1. Introduction
The quaternary Al-Zn-Mg-Cu wrought alloys offer advantageous physical and mechanical properties making them desirable for aerospace, automotive and defense applications. They gain their strength through various precipitates that are obtained through heat treatment. Due to their high alloy content, they are generally considered to have poor cast-ability, suffering from porosity, hot tear defects, poor fluidity and inhomogeneous cast microstructure [
1]. As a result, billets of cast Al-Zn-Mg-Cu typically undergo thermomechanical processing to obtain optimal mechanical properties and must be forged or machined to obtain a net shaped part. Squeeze casting may prove a useful technique to synthesize these alloys, considering that high cooling rates can be achieved resulting in a finer and more homogeneous microstructure, and the applied pressure is able to reduce porosity to produce a near net shape [
2,
3,
4,
5].
Four major intermetallic phases are expected in commercial Al-Zn-Mg-Cu alloys including η (MgZn
2), T (Al
2Zn
3Mg
3), S (Al
2CuMg) and θ (Al
2Cu) phase [
6]. High Zn content alloys typically exhibit high hardness and high strength; however, they have been shown to have a higher content of residual T phase that is not dissolved upon solutionizing which can lead to stress concentrations [
7]. Marlaud
et al. have shown that the composition of the precipitates may be tuned by adjusting the alloy composition and the heat treatment procedure due to the kinetics of the solution of Cu, Mg and Zn in aluminum [
