Prey Acceptability and Preference of Oenopia conglobata (Coleoptera: Coccinellidae), a Candidate for Biological Control in Urban Green Areas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Non-Choice Prey Acceptability
2.2. Choice (Cafeteria) Experiments
2.3. Data Analysis
2.3.1. Non-Choice Prey Acceptability
2.3.2. Choice (Cafeteria) Experiments
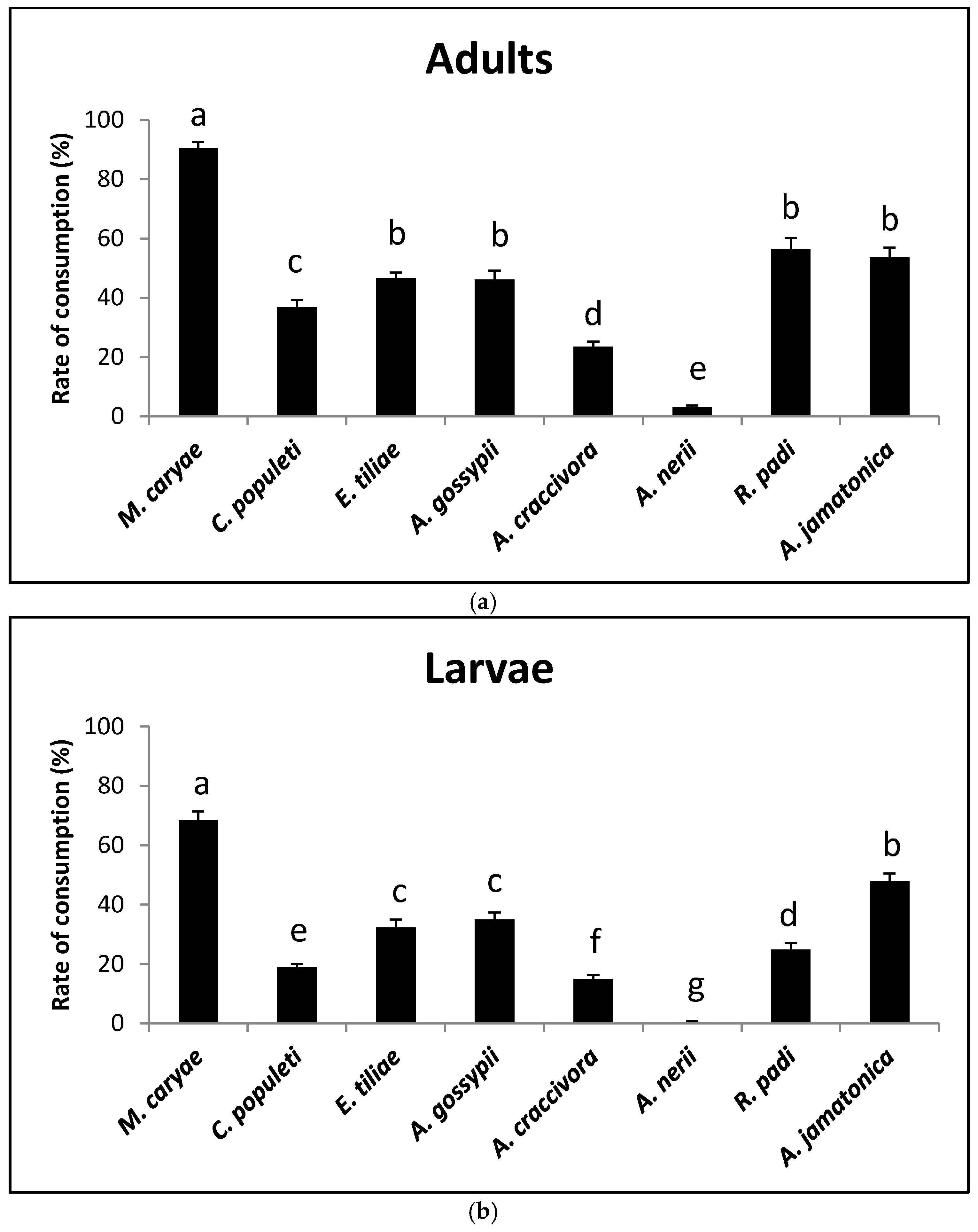
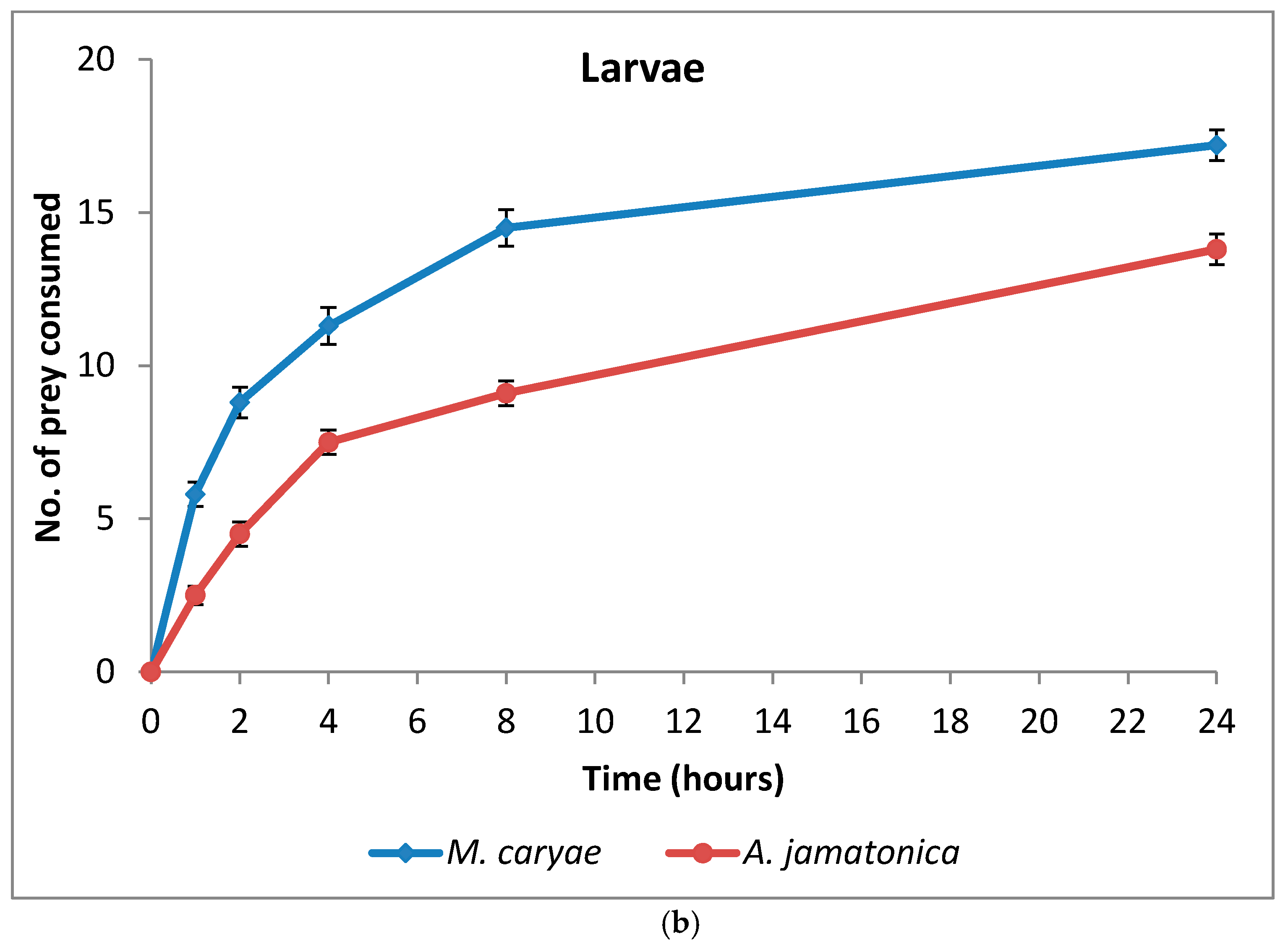
3. Results
3.1. Non-Choice Prey Acceptability
3.2. Choice (Cafeteria) Experiment
3.2.1. Preference among Aphid Species
3.2.2. Preference between Aphid and Psyllid
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Iperti, G. Biodiversity of predaceous coccinellidae in relation to bioindication and economic importance. Agric. Ecosyst. Environ. 1999, 74, 323–342. [Google Scholar] [CrossRef]
- Lumbierres, B.; Starý, P.; Pons, X. Parasitoids and predators of aphids associated with public green areas of Lleida (NE Iberian Peninsula). Adv. Hortic. Sci. 2005, 19, 69–75. [Google Scholar]
- Ameixa, O.M.C.C.; Honek, A.; Martinkova, Z.; Kindlmann, P. Position of Harmonia axyridis in aphidophagous guilds in the Czech Republic. IOBC/WPRS Bull. 2010, 58, 7–14. [Google Scholar]
