Increased Adult Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae) Abundance in a Dengue Transmission Hotspot, Compared to a Coldspot, within Kaohsiung City, Taiwan
Abstract
:1. Introduction
2. Materials and Methods
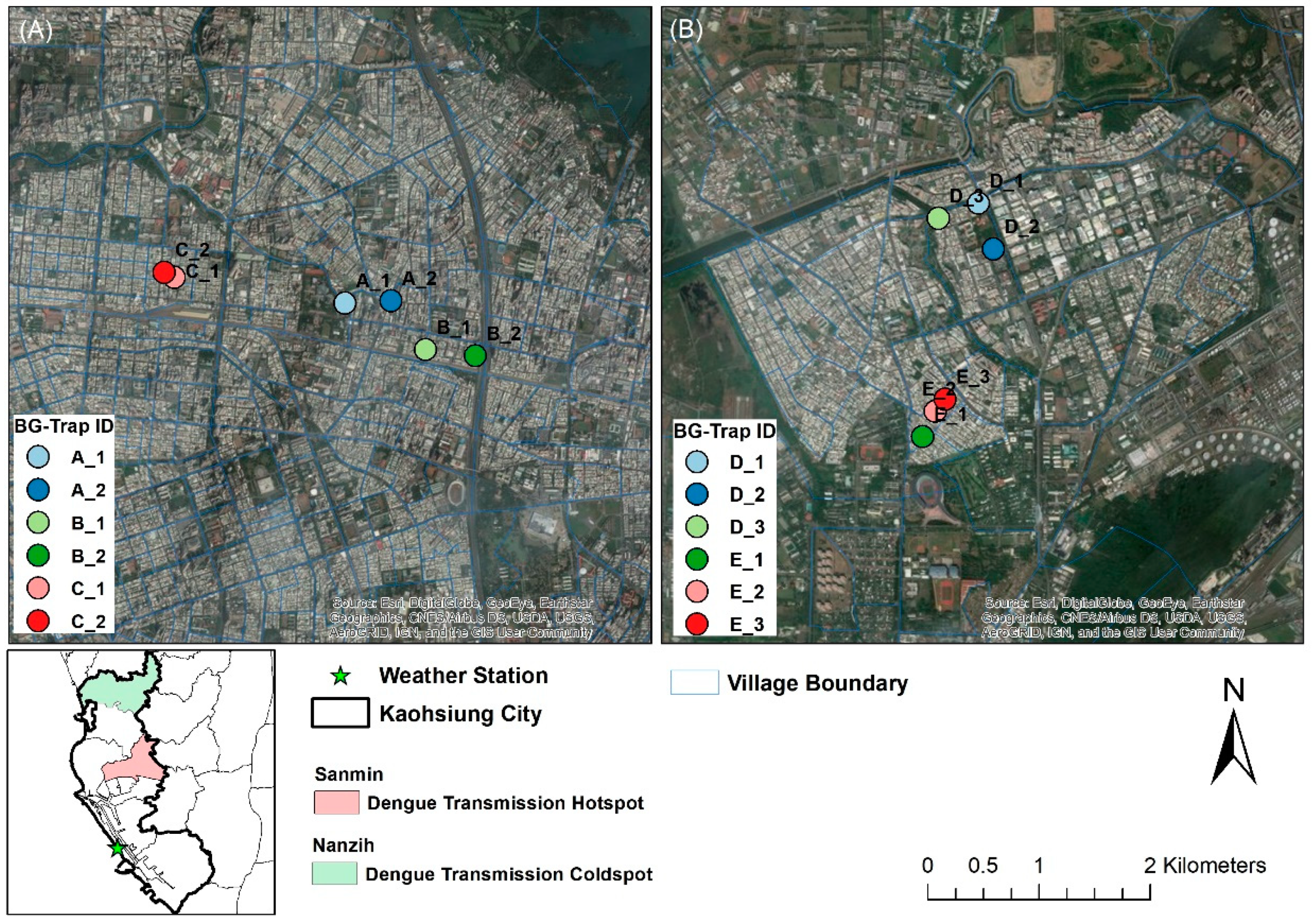
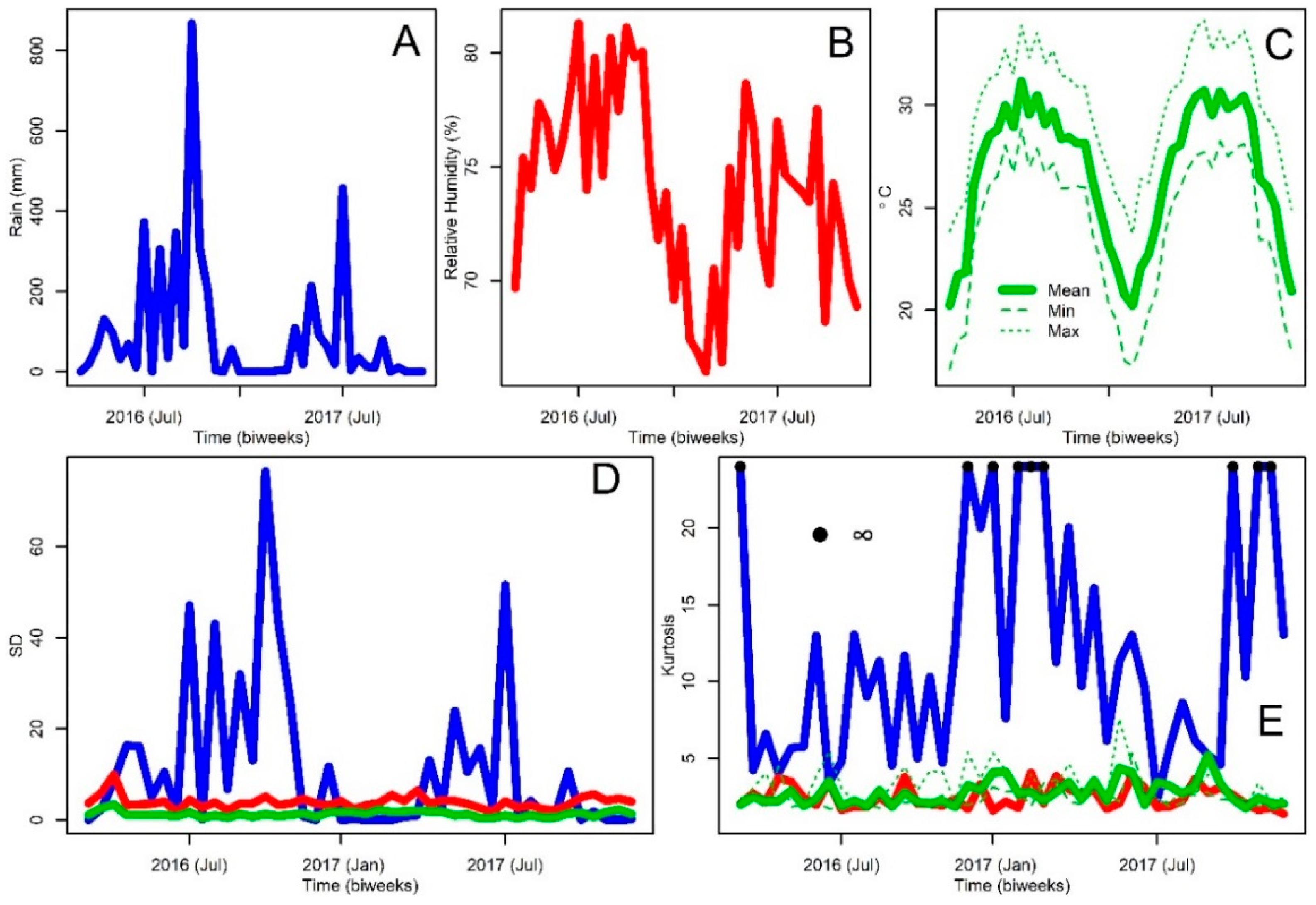
2.1. Study Area and Weather Data
2.2. Mosquito Sampling
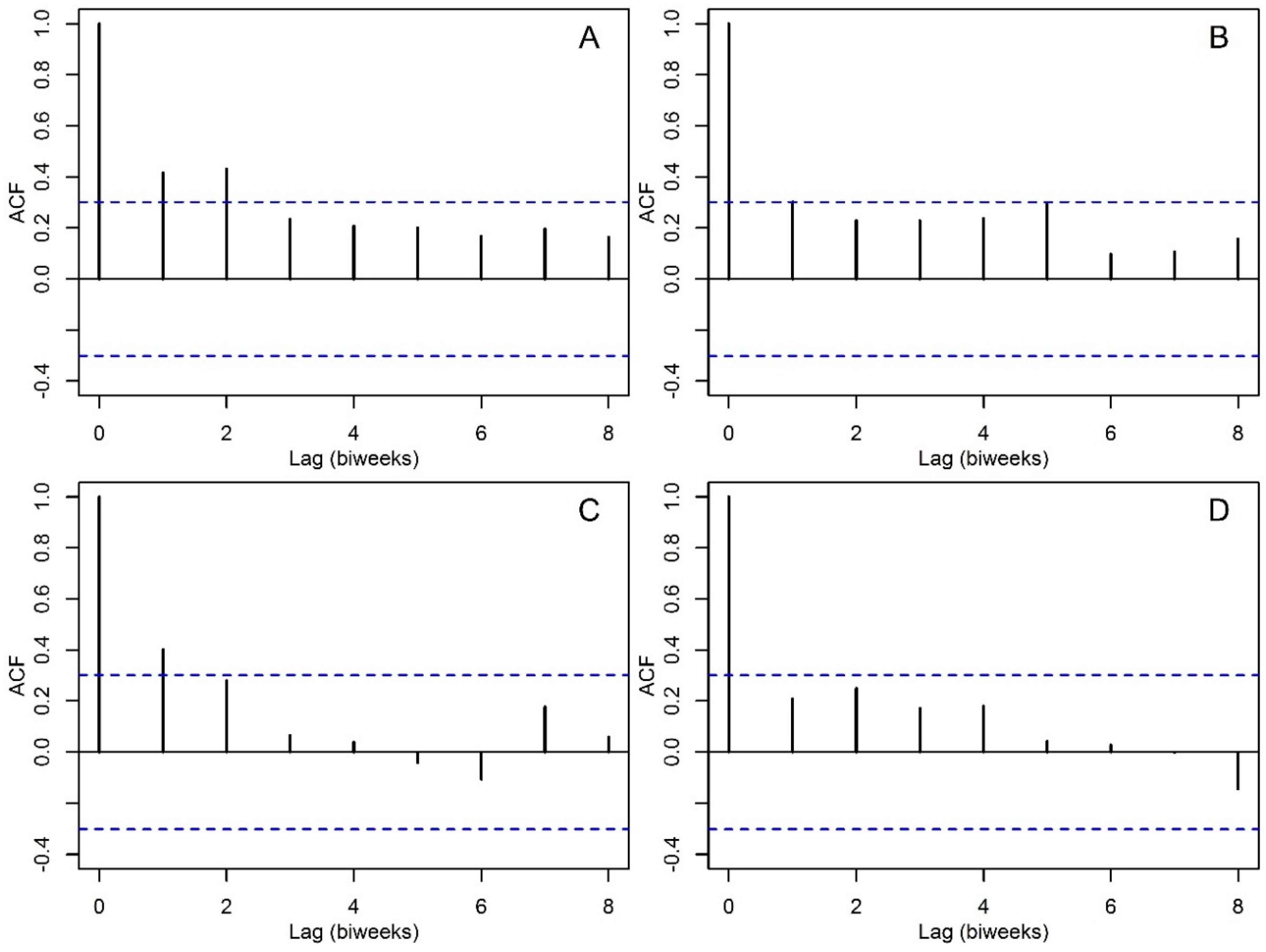
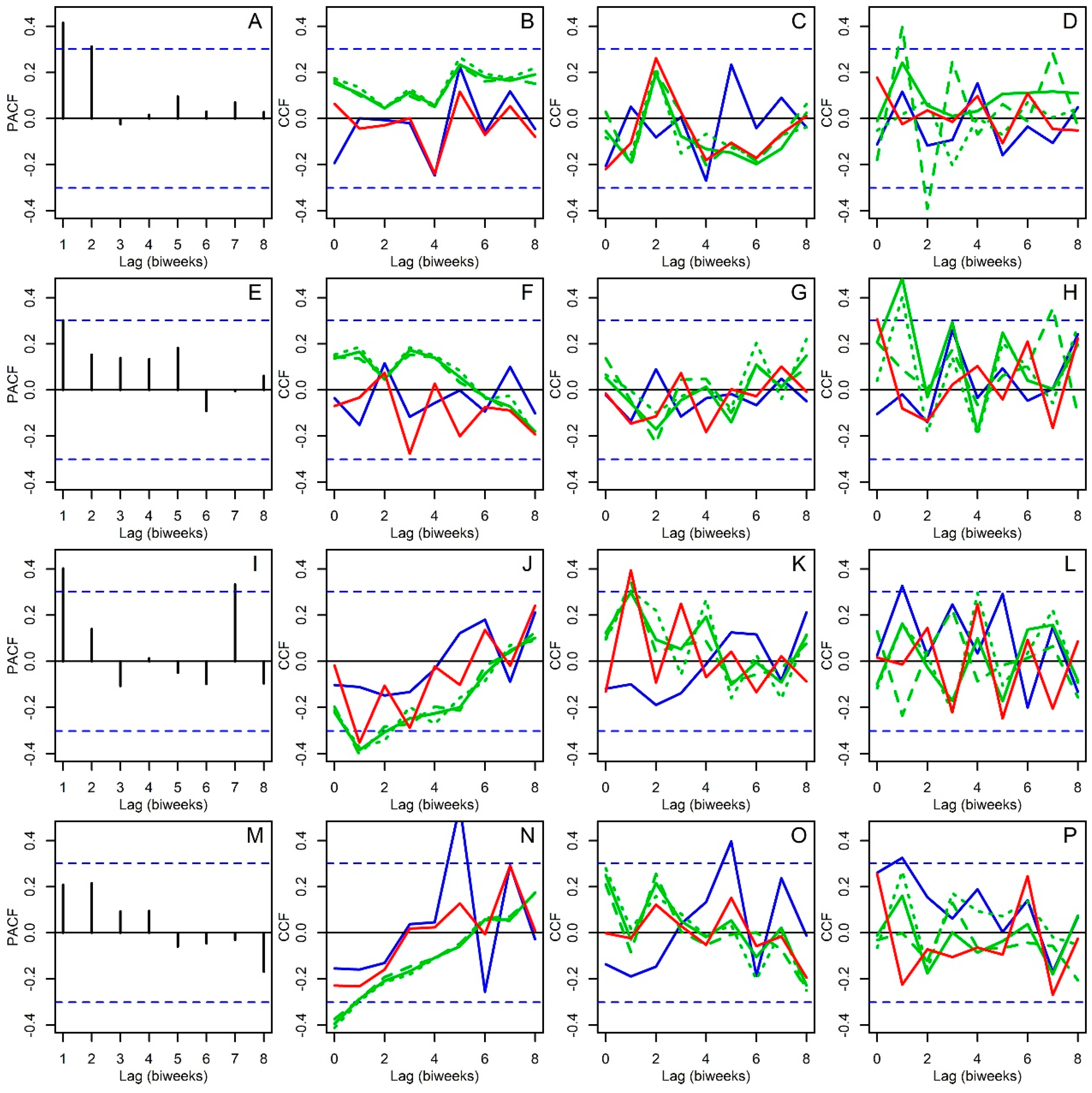
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Silver, J.B. Mosquito Ecology: Field Sampling Methods, 3rd ed.; Springer: New York, NY, USA, 2008. [Google Scholar]
- Burke, R.; Barrera, R.; Lewis, M.; Kluchinsky, T.; Claborn, D. Septic tanks as larval habitats for the mosquitoes Aedes albopictus and Culex quinquefasciatus in Playa-Playita, Puerto Rico. Med. Vet. Èntomol. 2010, 24, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Reisen, W.K. The contrasting bionomics of Culex mosquitoes in western North America. J. Am. Mosq. Control. Assoc. 2012, 28, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Russo, F.B.; Jungmann, P.; Beltrao-Braga, P.C.B. Zika infection and the development of neurological defects. Cell. Microbiol. 2017, 19, e12744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weaver, S.C.; Lecuit, M. Chikungunya virus and the global spread of a mosquito-borne disease. N. Engl. J. Med. 2015, 372, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Simmons, C.P.; Farrar, J.J.; van Nguyen, V.; Wills, B. Dengue. N. Engl. J. Med. 2012, 366, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Sasa, M. Mosquito as the vector of filariasis. Jpn. J. Sanit. Zoöl. 1965, 16, 171. [Google Scholar]
- Richards, S.L.; Lord, C.C.; Pesko, K.N.; Tabachnick, W.J. Environmental and Biological Factors Influencing Culex pipiens quinquefasciatus (Diptera: Culicidae) Vector Competence for West Nile Virus. Am. J. Trop. Med. Hyg. 2010, 83, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.L.; Lord, C.C.; Pesko, K.; Tabachnick, W.J. Environmental and Biological Factors Influencing Culex pipiens quinquefasciatus Say (Diptera: Culicidae) Vector Competence for Saint Louis Encephalitis Virus. Am. J. Trop. Med. Hyg. 2009, 81, 264–272. [Google Scholar] [PubMed]
- Edman, J.D. Disease control through manipulation of vector-host interaction: Some historical and evolutionary perspectives. In Proceedings of a Symposium: The Role of Vector-Host Interactions in Disease Tranmission; Scott, T.W., Grumstrup-Scott, J., Eds.; Entomological Society of America: Washington, DC, USA, 1988; pp. 43–50. [Google Scholar]
- Reisen, W.K.; Thiemann, T.; Barker, C.M.; Lu, H.L.; Carroll, B.; Fang, Y.; Lothrop, H.D. Effects of Warm Winter Temperature on the Abundance and Gonotrophic Activity of Culex (Diptera: Culicidae) in California. J. Med. Èntomol. 2010, 47, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.P.; Luther, C.; Moo-Llanes, D.; Ramsey, J.M.; Danis-Lozano, R.; Peterson, A.T. Climate change influences on global distributions of dengue and chikungunya virus vectors. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370. [Google Scholar] [CrossRef] [PubMed]