8]. In particular, the ratio of Zn + Cu atomic percentage to the Mg atomic percent has a strong effect on the residual solid solution concentration of Mg in the alloy, where a ratio greater than 2 results in near zero residual Mg and less than 2 results in a higher residual Mg content. The amount and type of precipitates formed as well as the residual solid solution concentration can have a profound effect on the mechanical properties of these alloys.
This work investigates the effect of composition on the solutionizing and artificial aging behavior of (1) variations of Cu and Zn that are within the limits of the AA-7034 specification and (2) variations of Mg beyond the limit of the AA-7034 specification.
2. Results and Discussion
2.1. Comparison to Casting Alloy Al-A206
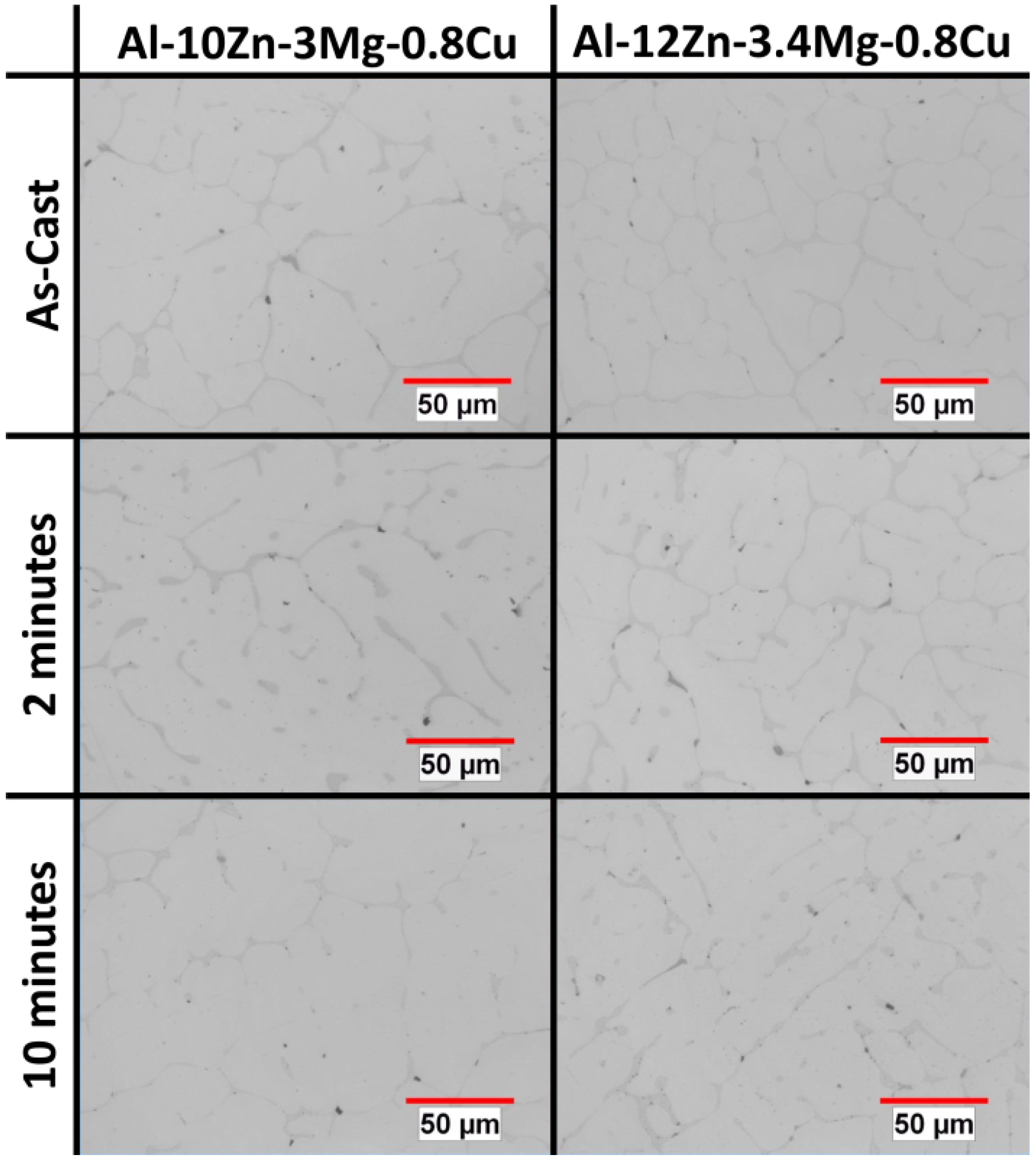
Figure 1 shows typical microstructures of these alloys. In as-cast samples, intermetallics are clearly visible in interdendritic regions. After 2 min of the solutionizing treatment the intermetallic material has begun to dissolve and by 10 min little of the intermetallic phases remain.
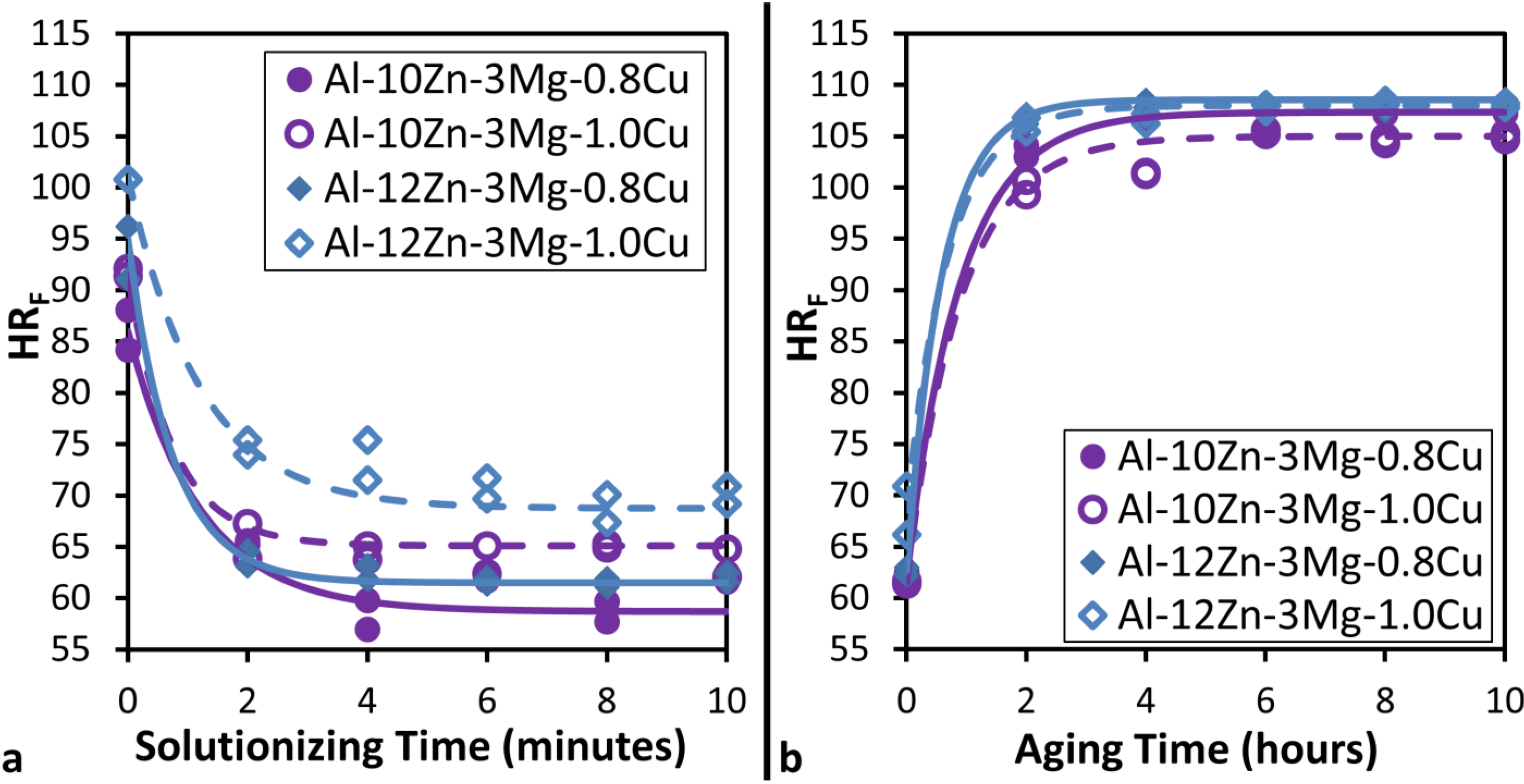
Figure 2a shows that the as-cast hardness for a fixed Cu Concentration is higher in the 12 wt% Zn samples than in the 10 wt% Zn samples. For a fixed Zn concentration 1 wt% Cu has a higher as-cast hardness than 0.8 wt% Cu. This is to be expected as the higher Zn and Cu content alloys will possess a higher concentration of intermetallic phases.
Figure 2a also shows that as solutionizing progresses, hardness rapidly drops for all alloys until reaching a minimum at about 8 min. Considering that the solutionized material at minimum hardness represents a supersaturated single phase material, it would be expected that solid solution strengthening will control the strength of the material. It has been proposed that solid solution strengthening depends on the size difference between the solvent atoms and the solute atoms to create a misfit strain field that impedes dislocation motion [
