The Role of Epigenetics on Dental Implant Therapy: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
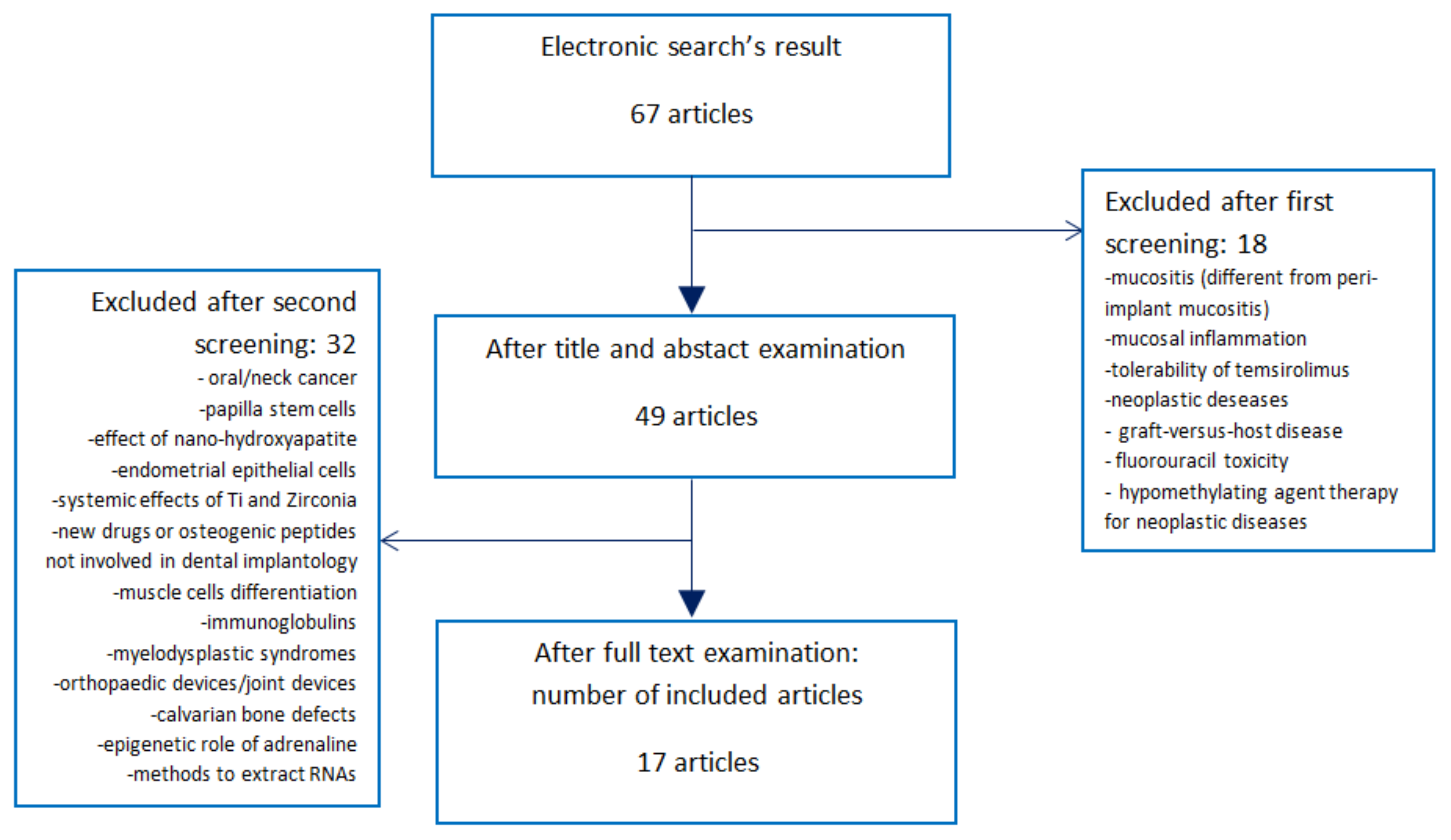
2.2. Selection of Studies
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.3. Development of the Review
3. Results
3.1. In Vivo and In Vitro Studies
3.2. Cell Population
3.3. Implant Surfaces
3.4. Comparative Analysis
3.5. Randomized Clinical Trial
3.6. Narrative Review
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| SLA | sandblasted acid-etched titanium surface |
| modSLA | SLA surface with an N2 protection and stored in an isotonic saline solution |
| MAO | microarch-oxidated titanium |
| CH/HA/mR-21 | chitosan/hyaluronic acid surface with miRNA-21 |
| Ti6A14V | micron-scale rough titanium alloy |
| EE | micro/nanostructured surface electrolytic etched |
| M | machined surface |
| TiO | TiOBlast; surface blasted with TiO2 |
| OS | osseospeed; surface blasted with TiO2 then treated with hydrofluoric acid |
| SMO | smooth polished surface |
| AT-1 | oxalic acid and hydrofluoric acid treated surface |
| AT-2 | oxalic acid treated surface |
| Ti6A14V | micron-scale rough titanium alloy (# indicates different dimension of roughness parameters) |
| TCPS | polystyrene surface |
| ALP | alkaline phosphatase |
| BMP | bone morphogenic protein |
| BSP | bone sialoprotein |
| Runx | runt-related transcription factor |
| OCN | Osteocalcin |
| PTHrp | parathyroid hormone-related protein |
| PTH | parathyroid hormone |
| BIC | bone-implant contact |
| POSTN | periostin related factor |
| VEGF | vascular endothelial growth factor |
| COL1 | collagen type Iα1 |
| COL3 | collagen type IIIα1 |
| OPN | Osteopontin |
| FGF | fibroblast growth factor |
| ITGA | integrin subunit |
| SHOX | short stature homeobox-containing gene |
| IGF | insulin-like grow factor |
| NOG | noggin gene |
| PRDX | Peroxiredoxin |
| ADAMTS | gene encoding for disintegrin and metalloproteinase with thrombospondin motifs |
| AMBN | Ameloblastin |
| PHEX | phosphate-regulating neutral endopeptidase |
| FBN | Fibrillin |
| CALCA | calcitonin related polypeptide alpha |