- Metzger, M.E.; Hardstone Yoshimizu, M.; Padgett, K.A.; Hu, R.; Kramer, V.L. Detection and Establishment of Aedes albopictus and Aedes albopictus (Diptera: Culicidae) Mosquitoes in California, 2011–2015. J. Med. Èntomol. 2017, 54, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.L.; Haque, U.; Monaghan, A.J.; Eisen, L.; Hahn, M.B.; Hayden, M.H.; Savage, H.M.; McAllister, J.; Mutebi, J.-P.; Eisen, R.J. Modeling the Environmental Suitability for Aedes (Stegomyia) aegypti and Aedes (Stegomyia) albopictus (Diptera: Culicidae) in the Contiguous United States. J. Med. Èntomol. 2017, 54, 1605–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebi, K.L.; Nealon, J. Dengue in a changing climate. Environ. Res. 2016, 151, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Messina, J.P.; Brady, O.J.; Scott, T.W.; Zou, C.; Pigott, D.M.; Duda, K.A.; Bhatt, S.; Katzelnick, L.; Howes, R.E.; Battle, K.E.; et al. Global spread of dengue virus types: Mapping the 70 year history. Trends Microbiol. 2014, 22, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.L.; Perkins, T.A.; Reiner, J.R.C.; Barker, C.M.; Niu, T.; Chaves, L.F.; Ellis, A.M.; George, D.B.; Le Menach, A.; Pulliam, J.R.C.; et al. Recasting the theory of mosquito-borne pathogen transmission dynamics and control. Trans. R. Soc. Trop. Med. Hyg. 2014, 108, 185–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, R.; Chaves, L.F.; Bergmann, L.; Ayres Lopes, C.F.J.; Hogerwerf, L.; Kock, R.; Wallace, R.G. Clear-Cutting Disease Control: Capital-Led Deforestation, Public Health Austerity, and Vector-Borne Infection; Springer: New York, NY, USA, 2018. [Google Scholar]
- Weaver, S.C.; Reisen, W.K. Present and future arboviral threats. Antivir. Res. 2010, 85, 328–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuang, T.W.; Chaves, L.F.; Chen, P.J. Effects of local and regional climatic fluctuations on dengue outbreaks in southern Taiwan. PLoS ONE 2017, 12, e0178698. [Google Scholar] [CrossRef] [PubMed]
- Bolling, B.G.; Barker, C.M.; Moore, C.G.; Pape, W.J.; Eisen, L. Seasonal patterns for entomological measures of risk for exposure to Culex vectors and West Nile virus in relation to human disease cases in northeastern Colorado. J. Med. Entomol. 2009, 46, 1519–1531. [Google Scholar] [CrossRef] [PubMed]
- Garrett-Jones, C. Prognosis for Interruption of Malaria Transmission through Assessment of Mosquitos Vectorial Capacity. Nature 1964, 204, 1173–1175. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.F.; Imanishi, N.; Hoshi, T. Population dynamics of Armigeres subalbatus (Diptera: Culicidae) across a temperate altitudinal gradient. Bull. Èntomol. Res. 2015, 105, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.F. Climate change and the biology of insect vectors of human pathogens. In Invertebrates and Global Climate Change; Johnson, S., Jones, H., Eds.; Wiley: Chichester, UK, 2017; pp. 126–147. [Google Scholar]
- Chaves, L.F.; Koenraadt, C.J.M. Climate Change and Highland Malaria: Fresh Air for a Hot Debate. Q. Rev. Boil. 2010, 85, 27–55. [Google Scholar] [CrossRef] [Green Version]
- Bar-Zeev, M. The effect of temperature on the growth rate and survival of the immature stages of Aedes albopictus (L.). Bull. Èntomol. Res. 1958, 49, 157–163. [Google Scholar] [CrossRef]