- Rondoni, G.; Onofri, A.; Ricci, C. Laboratory studies on intraguild and cannibalism among coccinellid larvae (Coleoptera: Coccinellidae). Eur. J. Entomol. 2012, 109, 353–362. [Google Scholar] [CrossRef]
- Yakhontov, V. Food specificity in Syrphidae and Coccinellidae of Central Asia. In Proceedings of the Ecology of Aphidophaga, Libice near Prague, Czech Republic, 27 September–1 October 1965; Hodek, I., Junk, W., Eds.; The Hague and Academia Publishing House: Prague, Czech Republic, 1966; pp. 35–36. [Google Scholar]
- Wakgari, M.; Rai, A.K. Effect of weather factors on Busseola fusca (Fuller) Lepidoptera: Noctuidae and its effect predator Oenopia conglobata (L.) (Coccinellidae) on sorghum in Ethiopia. Indian J. Entomol. 2011, 73, 331–337. [Google Scholar]
- Hodek, I.; Honěk, A. Ecology of Coccinellidae; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1966; p. 464. [Google Scholar]
- Mehrnejad, M.R.; Jalali, M.A. Life history parameters of the coccinellids beetle Oenopia conglobata contaminata, an important predator of the common pistachio psylla, Agonoscena pistaciae (Hemiptera: Psylloidea). Biocontrol Sci. Technol. 2004, 14, 701–711. [Google Scholar] [CrossRef]