| TFIP | tissue factor pathway inhibitor |
| OSX | osteoblast-specific transcription factor, osterix |
Appendix A
(("epigenomics"[MeSH Terms] OR "epigenomics"[All Fields] OR "epigenetics"[All Fields]) OR ("dna methylation"[MeSH Terms] OR ("dna"[All Fields] AND "methylation"[All Fields]) OR "dna methylation"[All Fields]) OR (("dna"[MeSH Terms] OR "dna"[All Fields]) AND methyl[All Fields] AND ("transferases"[MeSH Terms] OR "transferases"[All Fields] OR "transferase"[All Fields])) OR (("histones"[MeSH Terms] OR "histones"[All Fields] OR "histone"[All Fields]) AND deacetylation[All Fields]) OR ("histone deacetylases"[MeSH Terms] OR ("histone"[All Fields] AND "deacetylases"[All Fields]) OR "histone deacetylases"[All Fields] OR ("histone"[All Fields] AND "deacetylase"[All Fields]) OR "histone deacetylase"[All Fields]) OR (("histones"[MeSH Terms] OR "histones"[All Fields] OR "histone"[All Fields]) AND methyl[All Fields] AND ("transferases"[MeSH Terms] OR "transferases"[All Fields] OR "transferase"[All Fields])) OR (("histones"[MeSH Terms] OR "histones"[All Fields] OR "histone"[All Fields]) AND demethylase[All Fields]) OR ("micrornas"[MeSH Terms] OR "micrornas"[All Fields] OR ("micro"[All Fields] AND "rna"[All Fields]) OR "micro rna"[All Fields])) AND (("dental implants"[MeSH Terms] OR ("dental"[All Fields] AND "implants"[All Fields]) OR "dental implants"[All Fields] OR ("dental"[All Fields] AND "implant"[All Fields]) OR "dental implant"[All Fields]) OR ("dental implants"[MeSH Terms] OR ("dental"[All Fields] AND "implants"[All Fields]) OR "dental implants"[All Fields]) OR implantology[All Fields] OR ("Implant Dent"[Journal] OR ("implant"[All Fields] AND "dentistry"[All Fields]) OR "implant dentistry"[All Fields]) OR (implant[All Fields] AND failure[All Fields]) OR (platform[All Fields] AND shifting[All Fields]) OR (platform[All Fields] AND switching[All Fields]) OR (implant-abutment[All Fields] AND connection[All Fields]) OR ("osseointegration"[MeSH Terms] OR "osseointegration"[All Fields]) OR ("mucositis"[MeSH Terms] OR "mucositis"[All Fields]) OR perimplantitis[All Fields]).
