5110. [Google Scholar]
- Thelen, J.; Dickey, M.D.; Ward, T. A study of the production and reversible stability of EGaIn liquid metal microspheres using flow focusing. Lab. Chip 2012, 12, 3961–3967. [Google Scholar]
- Hutter, T.; Bauer, W.-A.C.; Elliott, S.R.; Huck, W.T.S. Formation of spherical and non-spherical eutectic gallium-indium liquid-metal microdroplets in microfluidic channels at room temperature. Adv. Funct. Mater. 2012, 22, 2624–2631. [Google Scholar]
- Scharmann, F.; Cherkashinin, G.; Breternitz, V.; Knedlik, C.; Hartung, G.; Weber, T.; Schaefer, J.A. Viscosity effect on GaInSn studied by XPS. Surf. Interface Anal. 2004, 36, 981–985. [Google Scholar]
- Kim, D.; Lee, D.-W.; Choi, W.; Lee, J.-B. A super-lyophobic 3-D PDMS channel as a novel microfluidic platform to manipulate oxidized galinstan. J. Microelectromech. Syst. 2013, 22, 1267–1275. [Google Scholar]
- Kim, D.; Thissen, P.; Viner, G.; Lee, D.-W.; Choi, W.; Chabal, Y.J.; Lee, J.-B. Recovery of nonwetting characteristics by surface modification of gallium-based liquid metal droplets using hydrochloric acid vapor. ACS Appl. Mater. Interfaces 2013, 5, 179–185. [Google Scholar]
- Doudrick, K.; Liu, S.; Mutunga, E.M.; Klein, K.L.; Damle, V.; Varanasi, K.K.; Rykaczewski, K. Different shades of oxide: From nanoscale wetting mechanisms to contact printing of gallium-based liquid metals. Langmuir 2014, 30, 6867–6877. [Google Scholar]
- Gozen, B.A.; Tabatabai, A.; Ozdoganlar, O.B.; Majidi, C. High-density soft-matter electronics with micron-scale line width. Adv. Mater. 2014, 26, 5211–5216. [Google Scholar]
- Dalmoro, A.; Barba, A.A.; d’Amore, M. Analysis of size correlations for microdroplets produced by ultrasonic atomization. Sci. World J. 2013, 2013, 482910. [Google Scholar]
- Nisisako, T.; Torii, T. Microfluidic large-scale integration on a chip for mass production of monodisperse droplets and particles. Lab Chip 2008, 8, 287–293. [Google Scholar]
- Wang, Y.; Xia, Y. Bottom-up and top-down approaches to the synthesis of monodispersed spherical colloids of low melting-point metals. Nano Lett. 2004, 4, 2047–2050. [Google Scholar]
- Kim, J.; Shen, W.; Latorre, L.; Kim, C.-J. A micromechanical switch with electrostatically driven liquid-metal droplet. Sens. Actuators Phys. 2002, 97–98, 672–679. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, M.G.; Xenakis, A.; Dickey, M.D. Production of Liquid Metal Spheres by Molding. Metals 2014, 4, 465-476. https://doi.org/10.3390/met4040465
Mohammed MG, Xenakis A, Dickey MD. Production of Liquid Metal Spheres by Molding. Metals. 2014; 4(4):465-476. https://doi.org/10.3390/met4040465
Chicago/Turabian StyleMohammed, Mohammed G., Alexis Xenakis, and Michael D. Dickey. 2014. "Production of Liquid Metal Spheres by Molding" Metals 4, no. 4: 465-476. https://doi.org/10.3390/met4040465