9]. X-ray Diffraction studies of supersaturated Al-Zn alloys show that increasing Zn content decreases the lattice parameter [
10]. Theoretical and experimental results show that increasing Cu concentration also decreases the lattice parameter [
11]. However, theoretical and experimental results show that increasing Mg concentration results in an increase in the lattice parameter [
11]. Therefore, if Al-Zn-Cu is considered the solvent and Mg is the solute, increasing Zn and Cu concentration will reduce the lattice parameter of the solvent and create larger misfit strains around the Mg. Thus, it would be expected that the minimum hardness would increase as Zn and Cu concentrations increase, which is consistent with the data in
Figure 2a. The plotted trend lines indicate that aging treatment results in a time-dependent hardness that follows the general form
HRF =
FRF Soln + (
HRFas−cast −
HRFSoln)
e−t∕τ, where
FRF Soln,
HRFas−cast,
t, and τ represent the solutionized hardness, the as-cast hardness, solutionizing time and characteristic time, respectively.
Figure 1.
Microstructures of representative alloys in the as-cast condition, and after a solutionizing heat treatment of 2 and 10 min at 753.15 K (480 °C).
Figure 2.
(a) Variation of Rockwell F Hardness (HRF) with solutionizing time at 753.15 K (480 °C) followed by a water quench and (b) Variation of Rockwell F Hardness (HRF) with aging time at 393 K (120 °C) in Al-(10 to 12)Zn-3Mg-(0.8 to 1)Cu alloys.