- Couret, J.; Benedict, M.Q. A meta-analysis of the factors influencing development rate variation in Aedes albopictus (Diptera: Culicidae). BMC Ecol. 2014, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- Couret, J. Meta-Analysis of Factors Affecting Ontogenetic Development Rate in the Culex pipiens (Diptera: Culicidae) Complex. Environ. Èntomol. 2013, 42, 614–626. [Google Scholar] [CrossRef] [PubMed]
- Rueda, L.M.; Patel, K.J.; Axtell, R.C.; Stinner, R.E. Temperature-Dependent Development and Survival Rates of Culex quinquefasciatus and Aedes albopictus (Diptera: Culicidae). J. Med. Èntomol. 1990, 27, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Watts, D.M.; Burke, D.S.; Harrison, B.A.; Whitmire, R.E.; Nisalak, A. Effect of temperature on the vector efficiency of Aedes albopictus for dengue 2 virus. Am. J. Trop. Med. Hyg. 1986, 36, 143–152. [Google Scholar] [CrossRef]
- Reisen, W.K.; Fang, Y.; Martinez, V.M. Effects of Temperature on the Transmission of West Nile Virus by Culex tarsalis (Diptera: Culicidae). J. Med. Èntomol. 2006, 43, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.F.; Scott, T.W.; Morrison, A.C.; Takada, T. Hot temperatures can force delayed mosquito outbreaks via sequential changes in Aedes albopictus demographic parameters in autocorrelated environments. Acta Trop. 2014, 129, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Berryman, A.; Barbosa, P.; Schultz, J. The theory and classification of outbreaks. In Insect Outbreaks; Barbosa, P., Schultz, J.C., Eds.; Academic Press: San Diego, CA, USA, 1987; pp. 3–30. [Google Scholar]
- Ye, Y.; Louis, V.R.; Simboro, S.; Sauerborn, R. Effect of meteorological factors on clinical malaria risk among children: An assessment using village-based meteorological stations and community-based parasitological survey. BMC Public Health 2007, 7, 101. [Google Scholar] [CrossRef] [PubMed]
- Day, J.F.; Curtis, G.A. When it rains they soar—And that makes Culex nigripalpus a dangerous mosquito. Am. Èntomol. 1994, 40, 162–167. [Google Scholar] [CrossRef]
- Dow, R.P.; Gerrish, G.M. Day-to-day change in relative humidity and activity of Culex nigripalpus (Diptera: Culicidae). Ann. Èntomol. Soc. Am. 1970, 63, 995–999. [Google Scholar] [CrossRef] [PubMed]
- Edman, J.D.; Scott, T.W.; Costero, A.; Morrison, A.C.; Harrington, L.C.; Clark, G.G. Aedes albopictus (Diptera: Culicidae) movement influenced by availability of oviposition sites. J. Med. Èntomol. 1998, 35, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.F. Globally invasive, withdrawing at home: Aedes albopictus and Aedes japonicus facing the rise of Aedes Flavopictus. Int. J. Biometeorol. 2016, 60, 1727–1738. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.F.; Morrison, A.C.; Kitron, U.D.; Scott, T.W. Nonlinear impacts of climatic variability on the density-dependent regulation of an insect vector of disease. Glob. Chang. Boil. 2012, 18, 457–468. [Google Scholar] [CrossRef]