- Pons, X.; Lumbierres, B. Control integrado de plagas en espacios verdes urbanos. In Proceedings of the 12th Symposium Sanidad Vegetal: Hacia la gestión integrada de plagas, Sevilla, Spain, 23–25 January 2013; pp. 145–184. [Google Scholar]
- Lumbierres, B. La marieta rosa (Oenopia conglobata). Lignosa 2016, 14, 7. Available online: http://arboretum.parcteclleida.es/ca/descarregues/lignosa-13/view (accessed on 27 December 2017).
- Krebs, C.J. Ecological Methodology, 2nd ed.; Addison Wesley Longman: Menlo Park, CA, USA, 1999. [Google Scholar]
- SAS/STAT User’s Guide; Version 9.1; SAS Institute: Cary, NC, USA, 2004.
- Blackman, R.L.; Eastop, V.F. Aphids on the World’s Plants. An Online Identification and Information Guide. 2014. Available online: http://www.aphidsonworldsplants.info (accessed on 31 October 2017).
- Pons, X.; Comas, J.; Albajes, R. Occurrence of holocyclic and anholocyclic populations of Rhopalosiphum padi and Sitobion avenae (Hom., Aphididae) in the northeast of Spain. J. Appl. Entomol. 1995, 119, 171–175. [Google Scholar] [CrossRef]
- Pons, X.; Lumbierres, B.; Madeira, F.; Starý, P. Aphid-parasitoid diversity in urban green areas: A background for conservative control strategies. Biodiversity. (accepted with minor revisions).