Figure 2b shows that hardness increases rapidly in the initial stages of the aging heat treatment, but maximum hardness is achieved at approximately 6 h. For a fixed Cu content it is clear that 12 wt% Zn results in a harder material than 10 wt% Zn. However, for a fixed Zn concentration, lower Cu content may provide slightly better hardness and seems to result in a slightly higher rate of hardening. The plotted trend lines indicate that aging treatment results in a time-dependent hardness that follows the general form
HRF =
HRF Soln + (
HRFmax −
HRFSoln)(1 −
e−t∕τ), where
HRFSoln,
HRFmax,
t, and τ represent the solutionized hardness, the maximum hardness, aging time and characteristic time, respectively.
2.2. Al-12Zn-(3.2 to 3.4)Mg-0.8Cu
There are few commercial Al-Zn-Mg-Cu alloys that exceed 3 wt% Mg, but those that do exceed this value typically contain considerably less Zn than AA-7034 [
12]. Typical tensile property data for various Al-Zn-Mg-Cu Alloys are available [
12] and show that, as a general rule, strength increases with increasing Mg concentration. As such the heat treating behavior of Al-12Zn-0.8Cu alloys with Mg concentrations exceeding 3 wt% would be expected to be higher in strength than AA7034, provided that the as-cast intermetallic phases could be completely dissolved and re-precipitated. However,
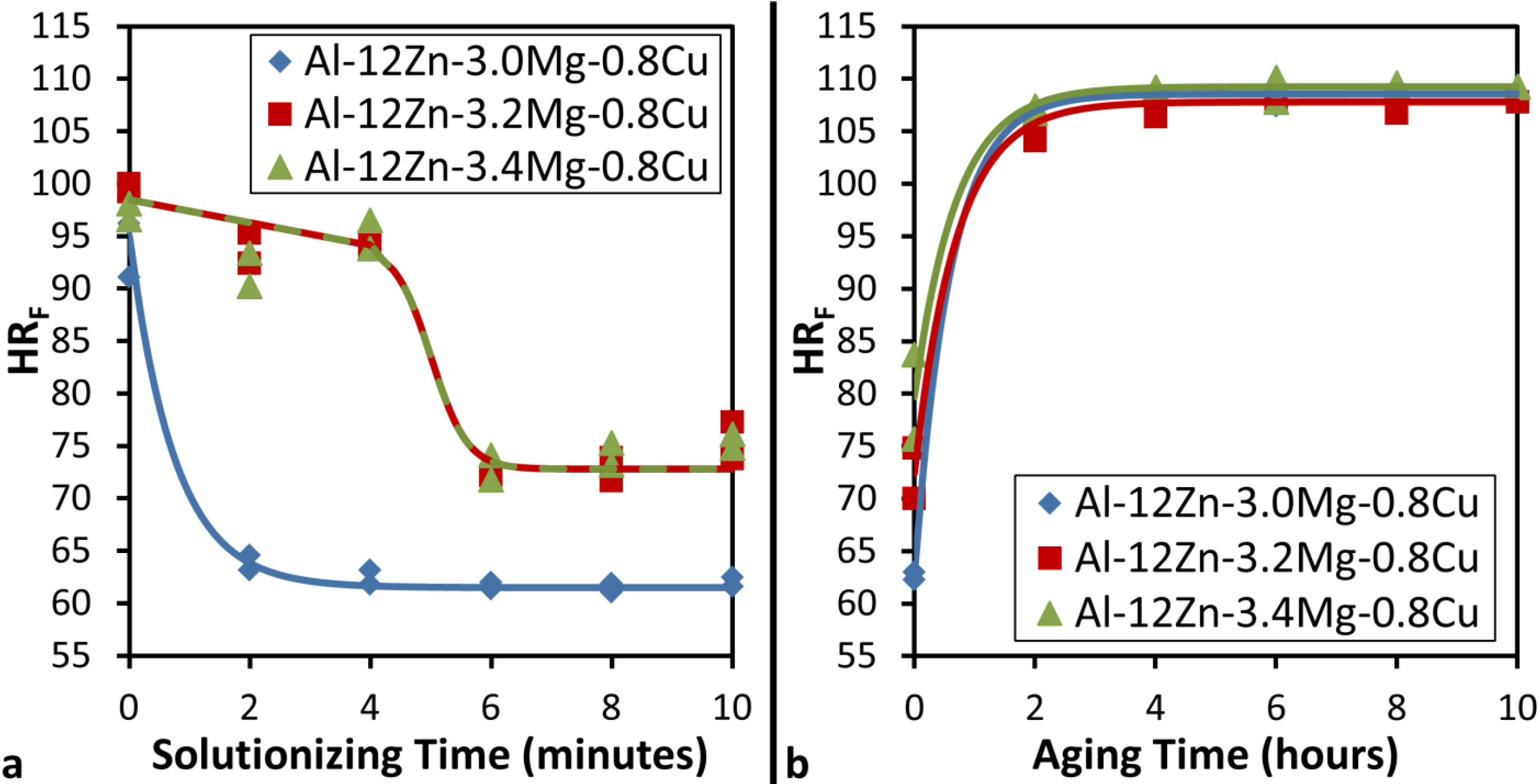
Figure 3a shows that though there is negligible difference in the solutionizing behavior of the 3.2 and 3.4 wt% Mg alloys, this behavior is significantly different from a similar alloy that contains only 3 wt% Mg. Both the 3.2 and 3.4 wt% Mg alloys show the same slight decrease in hardness for the first few minutes and then a sudden transition to the minimum hardness between 4 and 6 min. This indicates the slow dissolution of a phase that is relatively stable at high temperatures that results