- Chaves, L.F.; Moji, K. Density Dependence, Landscape, and Weather Impacts on Aquatic Aedes japonicus japonicus (Diptera: Culicidae) Abundance along an Urban Altitudinal Gradient. J. Med. Èntomol. 2018, 55, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Koenraadt, C.J.M.; Harrington, L.C. Flushing Effect of Rain on Container-Inhabiting Mosquitoes Aedes albopictus and Culex pipiens (Diptera: Culicidae). J. Med. Èntomol. 2008, 45, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Baak-Baak, C.M.; Moo-Llanes, D.A.; Cigarroa-Toledo, N.; Puerto, F.I.; Machain-Williams, C.; Reyes-Solis, G.; Nakazawa, Y.J.; Ulloa-Garcia, A.; Garcia-Rejon, J.E. Ecological Niche Model for Predicting Distribution of Disease-Vector Mosquitoes in Yucatan State, Mexico. J. Med. Entomol. 2017, 54, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Trewin, B.J.; Kay, B.H.; Darbro, J.M.; Hurst, T.P. Increased container-breeding mosquito risk owing to drought-induced changes in water harvesting and storage in Brisbane, Australia. Int. Health 2013, 5, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Barrera, R.; Avila, J.; Gonzalez-Tellez, S. Unreliable supply of potable water and elevated Aedes albopictus larval indices—A casual relationship. J. Am. Mosq. Control. Assoc. 1993, 9, 189–195. [Google Scholar] [PubMed]
- Hoshi, T.; Imanishi, N.; Higa, Y.; Chaves, L.F. Mosquito Biodiversity Patterns around Urban Environments in South-Central Okinawa Island, Japan. J. Am. Mosq. Control. Assoc. 2014, 30, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Torres, R.; Samudio, R.; Carrera, J.-P.; Young, J.; Márquez, R.; Hurtado, L.; Weaver, S.; Chaves, L.F.; Tesh, R.; Cáceres, L. Enzootic mosquito vector species at equine encephalitis transmission foci in the República de Panamá. PLoS ONE 2017, 12, e0185491. [Google Scholar] [CrossRef] [PubMed]
- Chaverri, L.G.; Dillenbeck, C.; Lewis, D.; Rivera, C.; Romero, L.M.; Chaves, L.F. Mosquito Species (Diptera: Culicidae) Diversity from Ovitraps in a Mesoamerican Tropical Rainforest. J. Med. Èntomol. 2018, 55, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Reisen, W.K.; Lothrop, H.D. Effects of sampling design on the estimation of adult mosquito abundance. J. Am. Mosq. Control. Assoc. 1999, 15, 105–114. [Google Scholar] [PubMed]
- Shand, L.; Brown, W.M.; Chaves, L.F.; Goldberg, T.L.; Hamer, G.L.; Haramis, L.; Kitron, U.; Walker, E.D.; Ruiz, M.O. Predicting West Nile Virus Infection Risk from the Synergistic Effects of Rainfall and Temperature. J. Med. Èntomol. 2016, 53, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.O.; Chaves, L.F.; Hamer, G.L.; Sun, T.; Brown, W.M.; Walker, E.D.; Haramis, L.; Goldberg, T.L.; Kitron, U.D. Local impact of temperature and precipitation on West Nile virus infection in Culex species mosquitoes in northeast Illinois, USA. Parasites Vectors 2010, 3, 19. [Google Scholar] [CrossRef] [PubMed]
- Scott, T.W.; Morrison, A.C.; Lorenz, L.H.; Clark, G.G.; Strickman, D.; Kittayapong, P.; Zhou, H.; Edman, J.D. Longitudinal studies of Aedes albopictus (Diptera: Culicidae) in Thailand and Puerto Rico: Population dynamics. J. Med. Èntomol. 2000, 37, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Reisen, W.K.; Meyer, R.P.; Shields, J.; Arbolante, C. Population ecology of preimaginal Culex tarsalis (Diptera: Culicidae) in Kern County, California. J. Med. Entomol. 1989, 26, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Reisen, W.K.; Milby, M.M.; Meyer, R.P. Population dynamics of adult Culex mosquitoes (Diptera: Culicidae) along the Kern River, Kern County, California, in 1990. J. Med. Entomol. 