- Sadeghi, S.E.; Mojib, H.G.Z.; Jalali, J.; Haji, Z.J. Investigation on the biology of lady beetle Oenopia conglobata (L.) on poplar aphid Chaitophorus leucomelas (Koch) in laboratory conditions. Pajouhesh Sazandegi (Natural Resources) 2004, 17, 20–24, (In Persian, abstract in English). [Google Scholar]
- Mojib, H.G.Z.; Jalali, S.J.; Sedeghi, S.E.; Yousefpour, M. Introduction of lady beetle Oenopia conglobata (L.) as predator of ulmus aphid Tinocallis saltans Nevski in Guilan province and biology of ladybeetle in laboratory conditions. Iran. J. Biol. 2009, 122, 363–370, (In Persian, abstract in English). [Google Scholar]
- Yaşar, B.; Özger, S. Functional response of Oenopia conglobata (L.) (Coleoptera: Coccinellidae) on Hyalopterus pruni (Geoffroy) (Homoptera: Aphididae) in three different size arenas. Türk Entomol. Derg. 2005, 29, 91–99. [Google Scholar]
- Rondoni, G.; Ielo, F.; Ricci, C.; Conti, E. Behavioural and physiological responses top rey-related cues reflect higher competitiveness of invasive vs. native ladybirds. Sci. Rep. 2017, 7, 3716. [Google Scholar] [CrossRef]
- Ninkovic, V.; Pettersson, J. Searching behaviour of the sevenspotted ladybird, Coccinella septempunctata—Effects of plant-plant odour interaction. Oikos 2003, 10, 65–70. [Google Scholar] [CrossRef]
- Iperti, G. Comportement naturel des Coccinelles aphidophages du Sud-Est de la France: Leur type de spécificité, leur action prédatrice sur Aphis fabae L. Entomophaga 1966, 11, 203–210. [Google Scholar] [CrossRef]
- Blackman, R.L. Selection of aphid prey by Adalia bipunctata L. and Coccinella 7-punctata L. Ann. App. Biol. 1967, 59, 331–338. [Google Scholar] [CrossRef]
- Nevded, O.; Salvucci, S. Ladybird Coccinella septempunctata (Coleoptera: Coccinellidae) prefers toxic prey in laboratory choice experiment. Eur. J. Entomol. 2008, 105, 431–436. [Google Scholar]
- Obrycki, J.J.; Orr, C.J. Suitability of three prey species for Neartic populations of Coccinella septempunctata, Hippodamia variegata and Propylea quatuordecimpunctata (Coleoptera: Coccinellidae). J. Econ. Entomol. 1990, 83, 1292–1297. [Google Scholar] [CrossRef]
- Kalushkov, P.; Hodek, I. The effects of thirteen species of aphids on some life history parameters of the ladybird Coccinella septempunctata. Biocontrol 2004, 49, 21–32. [Google Scholar] [CrossRef]
- Mignault, M.P.; Roy, M.; Brodeur, J. Soybean aphid predators in Quebec and suitability of Aphis glycines as prey for three Coccinellidae. Biocontrol 2006, 51, 89–106. [Google Scholar] [CrossRef]