in a large drop in strength when this phase is completely dissolved. The minimum hardness achieved in the solutionizing treatment is considerably higher than that attained in the alloy with only 3 wt% Mg. However, the negligible difference in minimum hardness between the 3.2 and 3.4 wt% Mg alloys is an indication that this hardness comes from an insoluble intermetallic phase. It is notable that with time there appears to be a slight, roughly linear increase in hardness that is absent from the 3 wt% Mg alloys. Because of the high Mg content, oxidation is more prevalent in these samples and contributes an additional hardness at the surface as this oxide was not removed prior to hardness testing.
Figure 3.
(a) Variation of Rockwell F Hardness (HRF) with solutionizing time at 753.15 K (480 °C) followed by a water quench and (b) Variation of Rockwell F Hardness (HRF) with aging time at 393 K (120 °C) in Al-(3 to 3.4)Mg-12Zn-0.8Cu alloys.
Figure 3b indicates that though there is considerably more Mg in these alloys, there is not much improvement in maximum hardness that can be achieved with aging treatments at 120 °C. This data indicates that increasing Mg concentration beyond 3 wt% Mg in these alloys results in a two phase material and limits the hardness that can be achieved by heat treating. The plotted trend lines indicate that, like the 3 wt% Mg alloys, the aging treatment follows the general form
HRF =
HRF Soln + (
HRFmax −
HRFSoln)(1 −
e−t∕τ).
The above results are consistent with the microstructures shown in
Figure 1, which shows typical microstructures of these alloys. In as-cast samples, intermetallics are clearly visible in interdendritic regions. After 2 min of the solutionizing treatment there is still a considerable amount of the intermetallic material and by 10 min, though some has dissolved, far more remains than in the lower Mg alloys.