1992, 29, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Cromwell, E.A.; Stoddard, S.T.; Barker, C.M.; Van Rie, A.; Messer, W.B.; Meshnick, S.R.; Morrison, A.C.; Scott, T.W. The relationship between entomological indicators of Aedes albopictus abundance and dengue virus infection. PLoS Negl. Trop. Dis. 2017, 11, e0005429. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.F.; Hamer, G.L.; Walker, E.D.; Brown, W.M.; Ruiz, M.O.; Kitron, U.D. Climatic variability and landscape heterogeneity impact urban mosquito diversity and vector abundance and infection. Ecosphere 2011, 2, 1–21. [Google Scholar] [CrossRef]
- Reisen, W.K. Landscape epidemiology of vector-borne diseases. Annu. Rev. Entomol. 2010, 55, 461–483. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.F.; Chang, K.; Loh, E.W.; Wang, W.H.; Tseng, S.P.; Lu, P.L.; Chen, Y.H.; Chen, Y.A. Consecutive large dengue outbreaks in Taiwan in 2014–2015. Emerg. Microbes Infect. 2016, 5, e123. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.F.; Wang, W.H.; Chang, K.; Chen, Y.H.; Tseng, S.P.; Yen, C.H.; Wu, D.C.; Chen, Y.M. Severe Dengue Fever Outbreak in Taiwan. Am. J. Trop. Med. Hyg. 2016, 94, 193–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, K.; Chen, C.D.; Shih, C.M.; Lee, T.C.; Wu, M.T.; Wu, D.C.; Chen, Y.H.; Hung, C.H.; Wu, M.C.; Huang, C.C.; et al. Time-Lagging Interplay Effect and Excess Risk of Meteorological/Mosquito Parameters and Petrochemical Gas Explosion on Dengue Incidence. Sci. Rep. 2016, 6, 35028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Su, X.; Zhou, G.; Zhang, H.; Puthiyakunnon, S.; Shuai, S.; Cai, S.; Gu, J.; Zhou, X.; Yan, G.; et al. Comparative evaluation of the efficiency of the BG-Sentinel trap, CDC light trap and Mosquito-oviposition trap for the surveillance of vector mosquitoes. Parasit Vectors 2016, 9, 446. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.A.; Larson, R.T.; Richardson, A.G.; Cote, N.M.; Stoops, C.A.; Clark, M.; Obenauer, P.J. Comparison of BG-Sentinel(R) Trap and Oviposition Cups for Aedes albopictus and Aedes albopictus Surveillance in Jacksonville, Florida, USA. J. Am. Mosq. Control. Assoc. 2015, 31, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Degener, C.M.; Azara, T.M.; Roque, R.A.; Codeco, C.T.; Nobre, A.A.; Ohly, J.J.; Geier, M.; Eiras, A.E. Temporal abundance of Aedes albopictus in Manaus, Brazil, measured by two trap types for adult mosquitoes. Mem. Inst. Oswaldo Cruz 2014, 109, 1030–1040. [Google Scholar] [CrossRef] [PubMed]
- Maciel-de-Freitas, R.; Eiras, Á.E.; Lourenço-de-Oliveira, R. Field evaluation of effectiveness of the BG-Sentinel, a new trap for capturing adult Aedes albopictus (Diptera: Culicidae). Mem. Inst. Oswaldo Cruz 2006, 101, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Harrington, L.C.; Scott, T.W.; Lerdthusnee, K.; Coleman, R.C.; Costero, A.; Clark, G.G.; Jones, J.J.; Kitthawee, S.; Kittayapong, P.; Sithiprasasna, R. Dispersal of the dengue vector Aedes albopictus within and between rural communities. Am. J. Trop. Med. Hyg. 2005, 72, 209–220. [Google Scholar] [PubMed]