References
- Berglundh, T.; Persson, L.; Klinge, B. A systematic review of the incidence of biological and technical complications in implant dentistry reported in prospective longitudinal studies of at least 5 years. J. Clin. Periodontol. 2002, 29 (Suppl. S3), 197–212. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, A.; Pohler, O.; Sutter, F. Tissue reaction to an implant of a titanium hollow cylinder with a titanium surface spray layer. SSO Schweiz. Monatsschr. Zahnheilkd. 1976, 86, 713–727. [Google Scholar] [PubMed]
- Adell, R.; Lekholm, U.; Rockler, B.; Brånemark, P.I. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int. J. Oral Surg. 1981, 10, 387–416. [Google Scholar] [CrossRef]
- Roos-Jansåker, A.M.; Lindahl, C.; Renvert, H.; Renvert, S. Nine to fourteen-year follow-up of implant treatment. Part I: Implant loss and associations to various factors. J. Clin. Periodontol. 2006, 33, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Roos-Jansåker, A.M.; Lindahl, C.; Renvert, H.; Renvert, S. Nine to fourteen-year follow-up of implant treatment. Part II: Presence of peri-implant lesions. J. Clin. Periodontol. 2006, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Lindhe, J.; Meyle, J. Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. J. Clin. Periodontol. 2008, 35 (Suppl. S8), 282–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broggini, N.; McManus, L.M.; Hermann, J.S.; Medina, R.; Schenk, R.K.; Buser, D.; Cochran, D.L. Peri-implant inflammation defined by the implant-abutment interface. J. Dent. Res. 2006, 85, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Piattelli, A.; Vrespa, G.; Petrone, G.; Iezzi, G.; Annibali, S.; Scarano, A. Role of the microgap between implant and abutment: A retrospective histologic evaluation in monkeys. J. Periodontol. 2003, 74, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A.; Buser, D.; Lang, N.P. Colonization of osseointegrated titanium implants in edentulous patients. Early results. Oral Microbiol. Immunol. 1988, 3, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A. Microbiology and antimicrobial therapy of peri-implantitis. Periodontology 2000 2002, 28, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Thomsen, P.; Ericson, L.E.; Lekholm, U. Histopathologic observations on early oral implant failures. Int. J. Oral Maxillofac. Implant. 1999, 14, 798–810. [Google Scholar]
- Esposito, M.; Hirsch, J.; Lekholm, U.; Thomsen, P. Differential diagnosis and treatment strategies for biologic complications and failing oral implants: A review of the literature. Int. J. Oral Maxillofac. Implant. 1999, 14, 473–490. [Google Scholar]
- Salcetti, J.M.; Moriarty, J.D.; Cooper, L.F.; Smith, F.W.; Collins, J.G.; Socransky, S.S.; Offenbacher, S. The clinical, microbial, and host response characteristics of the failing implant. Int. J. Oral Maxillofac. Implant. 1997, 12, 32–42. [Google Scholar]
- Berger, S.L.; Kouzarides, T.; Shiekhattar, R.; Shilatifard, A. An operational definition of epigenetics. Genes Dev. 2009, 23, 781–783. [Google Scholar] [CrossRef] [PubMed]
- Martos, S.N.; Tang, W.Y.; Wang, Z. Elusive inheritance: Transgenerational effects and epigenetic inheritance in human environmental disease. Prog. Biophys. Mol. Biol. 2015, 118, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Fazzari, M.J.; Greally, J.M. Epigenomics: Beyond CpG islands. Nat. Rev. Genet. 2004, 5, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Rothstein, M.A.; Cai, Y.; Marchant, G.E. The ghost in our genes: Legal and ethical implications of epigenetics. Health Matrix Clevel. 2009, 19, 1–62. [Google Scholar] [PubMed]
- Williams, S.D.; Hughes, T.E.; Adler, C.J.; Brook, A.H.; Townsend, G.C. Epigenetics: A new frontier in dentistry. Aust. Dent. J. 2014, 59 (Suppl. S1), 23–33. [Google Scholar] [CrossRef] [PubMed]
- Barros, S.P.; Offenbacher, S. Epigenetics: Connecting environment and genotype to phenotype and disease. J. Dent. Res. 2009, 88, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, Z. Adrenaline inhibits osteogenesis via repressing miR-21 expression. Cell Biol. Int. 2017, 41, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Bishop, K.S.; Ferguson, L.R. The interaction between epigenetics, nutrition and the development of cancer. Nutrients 2015, 7, 922–947. [Google Scholar] [CrossRef] [PubMed]