2.3. Comparison to Casting Alloy Al-A206
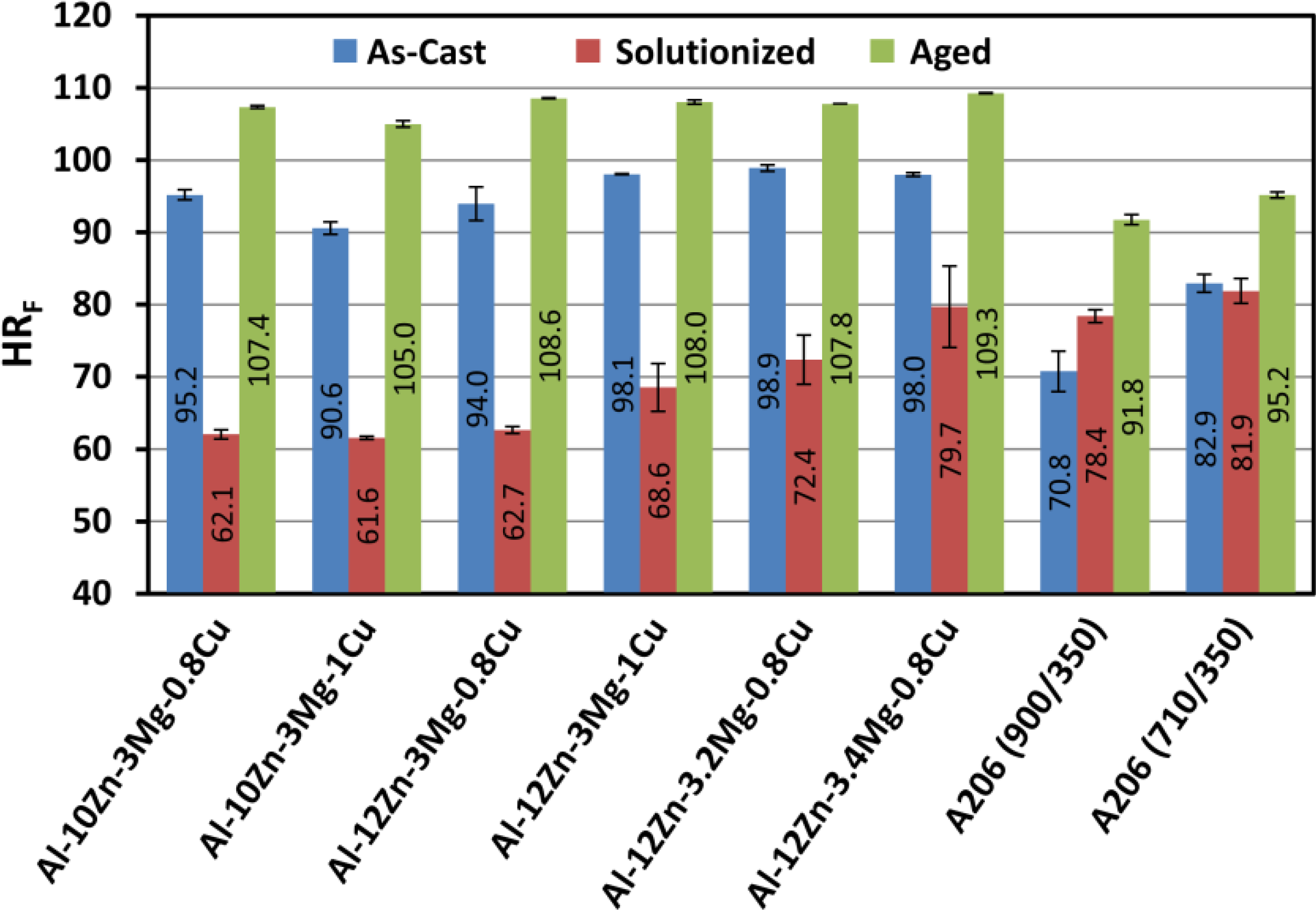
Figure 4 shows the as-cast, solutionized, and aged hardness values for the various alloys studied in this work. These values can be compared to values attained for squeeze cast Al-A206 casting alloy. Though the hardness is higher in the Al-A206 sample poured at 983.15 K (710 °C) due to smaller grain size than the Al-A206 sample poured at 1173.15 K (900 °C), it is clear that the solutionizing and aging heat treatments applied to the squeeze cast Al-Zn-Mg-Cu samples resulted in hardness values considerably higher than Al-A206 in the T7 condition. This indicates that squeeze casting may be a viable technique for fabricating near net shape components from high-strength alloys. Future investigations are necessary to investigate the tensile properties of squeeze cast Al-Zn-Mg-Cu alloys.
Figure 4.
Comparison of the Rockwell F Hardness (HRF) of various alloys in the As-cast, Solutionized, and Aged Conditions (Note: Data for Al-Zn-Mg-Cu Aged corresponds to 10 h aging treatment. Data for A206 Aged is for the T7 condition and numbers in parentheses denote pouring temperature and mold temperatures in degrees Celsius, respectively).