- Medeiros, M.C.I.; Boothe, E.C.; Roark, E.B.; Hamer, G.L. Dispersal of male and female Culex quinquefasciatus and Aedes albopictus mosquitoes using stable isotope enrichment. PLoS Negl. Trop. Dis. 2017, 11, e0005347. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.F.; McPhaden, M.J. How the July 2014 easterly wind burst gave the 2015–2016 El Niño a head start. Geophys. Res. Lett. 2016, 43, 6503–6510. [Google Scholar] [CrossRef]
- McPhaden, M.J. Playing hide and seek with El Nino. Nat. Clim Chang. 2015, 5, 791–795. [Google Scholar] [CrossRef]
- Chaves, L.F. Mosquito Species (Diptera: Culicidae) Persistence and Synchrony across an Urban Altitudinal Gradient. J. Med. Èntomol. 2017, 54, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.M. Introduction to Probability Models; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Irish, S.R.; Chandre, F.; N’Guessan, R. Comparison of Octenol- And BG Lure®-Baited Biogents Sentinel Traps and an Encephalitis Virus Surveillance Trap in Portland, OR. J. Am. Mosq. Control. Assoc. 2008, 24, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.F.; Higa, Y.; Lee, S.H.; Jeong, J.Y.; Heo, S.T.; Kim, M.; Minakawa, N.; Lee, K.H. Environmental Forcing Shapes Regional House Mosquito Synchrony in a Warming Temperate Island. Environ. Èntomol. 2013, 42, 605–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritchie, S.A.; Moore, P.; Carruthers, M.; Williams, C.; Montgomery, B.; Foley, P.; Ahboo, S.; Van Den Hurk, A.F.; Lindsay, M.D.; Cooper, B.; et al. Discovery of a Widespread Infestation of Aedes albopictus in the Torres Strait, Australia. J. Am. Mosq. Control. Assoc. 2006, 22, 358–365. [Google Scholar] [CrossRef]
- Kröckel, U.; Rose, A.; Eiras, Á.E.; Geier, M. New tools for surveillance of adult yellow fever mosquitoes: Comparison of trap catches with human landing rates in an urban environment. J. Am. Mosq. Control. Assoc. 2006, 22, 229–238. [Google Scholar] [CrossRef]
- Farajollahi, A.; Kesavaraju, B.; Price, D.C.; Williams, G.M.; Healy, S.P.; Gaugler, R.; Nelder, M.P. Field efficacy of BG-Sentinel and industry-standard traps for Aedes albopictus (Diptera: Culicidae) and West Nile virus surveillance. J. Med. Èntomol. 2009, 46, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Lien, J.C. Pictorial Keys to Mosquitoes of Taiwan; Yi Hsien Publishing Co., Ltd.: New Taipei, Taiwan, 2004. [Google Scholar]
- Shumway, R.H.; Stoffer, D.S. Time Series Analysis and Its Applications, 3rd ed.; Springer: New York, NY, USA, 2011. [Google Scholar]
- Cleveland, W.S.; Devlin, S.J. Locally Weighted Regression: An Approach to Regression Analysis by Local Fitting. J. Am. Stat. Assoc. 1988, 83, 596–610. [Google Scholar] [CrossRef]
- Hoshi, T.; Higa, Y.; Chaves, L.F. Uranotaenia novobscura ryukyuana (Diptera: Culicidae) Population Dynamics Are Denso-Dependent and Autonomous from Weather Fluctuations. Ann. Èntomol. Soc. Am. 2014, 107, 136–142. [Google Scholar] [CrossRef]