- Egger, G.; Liang, G.; Aparicio, A.; Jones, P.A. Epigenetics in human disease and prospects for epigenetic therapy. Nature 2004, 429, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.A.; Liang, G. Rethinking how DNA methylation patterns are maintained. Nat. Rev. Genet. 2009, 10, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Hark, A.T.; Schoenherr, C.J.; Katz, D.J.; Ingram, R.S.; Levorse, J.M.; Tilghman, S.M. CTCF mediates methylation-sensitive enhancer-blocking activity at the H19/Igf2 locus. Nature 2000, 405, 486–489. [Google Scholar] [PubMed]
- Vrtačnik, P.; Marc, J.; Ostanek, B. Epigenetic mechanisms in bone. Clin. Chem. Lab. Med. 2014, 52, 589–608. [Google Scholar] [CrossRef] [PubMed]
- Bayarsaihan, D. Epigenetic mechanisms in inflammation. J. Dent. Res. 2011, 90, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Razidlo, D.F.; Whitney, T.J.; Casper, M.E.; McGee-Lawrence, M.E.; Stensgard, B.A.; Li, X.; Secreto, F.J.; Knutson, S.K.; Hiebert, S.W.; Westendorf, J.J. Histone deacetylase 3 depletion in osteo/chondroprogenitor cells decreases bone density and increases marrow fat. PLoS ONE 2010, 5, e11492. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.S.; Alliston, T.; Delston, R.; Derynck, R. Repression of Runx2 function by TGF-β through recruitment of class II histone deacetylases by Smad3. EMBO J. 2005, 24, 2543–2555. [Google Scholar] [CrossRef] [PubMed]
- Harakalova, M.; Van den Boogaard, M.J.; Sinke, R.; Van Lieshout, S.; Van Tuil, M.C.; Duran, K.; Renkens, I.; Terhal, P.A.; De Kovel, C.; Nijman, I.J.; et al. X-exome sequencing identifies a HDAC8 variant in a large pedigree with X-linked intellectual disability, truncal obesity, gynaecomastia, hypogonadism and unusual face. J. Med. Genet. 2012, 49, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Suh, J.H.; Kim, A.Y.; Lee, Y.S.; Park, S.Y.; Kim, J.B. Histone deacetylase 1-mediated histone modification regulates osteoblast differentiation. Mol. Endocrinol. 2006, 20, 2432–2443. [Google Scholar] [CrossRef] [PubMed]
- Shookhoff, J.M.; Gallicano, G.I. The emerging role of microRNAs in adult stem cells. In Stem Cell Biology and Regenerative Medicine; Turksen, K., Ed.; Humana Press: New York, NY, USA; Springer: Berlin, Germany, 2011; Volume 1, pp. 57–94. [Google Scholar]
- Fakhry, M.; Hamade, E.; Badran, B.; Buchet, R.; Magne, D. Molecular mechanisms of mesenchymal stem cell differentiation towards osteoblasts. World J. Stem Cells 2013, 5, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xie, R.L.; Croce, C.M.; Stein, J.L.; Lian, J.B.; Van Wijnen, A.J.; Stein, G.S. A program of microRNAs controls osteogenic lineage progression by targeting transcription factor Runx2. Proc. Natl. Acad. Sci. USA 2011, 108, 9863–9868. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhao, L.; Xing, L.; Chen, D. MicroRNA-204 regulates Runx2 protein expression and mesenchymal progenitor cell differentiation. Stem Cells 2010, 28, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Kang, I.H.; Lee, J.W.; Jang, W.G.; Koh, J.T. MiR-433 mediates ERRγ-suppressed osteoblast differentiation via direct targeting to Runx2 mRNA in C3H10T1/2 cells. Life Sci. 2013, 92, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Andia, D.C.; De Oliveira, N.F.; Casarin, R.C.; Casati, M.Z.; Line, S.R.; De Souza, A.P. DNA methylation status of the IL-8 gene promoter in aggressive periodontitis. J. Periodontol. 2010, 81, 1336–1341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Barros, S.P.; Niculescu, M.D.; Moretti, A.J.; Preisser, J.S.; Offenbacher, S. Alteration of PTGS2 promoter methylation in chronic periodontitis. J. Dent. Res. 2010, 89, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Crivello, A.; Offenbacher, S.; Moretti, A.; Paquette, D.W.; Barros, S.P. Interferon-gamma promoter hypomethylation and increased expression in chronic periodontitis. J. Clin. Periodontol. 2010, 37, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.O.; An, J.Y.; Yin, L.; Hacker, B.M.; Rohani, M.G.; Dommisch, H.; DiJulio, D.H. Interplay of protease activated receptors and NOD pattern recognition receptors in epithelial innate immune responses to bacteria. Immunol. Lett. 2010, 131, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Darveau, R.P.; Pham, T.T.; Lemley, K.; Reife, R.A.; Bainbridge, B.W.; Coats, S.R.; Howald, W.N.; Way, S.S.; Hajjar, A.M. Porphyromonas gingivalis lipopolysaccharide contains multiple lipid A species that functionally interact with both toll-like receptors 2 and 4. Infect. Immun. 