3. Experimental Section
Alloys listed in
Table 1 were produced for this study starting from commercially pure Al (Al-0.05%Si-0.06%Fe) obtained from NT Ruddock (Cleveland, OH, USA). The Al was first melted in A3 clay graphite crucibles at 1073.15 K (800 °C) and 99.98% pure Cu shot obtained from Fisher Chemical (Waltham, MA, USA) of the necessary weight to create the desired alloy composition was added to the melt and intermittently stirred by hand to ensure that the copper was melted and completely dissolved into the aluminum. After complete dissolution of the Cu, 99.9% pure Zn shot obtained from Fisher Chemical (USA) of the required quantity was added to the melt. After dissolution of the Zn, 99.8% pure Mg round stock obtained from Alfa Aesar (Ward Hill, MA, USA) of the required quantity was added to the melt. To compare the properties of the various Al-Zn-Mg-Cu alloys to a high strength, heat-treatable casting alloy, Aluminum Alloy A206 obtained from NT Ruddock (Cleveland, OH, USA) was also melted in A3 clay graphite crucibles at 983.15 K (710 °C) and 1173.15 K (900 °C).
Table 1.
Alloy compositions and processing conditions.
| Alloy | Composition | Melt Temp K/(°C) | Mold Temp K/(°C) |
|---|
| Al | Zn | Mg | Cu |
|---|
| AA7034 | Balance | 10 | 3 | 0.8 | 1073.15 (800) | 623.15 (350) |
| Balance | 10 | 3 | 1 | 1073.15 (800) | 623.15 (350) |
| Balance | 12 | 3 | 0.8 | 1073.15 (800) | 623.15 (350) |
| Balance | 12 | 3 | 1 | 1073.15 (800) | 623.15 (350) |
| AA7034 + High Mg | Balance | 12 | 3.2 | 0.8 | 1073.15 (800) | 623.15 (350) |
| Balance | 12 | 3.4 | 0.8 | 1073.15 (800) | 623.15 (350) |
| A206-T7 | Balance | 0 | 0.2–0.5 | 4.2–5.0 | 1173.15 (900) | 623.15 (350) |
| Balance | 0 | 0.2–0.5 | 4.2–5.0 | 983.15 (710) | 623.15 (350) |
All alloys were cast by pouring the liquid into a permanent steel mold preheated to 623.15 K (350 °C) and immediately squeezed at 24 MPa until solid and a billet of 100.6 mm × 19 mm × 55 mm was formed. Square coupons (dimensions 19 mm × 19 mm × 6.4 mm) were cut from the bottom 7 mm of the sample for heat treatment testing. To determine the solutionizing behavior of the Al-Zn-Mg-Cu alloys, samples were placed in a Thermoscientific Thermolyne heat treatment furnace at a temperature of 753.15 K (480 °C) for 2 min intervals, then quenched in 293.15 K (20 °C) quiescent water, after which the two Rockwell F Hardness (HRF) measurements were immediately determined. This process was repeated until a total solutionizing time of 10 min was reached. To determine the aging behavior, samples were solutionized at 753.15 K (480 °C) for 10 min, quenched in 293.15 K (20 °C) quiescent water, and then aged at 393.15 K (120 °C) for 2 h intervals. After each of these intervals, two Rockwell F Hardness (HRF) measurements were immediately determined. The samples were returned to the heat treat furnace for further aging until a time of 10 h was reached. Al-A206 was heat treated to T7 condition according to Alcan’s (Montreal, Quebec, Canada) specification.
Optical micrographs were obtained by polishing sample to a mirror finish for characterization using a Nikon Eclipse TS100 optical microscopy (Tokyo, Japan).
4. Conclusions
For Al-(10 to 12)Zn-3Mg-(0.8 to 1.0)Cu alloys:
Minimum hardness was achieved after approximately 8 min at 753.15 K (480 °C) followed by water quenching.
The minimum hardness increased with increasing Cu and Zn content.
Artificial aging of solutionized samples at 393.15 K (120 °C) resulted in maximum hardness after approximately 6 h.
Higher Zn content resulted in higher maximum hardness, but higher Cu content did not significantly improve hardness.