- Tsai, P.J.; Teng, H.J. Role of Aedes albopictus (Linnaeus) and Aedes albopictus (Skuse) in local dengue epidemics in Taiwan. BMC Infect. Dis. 2016, 16, 662. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.F.; Hou, J.N.; Chen, T.H.; Chen, W.J. Discriminable roles of Aedes albopictus and Aedes albopictus in establishment of dengue outbreaks in Taiwan. Acta Trop. 2014, 130, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J. Seasonal changes in population structure of Culex pipiens quinquefasciatus Say (Diptera: Culicidae): Study of an isolated population. J. Med. Èntomol. 1975, 12, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.; Hsi, B.P. Interrelationships between selected meteorologic phenomena and immature stages of Culex pipiens quinquefasciatus Say: Study of an isolated population. J. Med. Èntomol. 1975, 12, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.; Downs, T.D. Seasonal changes in an isolated population of Culex pipiens quinquefasciatus (Diptera: Culicidae): A time series analysis. J. Med. Èntomol. 1980, 17, 63–69. [Google Scholar] [CrossRef]
- Shaman, J.; Day, J.F. Reproductive Phase Locking of Mosquito Populations in Response to Rainfall Frequency. PLoS ONE 2007, 2, e331. [Google Scholar] [CrossRef] [PubMed]
- Focks, D.A.; Haile, D.G.; Daniels, E.; Mount, G.A. Dynamic Life Table Model for Aedes albopictus (Diptera, Culcidae)—Analysis of the Literature and Model Development. J. Med. Èntomol. 1993, 30, 1003–1017. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.; Williams-Newkirk, A.J.; Kitron, U.D.; Chaves, L.F. Seasonal weather, nutrient dynamics and conspecific presence impacts on the southern house mosquito oviposition dynamics in combined sewage overflows. J. Med. Èntomol. 2012, 49, 1328–1338. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.F.; Kitron, U.D. Weather variability impacts on oviposition dynamics of the southern house mosquito at intermediate time scales. Bull. Èntomol. Res. 2011, 101, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.F.; Keogh, C.L.; Nguyen, A.M.; Decker, G.M.; Vazquez-Prokopec, G.M.; Kitron, U.D. Combined sewage overflow accelerates immature development and increases body size in the urban mosquito Culex Quinquefasciatus. J. Appl. Èntomol. 2011, 135, 611–620. [Google Scholar] [CrossRef]
- Scott, T.W.; Amerasinghe, P.H.; Morrison, A.C.; Lorenz, L.H.; Clark, G.G.; Strickman, D.; Kittayapong, P.; Edman, J.D. Longitudinal studies of Aedes albopictus (Diptera: Culicidae) in Thailand and Puerto Rico: Blood feeding frequency. J. Med. Èntomol. 2000, 37, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Cazelles, B.; Chavez, M.; McMichael, A.J.; Hales, S. Nonstationary influence of El Nino on the synchronous dengue epidemics in Thailand. PLoS Med. 2005, 2, e106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansson, M.A.; Cummings, D.A.; Glass, G.E. Multiyear climate variability and dengue—El Nino southern oscillation, weather, and dengue incidence in Puerto Rico, Mexico, and Thailand: A longitudinal data analysis. PLoS Med. 2009, 6, e1000168. [Google Scholar] [CrossRef] [PubMed]
- Thai, K.T.D.; Cazelles, B.; Nguyen, N.V.; Vo, L.T.; Boni, M.F.; Farrar, J.; Simmons, C.P.; van Doorn, H.R.; de Vries, P.J. Dengue Dynamics in Binh Thuan Province, Southern Vietnam: Periodicity, Synchronicity and Climate Variability. PLoS Negl. Trop. Dis. 2010, 4, e747. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Valderrama, A.; Gottdenker, N.; Cerezo, L.; Minakawa, N.; Saldaña, A.; Calzada, J.E.; Chaves, L.F. Macroecological patterns of American Cutaneous Leishmaniasis transmission across the health areas of Panamá (1980–2012). Parasite Epidemiol. Control. 