| Number of Individuals Consumed | ||||
|---|---|---|---|---|
| Prey | 1 h | 2 h | 4 h | 8 h |
| ADULTS | ||||
| M. caryae | 10.7 ± 0,5 a * | 13.0 ± 0,5 a | 15.5 ± 0,5 a | 18.1 ± 0,4 a |
| C. populeti | 2.6 ± 0.3 bcd | 4.8 ± 0.3 b | 6.0 ± 0.4 c | 7.3 ± 0.5 c |
| E. tiliae | 4.0 ± 0.4 b | 5.4 ± 0,4 b | 7.2 ± 0.5 bc | 9.3 ± 0.4 bc |
| A. gossypii | 2.5 ± 0.4 cd | 4.1 ± 0.4 b | 6.5 ± 0.5 bc | 9.2 ± 0.6 bc |
| A. craccivora | 1.5 ± 02 d | 2.3 ± 0.2 c | 3.4 ± 0.2 d | 4.7 ± 0.3 d |
| A. nerii | 0.1 ± 0.1 e | 0.2 ± 0.1 d | 0.2 ± 0.1 e | 0.6 ± 0.1 e |
| R. padi | 3.2 ± 0.3 bc | 5.5 ± 0.4 b | 8.0 ± 0.5 b | 11.3 ± 0.8 b |
| A. jamatonica | 3.6 ± 0.4 bc | 5.4 ± 0.5 b | 8.0 ± 0.6 b | 10.7 ± 0.7 bc |
| LARVAE | ||||
| M. caryae | 6.0 ± 0.6 a | 8.3 ± 0.7 a | 10.5 ± 0.8 a | 13.7 ± 0.6 a |
| C. populeti | 1.6 ± 0.2 bc | 2.2 ± 0.2 cd | 2.9 ± 0.2 de | 3.8 ± 0.2 de |
| E. tiliae | 2.3 ± 0.3 b | 3.3 ± 0.3 bc | 4.4 ± 0.4 cd | 6.5 ± 0.5 cd |
| A. gossypii | 2.5 ± 0.2 b | 3.6 ± 0.2 bc | 5.1 ± 0.3 c | 7.0 ± 0.5 c |
| A. craccivora | 0.8 ± 0.1 cd | 1.4 ± 0.2 de | 2.0 ± 0.3 e | 3.0 ± 0.3 e |
| A. nerii | 0.0 ± 0.0 d | 0.0 ± 0.0 e | 0.1 ± 0.1 f | 0.1 ± 0.1 f |
| R. padi | 1.7 ± 0.2 bc | 2.7 ± 0.2 cd | 3.8 ± 0.3 cde | 5.0 ± 0.4 d |
| A. jamatonica | 2.7 ± 0.3 b | 4.5 ± 0.3 b | 7.2 ± 0.5 b | 9.6 ± 0.5 b |
| Prey | Adults | Larvae | Both Stages |
|---|---|---|---|
| M. caryae | 1.00 ± 0.00 a * | 1.00 ± 0.00 a | 1.00 ± 0.00 a |
| E. tiliae | 0.63 ± 0.02 b | 0.61 ± 0.03 b | 0.62 ± 0.02 b |
| C. populeti | 0.59 ± 0.03 b | 0.62 ± 0.03 b | 0.61 ± 0.02 b |
| Prey | Adults | Larva | Both Stages |
|---|---|---|---|
| M. caryae | 1.00 ± 0.00 a * | 0.98 ± 0.01 a | 0.99 ± 0.01 a |
| A. jamatonica | 0.65 ± 0.02 b | 0.70 ± 0.03 b | 0.67 ± 0.02 b |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lumbierres, B.; Madeira, F.; Pons, X. Prey Acceptability and Preference of Oenopia conglobata (Coleoptera: Coccinellidae), a Candidate for Biological Control in Urban Green Areas. Insects 2018, 9, 7. https://doi.org/10.3390/insects9010007
Lumbierres B, Madeira F, Pons X. Prey Acceptability and Preference of Oenopia conglobata (Coleoptera: Coccinellidae), a Candidate for Biological Control in Urban Green Areas. Insects. 2018; 9(1):7. https://doi.org/10.3390/insects9010007
Chicago/Turabian StyleLumbierres, Belén, Filipe Madeira, and Xavier Pons. 2018. "Prey Acceptability and Preference of Oenopia conglobata (Coleoptera: Coccinellidae), a Candidate for Biological Control in Urban Green Areas" Insects 9, no. 1: 7. https://doi.org/10.3390/insects9010007