2004, 72, 5041–5051. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.O.; Hansen, S.R.; Rao, D.; Dale, B.A. Protease-activated receptor signaling increases epithelial antimicrobial peptide expression. J. Immunol. 2004, 173, 5165–5170. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, N.F.; Damm, G.R.; Andia, D.C.; Salmon, C.; Nociti, F.H., Jr.; Line, S.R.; De Souza, A.P. DNA methylation status of the IL8 gene promoter in oral cells of smokers and non-smokers with chronic periodontitis. J. Clin. Periodontol. 2009, 36, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Chung, W.O. Epigenetic regulation of human ß-defensin 2 and CC chemokine ligand 20 expression in gingival epithelial cells in response to oral bacteria. Mucosal Immunol. 2011, 4, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.J.; Mulrow, C.D.; Haynes, R.B. Systematic reviews: Synthesis of best evidence for clinical decisions. Ann. Intern. Med. 1997, 126, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Mulrow, C.; Langhorne, P.; Grimshaw, J. Integrating heterogeneous pieces of evidence in systematic reviews. Ann. Intern. Med. 1997, 127, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Giannoni, P.; Muraglia, A.; Giordano, C.; Narcisi, R.; Cancedda, R.; Quarto, R.; Chiesa, R. Osteogenic differentiation of human mesenchymal stromal cells on surface-modified titanium alloys for orthopedic and dental implants. Int. J. Artif. Organs 2009, 32, 811–820. [Google Scholar] [PubMed]
- Iaculli, F.; Di Filippo, E.S.; Piattelli, A.; Mancinelli, R.; Fulle, S. Dental pulp stem cells grown on dental implant titanium surfaces: An in vitro evaluation of differentiation and microRNAs expression. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 953–965. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Zhou, Y.; Zhang, Y.; Cai, Q.; Yang, L.; Wang, B. Effects of hierarchical micro/nano-textured titanium surface features on osteoblast-specific gene expression. Implant Dent. 2013, 22, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Thalji, G.; Cooper, L.F.; Nares, S. Gene Expression Profiles of Early Implant Adherent Cells in Smokers and Nonsmokers. J. Oral Implantol. 2015, 41, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Zhou, L.; Wang, J.; Zhao, Q.; Lin, X.; Gao, Y.; Li, S.; Wu, J.; Rong, M.; Guo, Z.; et al. The effects of hierarchical micro/nanosurfaces decorated with TiO2 nanotubes on the bioactivity of titanium implants in vitro and in vivo. Int. J. Nanomed. 2015, 10, 6955–6973. [Google Scholar]
- Chakravorty, N.; Ivanovski, S.; Prasadam, I.; Crawford, R.; Oloyede, A.; Xiao, Y. The microRNA expression signature on modified titanium implant surfaces influences genetic mechanisms leading to osteogenic differentiation. Acta Biomater. 2012, 8, 3516–3523. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, G.; Feng, Z.; Bai, S.; Dong, Y.; Wu, G.; Zhao, Y. Microarc-oxidized titanium surfaces functionalized with microRNA-21-loaded chitosan/hyaluronic acid nanoparticles promote the osteogenic differentiation of human bone marrow mesenchymal stem cells. Int. J. Nanomed. 2015, 10, 6675–6687. [Google Scholar]
- Palmieri, A.; Pezzetti, F.; Brunelli, G.; Lo Muzio, L.; Scarano, A.; Scapoli, L.; Martinelli, M.; Arlotti, M.; Guerzoni, L.; Rubini, C.; et al. Short-period effects of zirconia and titanium on osteoblast microRNAs. Clin. Implant Dent. Relat. Res. 2008, 10, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Thalji, G.; Gretzer, C.; Cooper, L.F. Comparative molecular assessment of early osseointegration in implant-adherent cells. Bone 2013, 52, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Wimmers Ferreira, M.R.; Rodrigo Fernandes, R.; Freire Assis, A.; Dernowsek, J.A.; Passos, G.A.; Variola, F.; Fittipaldi Bombonato-Prado, K. Oxidative nanopatterning of titanium surface influences mRNA and MicroRNA expression in human alveolar bone osteoblastic cells. Int. J. Biomater. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, A.; Pezzetti, F.; Brunelli, G.; Zollino, I.; Lo Muzio, L.; Martinelli, M.; Scapoli, L.; Arlotti, M.; Masiero, E.; Carinci, F. Zirconium oxide regulates RNA interfering of osteoblast-like cells. J. Mater. Sci. Mater. Med. 2008, 19, 2471–2476. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, A.; Pezzetti, F.; Brunelli, G.; Arlotti, M.; Lo Muzio, L.; Scarano, A.; Rubini, C.; Sollazzo, V.; Massari, L.; Carinci, F. Anatase nanosurface regulates microRNAs. J. Craniofac. Surg. 2008, 19, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Song, W.; Zhao, L.; Liu, M.; Yan, J.; Andersen, M.Ø.; Kjems, J.; Gao, S.; Zhang, Y. MicroRNA functionalized microporous titanium oxide surface by lyophilization with enhanced osteogenic activity. ACS Appl. Mater. Interfaces 2013, 5, 2733–2744. [Google Scholar] [CrossRef] [PubMed]
- Marinucci, L.; Balloni, S.; Becchetti, E.; Belcastro, S.; Guerra, M.; Calvitti, M.; Lilli, C.; Calvi, E.M.; Locci, P. Effect of titanium surface roughness on human osteoblast proliferation and gene expression in vitro. Int. J. Oral Maxillofac. Implant. 2006, 21, 719–725. [Google Scholar]
- Olivares-Navarrete, R.; Hyzy, S.L.; Berg, M.E.; Schneider, J.M.; Hotchkiss, K.; Schwartz, Z.; Boyan, B.D. Osteoblast lineage cells can discriminate microscale topographic features on titanium-aluminum-vanadium surfaces. Ann. Biomed. Eng. 2014, 42, 2551–2561. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.H.; Rios, H.; Al-Hezaimi, K.; Oh, T.J.; Benavides, E.; Wang, H.L. A randomized clinical trial evaluating the efficacy of the sandwich bone augmentation technique in increasing buccal bone thickness during implant placement. II. Tomographic, histologic, immunohistochemical, and RNA analyses. Clin. Oral Implant. Res. 2015, 26, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Razzouk, S.; Sarkis, R. Smoking and Diabetes Epigenetics Involvement in Osseointegration. N. Y. State Dent. J. 2013, 79, 27–30. [Google Scholar] [PubMed]
- Lozano, D.; De Castro, L.F.; Dapia, S.; Andrade-Zapata, L.; Manzarbeitia, F.; Alvarez-Arroyo, M.V.; Gómez-Barrena, E.; Esbrit, P. Role of parathyroid hormone-related protein in the decreased osteoblast function in diabetes-related osteopenia. Endocrinology 2009, 150, 2027–2035. [Google Scholar] [CrossRef] [PubMed]
- Ferron, M.; Wei, J.; Yoshizawa, T.; Del Fattore, A.; DePinho, R.A.; Teti, A.; Ducy, P.; Karsenty, G. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell 2010, 142, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Mangano, C.; Paino, F.; d’Aquino, R.; De Rosa, A.; Iezzi, G.; Piattelli, A.; Laino, L.; Mitsiadis, T.; Desiderio, V.; Mangano, F.; et al. Human dental pulp stem cells hook into biocoral scaffold forming an engineered biocomplex. PLoS ONE 2011, 6, e18721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, R.S.; Bumann, A.; Schaffer, J.L.; Gerstenfeld, L.C. Pre-dominant integrin ligands expressed by osteoblasts show preferential regulation in response to both cell adhesion and mechanical perturbation. J. Cell. Biochem. 2002, 84, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Pieralli, S.; Kohal, R.J.; Jung, R.E.; Vach, K.; Spies, B.C. Clinical Outcomes of Zirconia Dental Implants. J. Dent. Res. 2017, 96, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Cionca, N.; Hashim, D.; Mombelli, A. Zirconia dental implants: Where are we now, and where are we heading? Periodontology 2000 2017, 73, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, Y.; Cheng, L. miR-21 expression is related to particle-induced osteolysis pathogenesis. J. Orthop. Res. 2012, 30, 1837–1842. [Google Scholar] [CrossRef] [PubMed]

| DNA Methylation | Histone Modification | MicroRNA |
|---|---|---|
|
|
|
| Effects of Different Surfaces on Genetic Expression | ||||||||
| Author | Year | Type | Surfaces Tested | Type of Cells | Evaluation of miRNAs | Gene Expression and Molecular Mechanism | Conclusion | |
| Giannoni P. [46] | 2009 | In vitro, research support. | KOH alkali-etched; NaOH alkali-etched; not etched surface. | Bone marrow stromal cells from iliac crest. | not provided (n.p.) | High expression of BSP was found on KOH etched surfaces. | KOH modifications seem to allow the best osteogenic differentiation of human mesenchymal stromal cells. | |