2016, 1, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.F.; Calzada, J.E.; Valderama, A.; Saldaña, A. Cutaneous Leishmaniasis and Sand Fly fluctuations are associated with El Niño in Panamá. PLoS Negl. Trop. Dis. 2014, 8, e3210. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.F.; Jian, J.-Y.; Moji, K. Overwintering in the Bamboo Mosquito Tripteroides bambusa (Diptera: Culicidae) During a Warm, But Unpredictably Changing, Winter. Environ. Èntomol. 2018, 47, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.F.; Pascual, M. Comparing Models for Early Warning Systems of Neglected Tropical Diseases. PLoS Negl. Trop. Dis. 2007, 1, e33. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liu, X.; Kok, S.-Y.; Rajarethinam, J.; Liang, S.; Yap, G.; Chong, C.-S.; Lee, K.-S.; Tan, S.S.; Chin, C.K.Y. Three-month real-time dengue forecast models: An early warning system for outbreak alerts and policy decision support in Singapore. Environ. Health Perspect. 2016, 124, 1369–1375. [Google Scholar] [CrossRef] [PubMed]
- Hii, Y.L.; Rocklöv, J.; Wall, S.; Ng, L.C.; Tang, C.S.; Ng, N. Optimal Lead Time for Dengue Forecast. PLoS Negl. Trop. Dis. 2012, 6, e1848. [Google Scholar] [CrossRef] [PubMed]

| Parameters (lag) | Sanmin | Nanzih | ||||||
|---|---|---|---|---|---|---|---|---|
| Estimates | S.E. | z | p-Value | Estimates | S.E. | z | p-Value | |
| Intercept | 9.0337 | 1.4571 | 6.19 | 6.65 × 10−10 * | 6.2149 | 0.4716 | 13.18 | 1.18 × 10−39 * |
| AR (1) | 0.5537 | 0.1287 | 4.30 | 1.69 × 10−5 * | - | - | - | - |
| KTmin (1) | 2.0087 | 0.8928 | 2.25 | 0.024 * | - | - | - | - |
| KTmin (2) | −2.0886 | 0.8976 | −2.33 | 0.019 * | - | - | - | - |
| KTmean (1) | - | - | - | - | 2.6512 | 0.591 | 4.49 | 7.26 × 10−6 * |
| Error Variance | 18.77 | - | - | - | 9.33 | - | - | - |
| Parameter (lag) | Sanmin | Nanzih | ||||||
|---|---|---|---|---|---|---|---|---|
| Estimates | S.E. | z | p-Value | Estimates | S.E. | z | p-Value | |
| Intercept | 142.5738 | 14.3782 | 9.92 | 3.55 × 10−23 * | 63.3702 | 8.71 | 7.28 | 3.45 × 10−13 * |
| Tmax (1) | −22.0949 | 5.1298 | −4.31 | 1.65 × 10−5 * | - | - | - | - |
| SDR (5) | - | - | - | - | 1.7311 | 0.4884 | 3.54 | 0.00039 * |
| Tmax (0) | - | - | - | - | −11.8535 | 2.9783 | −3.98 | 6.89 × 10−5 * |
| Error Variance | 7743 | - | - | - | 2882 | - | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, K.-C.; Chaves, L.F.; Tsai, K.-H.; Chuang, T.-W. Increased Adult Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae) Abundance in a Dengue Transmission Hotspot, Compared to a Coldspot, within Kaohsiung City, Taiwan. Insects 2018, 9, 98. https://doi.org/10.3390/insects9030098
Ng K-C, Chaves LF, Tsai K-H, Chuang T-W. Increased Adult Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae) Abundance in a Dengue Transmission Hotspot, Compared to a Coldspot, within Kaohsiung City, Taiwan. Insects. 2018; 9(3):98. https://doi.org/10.3390/insects9030098
Chicago/Turabian StyleNg, Ka-Chon, Luis Fernando Chaves, Kun-Hsien Tsai, and Ting-Wu Chuang. 2018. "Increased Adult Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae) Abundance in a Dengue Transmission Hotspot, Compared to a Coldspot, within Kaohsiung City, Taiwan" Insects 9, no. 3: 98. https://doi.org/10.3390/insects9030098