Maximum hardness was achieved more rapidly in alloys with lower Cu concentrations
For Al-12Zn-(3.2 to 3.4)Mg-0.8Cu alloys:
Al-12Zn-(3.2 to 3.4)Mg-0.8Cu alloys showed a different behavior from similar alloys with only 3 wt% Mg, where there was a slow dissolution of a phase that is relatively stable at high temperatures followed by a significant decrease in hardness when this phase was completely dissolved.
After 10 min of the solutionizing treatment, optical microscopy showed that a second phase still remained.
Maximum hardness after 10 h of aging was not significantly higher in the 3.2 and 3.4 wt% alloys when compared to the 3 wt% Mg alloy of similar composition.
All of the squeeze cast Al-Zn-Mg-Cu alloys attained higher maximum hardness than what was obtained by heat treating squeeze cast Al-A206 to the T7 condition, suggesting that squeeze casting may be a viable technique for fabricating near net shape components from these high-strength alloys.
Acknowledgments
This research was supported by the U.S. Army-TARDEC through TACOM R&D Contract# W56HZV-08-C-0716. Disclaimer: Reference herein to any specific commercial company, product, process, or service by trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or the Department of the Army (DoA). The opinions of the authors expressed herein do not necessarily state or reflect those of the United States Government or the DoA, and shall not be used for advertising or product endorsement purposes.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, H.; Xu, J.; Kang, Y.; Tang, M.; Zhang, Z. Study on inhomogeneous characteristics and optimize homogenization treatment parameter for large size DC ingots of Al-Zn-Mg-Cu alloys. J. Alloys Compd. 2014, 585, 19–24. [Google Scholar] [CrossRef]
- Fan, C.H.; Zhen, Z.H.; He, W.Q.; Chen, J.H.; Chen, D. Effects of the casting temperature on microstructure and mechanical properties of the squeeze-cast Al-Zn-Mg-Cu alloy. J. Alloys Compd. 2010, 504, L42–L45. [Google Scholar] [CrossRef]
- Hajjari, E.; Divandari, M. An investigation on the microstructure and tensile properties of direct squeeze cast and gravity die cast 2024 wrought Al alloy. Mater. Des. 2008, 29, 1685–1689. [Google Scholar] [CrossRef]
- Yue, T.M. Squeeze casting of high-strength aluminium wrought alloy AA7010. J. Mater. Proc. Technol. 1997, 66, 179–185. [Google Scholar] [CrossRef]
- Sukumaran, K.; Ravikumar, K.K.; Pillai, S.G.K.; Rajan, T.P.D.; Ravi, M.; Pillai, R.M.; Pai, B.C. Studies on squeeze casting of Al 2124 alloy and 2124-10% SiCp metal matrix composite. Mater. Sci. Eng. A 2008, 490, 235–241. [Google Scholar] [CrossRef]
- Li, X.M.; Starink, M.J. The effect of compositional variations on the characteristics of coarse intermetallic particles in over-aged 7xxx Al alloys. Mater. Sci. Technol. 2001, 17, 1324–1328. [Google Scholar] [CrossRef]
- Chen, Z.; Mo, Y.; Nie, Z. Effect of Zn content on the microstructure and properties of super-high strength Al-Zn-Mg-Cu alloys. Metall. Mater. Trans. A 2013, 44, 3910–3920. [Google Scholar] [CrossRef]
- Marlaud, T.; Deschamps, A.; Bley, F.; Lefebvre, W.; Baroux, B. Influence of alloy composition and heat treatment on precipitate composition in Al-Zn-Mg-Cu alloys. Acta Mater. 2010, 58, 248–260. [Google Scholar] [CrossRef]
- Verhoeven, J.D. Fundamentals of Physical Metallurgy, 1st ed.; John Wiley & Sons: New York, NY, USA, 1975; pp. 518–519. [Google Scholar]
- Skoko, Z.; Popovic, Z.; Stefanic, G. Microstructure of Al-Zn and Zn-Al alloys. Croat. Chem. Acta. 2009, 82, 405–420. [Google Scholar]
- Lubarda, V.A. On the effective lattice parameter of binary alloys. Mech. Mater. 2003, 35, 53–68. [Google Scholar] [CrossRef]
- Kaufman, J.G. Aluminum Alloy Database; Knovel: Norwich, NY, USA, 2009. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).