| Iaculli F. [47] | 2016 | In vitro, research support. | SLA surface without ionization (control); Ionized SLA surface (test). | Dental pulp stem cells. | Provided | miRNA-133a, miRNA-133b, and miRNA-135 influenced the expression of Runx2 and Smad5 genes. | Ionized sandblasted and acid-etched surface seemed to markedly enhance the development and differentiation of osteoblast cells. | |
| Meng W. [48] | 2013 | In vitro, research support. | EE; SLA; M. | MG-63 osteosarcoma cells. | n.p. | Gene expression is related to features of implant surface and the level of osteoblast differentiation. | Hierarchical micro-/nanostructured titanium surface treated by EE enhanced the ALP, OCN, Runx2, OPN activity, and COL1 mRNA gene expression of osteoblast. | |
| Thalji G. [49] | 2015 | In vivo (human). | TiO versus OS; smoker versus non-smoker. | Implant adherent cells (alveolar bone cells). | n.p. | The variable of time influences gene expression more that the effect of nicotine. Modified surfaces could soften the negative effect of nicotine. High number of genes has been investigated. | At early time points, similar trends in gene expression were noted in implant-adherent cells regardless of implant surface and smoking status. | |
| Ding X. [50] | 2015 | In vitro and in vivo (animal, beagle dog). | SLA + 30 nm; SLA + 50 nm; SLA + 80 nm; SLA without nanotubes. | MG-63 osteosarcoma cells (in vitro study). Beagles’ tibias cells (in vivo study). | n.p. | Nanotube diameters influence cell phenotype (filopodia and lamellipodia) and ALP, Runx2, and OCN genes expression. | SLA + 80 nm surface is the most favorable for promoting the activity of osteoblasts and early bone bonding. | |
| Chakravorty N. [51] | 2012 | In vitro, research support. | SLA; ModSLA; SMO. | Human alveolar bone cells. | Provided | Highest level of bone expression is documented on modSLA and SLA. 35 different types of miRNA were downregulated in modSLA, and 32 in SLA surfaces. High number of genes and miRNAs has been investigated. | The majority of miRNA were downregulated in response to the SLA and modSLA surfaces compared to the SMO one, with only relative changes found between SLA and modSLA. | |
| Wang Z. [52] | 2015 | In vitro, research support. | MAO; CS/HA/miR-21. | Human bone marrow mesenchymal stem cells (alveolar cells). | Provided only miRNA-21 | miRNA-21 induces upregulation of osteogenesis-related genes like COL1, COL3, Runx2, OPN, and OCN. | Titanium surfaces functionalized with miRNA-21 presented a significantly higher expression of osteogenic genes. | |
| Palmieri A. [53] | 2008 a | In vitro, research support. | Machined Ti; Zirconia. | MG-63 cells. | Provided | Six miRNAs were found upregulated in zirconia compared to Titanium (miR-214, miR-337, miR-423, miR-339, miR377, and miR-193b), and four downregulated (miR-143, miR-17-5p, miR-24, and miR-22). Bone related genes BMP4 and 7 were more expressed in osteoblasts exposed to Ti surface. | Ti surfaces could provide some advantages to earlier osteogenesis useful for immediate loading. | |
| Thalji G. [54] | 2013 | In vivo (animal, rat tibia model). | AT-1; AT-2. | Rat’s tibia cells. | n.p. | Significant differences at the gene level were not noted when comparing the two implant surfaces at each timepoint. However, genes were differentially regulated at day 4 vs. day 2 for both implant surfaces. High number of genes and miRNAs has been investigated. | The number of genes that were associated with the inflammatory or immune response category was greater for AT-1 than AT-2. | |
| Wimmers Ferreira M.R. [55] | 2016 | In vitro, research support. | Nanotextured; NanoSubmicrotextured; Rough microtextured; Smooth surface. | Human alveolar bone cells. | Provided | The nanotextured surface group showed the highest alkaline phosphatase activation. The rough microtextured surface group had the greatest amount of calcium produced. NOTCH1 gene increased its expression in nanosubmicrotexture surfaces. | Oxidative nanopatterning of titanium surfaces induces changes in the metabolism of osteoblastic cells and cell responses. | |
| Palmieri A. [56] | 2008 b | In vitro, research support. | Zirconium Oxide; Control group not provided. | MG-63 cells. | Provided | Zirconia disks upregulated 18 miRNAs and downregulated 3 miRNAs related to osseogenetic genes. The most notable osseogenic genes influences by zirconia are NOG, SHOX, IGF1, BMP1, and FGFR1. | Zirconium oxide surfaces influence genic expression, however speculations about clinical outcomes of zirconia implants were not provided. | |
| Palmieri A. [57] | 2008 c | In vitro, research support. | Anatase coating; Control group not provided. | MG-63 cells | Provided | There were 9 upregulated miRNAs and 10 downregulated miRNAs. PRDX1, COL9A2, ADAMTS4, SHOX and ALPL, AMBN, andTUFT1 were upregulated. PHEX, FBN1, IGFBP4, CALCA, TFIP1 and PTH were downregulated. | Anatase colloidal solution regulates osteogenic genes and miRNAs, however clinical speculations were not provided. | |
| Wu K. [58] | 2013 | In vitro, research support. | MAO + miR29b; MAO + antimiR138; MAO surface. | Rat bone marrow cells. | provided | For the genes BMP, OCN, OSX, and Runx2, the antimiR-138 functionalized surface induces higher expression. For COL1, the miR-29b functionalized surface induces higher expression than using antimiR-138, whereas this trend is reversed after 14 days of culture. The miR-29b functionalized surface induces higher expression of ALP. | MicroRNA-29b enhances osteogenic activity and antimicroRNA-138 inhibits miR-138, inhibitors of endogenous osteogenesis. Clear stimulation of osteogenic process was observed, in terms of upregulating osteogenic expression and enhancing alkaline phosphatase production, collagen secretion and mineralization. | |
| Marinucci L. [59] | 2006 | In vitro, research support. | Machined; Micro-sandblasted; Macro-sandblasted. | Human alveolar bone cells. | n.p. | All blasted surfaces showed significantly higher DNA synthesis than the machined surfaces. Other mRNA transcripts were increased in osteoblasts cultured on rough titanium surfaces, particularly the macro-sandblasted surface. | Macro-sandblasted titanium showed best results in favoring osteoblast differentiation. | |
| Olivares-Navarette R. [60] | 2014 | In vitro, research support. | TCPS; Ti6A14V #5; Ti6A14V #9; Ti6A14V #12. | Human mesenchymal stem cells; human osteoblasts. | n.p. | Test #9 showed greater ALP activity, OCN and osteoprotegerin production. BMP2 and BMP4 were highest in cultures grown on #9, as were VEGF-A and FGF-2. Integrin expression also varied with the surface. mRNAs for all integrin subunits except ITGA-5 were higher when cells were cultured on test substrates than on TCPS. | Osteoblast lineage cells are sensitive to specific micro/nanostructures. | |
| Comparison of Clinical Treatments | ||||||||
| Author | Year | Type | No. of Patients | Bone Defect | Clinical Treatments Tested | Evaluation of miRNAs | Results | Conclusion |
| Fu J.H. [61] | 2015 | RCT with immuno-histochemical and RNA analyses. | 26 patients 13 (test) 13 (contr) | Buccal implant dehiscences in maxilla. | Defects treated with bone particulate allograft (control) or bone and pericardium membrane (test). | Not provided. | No significant differences in POSTN, Runx2, and VEGF expressions between test and control groups were found. Epigenetic mechanism was not provided. | Bone preserved with the membrane was bigger in volume but less mineralized and more fibrous. No significant differences in mRNA expression between the two groups were found. |
| Narrative Review | ||||||||
| Author | Year | Type | Epigenetic Target. | Mechanism | ||||
| Razzouk S. [62] | 2013 | Narrative review. | DNA methylation, Histone deacetylation. | Smoking downregulates osteopontin, Type 2 collagen, BMP-2, and osteoprotegerin. Diabetes influences the expression of PTHrP, OCN, Runx2, and OSX. | ||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Gianfilippo, R.; Di Gianfilippo, C.; Prato, G.P.P. The Role of Epigenetics on Dental Implant Therapy: A Systematic Review. Epigenomes 2017, 1, 12. https://doi.org/10.3390/epigenomes1020012
Di Gianfilippo R, Di Gianfilippo C, Prato GPP. The Role of Epigenetics on Dental Implant Therapy: A Systematic Review. Epigenomes. 2017; 1(2):12. https://doi.org/10.3390/epigenomes1020012
Chicago/Turabian StyleDi Gianfilippo, Riccardo, Carmine Di Gianfilippo, and Giovan Paolo Pini Prato. 2017. "The Role of Epigenetics on Dental Implant Therapy: A Systematic Review" Epigenomes 1, no. 2: 12. https://doi.org/10.3390/epigenomes1020012






