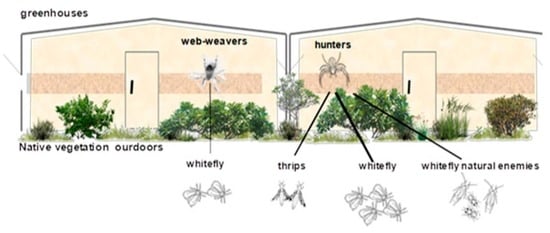
Spider Communities and Biological Control in Native Habitats Surrounding Greenhouses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Plant Species
2.2. Arthropod Collection
2.3. Statistical Analysis
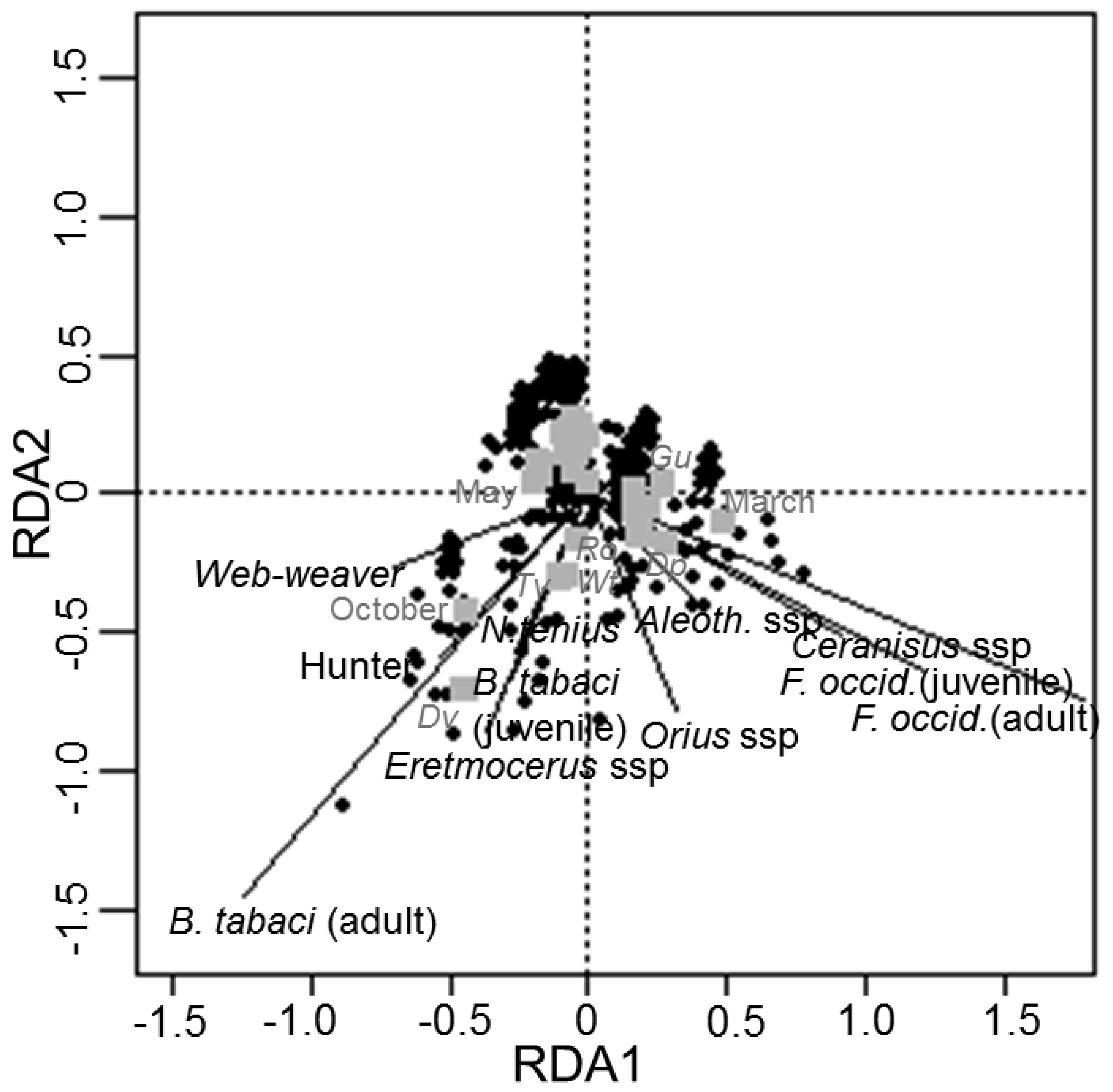
3. Results
3.1. Spider Composition in Native Plants
3.2. Redundancy Analysis of Spiders, Pests and Other NEs in Native Plants
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bommarco, R.; Kleijn, D.; Potts, S.G. Ecological intensification: Harnessing ecosystem services for food security. Trends Ecol. Evol. 2013, 28, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Gurr, G.M.; Lu, Z.; Zheng, X.; Xu, H.; Zhu, P.; Chen, G.; Yao, X.; Cheng, J.; Zhu, Z.; Catindig, J.L.; et al. Multi-country evidence that crop diversification promotes ecological intensification of agriculture. Nat. Plants 2016, 2, 16014. [Google Scholar] [CrossRef] [PubMed]
- Rusch, A.; Chaplin-Kramer, R.; Gardiner, M.M.; Hawro, V.; Holland, J.; Landis, D.; Thies, C.; Tscharntke, T.; Weisser, W.W.; Winqvist, C.; et al. Agricultural landscape simplification reduces natural pest control: A quantitative synthesis. Agric. Ecosyst. Environ. 2016, 221, 198–204. [Google Scholar] [CrossRef]
- Bianchi, F.J.J.A.; Booij, C.J.H.; Tscharntke, T. Sustainable pest regulation in agricultural landscapes: A review on landscape composition, biodiversity and natural pest control. Proc. R. Soc. Lond. B Biol. Sci. 2006, 273, 1715–1727. [Google Scholar] [CrossRef] [PubMed]
- Landis, D.A.; Wratten, S.D.; Gurr, G.M. Habitat management to conserve natural enemies of arthropod pests in agriculture. Annu. Rev. Entomol. 2000, 45, 175–201. [Google Scholar] [CrossRef] [PubMed]
- Snyder, W.E.; Tylianakis, J.M. The ecology of biodiversity-biocontrol relationships. In Biodiversity and Insect Pests—Key Issues for Sustainable Management; Gurr, G.M., Wratten, S.D., Snyder, W.E., Read, D.M.Y., Eds.; Wiley: Chichester, UK, 2012; pp. 23–40. ISBN 978-0-470-65686-0. [Google Scholar]
- Straub, C.S.; Finke, D.L.; Snyder, W.E. Are the conservation of natural enemy biodiversity and biological control compatible goals? Biol. Control 2008, 45, 225–237. [Google Scholar] [CrossRef]
- Gaba, S.; Bretagnolle, F.; Rigaud, T.; Philippot, L. Managing biotic interactions for ecological intensification of agroecosystems. Front. Ecol. Environ. 2014, 2, 29. [Google Scholar] [CrossRef]
- Giagnocavo, C.; Headquarters, U. The Almería Agricultural Cooperative Model: Creating Successful Economic and Social Communities; International Year of Cooperatives Side-Event with the Commission for Social Development “The Role of Coopera-Tives in Poverty Eradication”; UN Headquarters: New York, NY, USA, 2012. [Google Scholar]
- Mendoza-Fernández, A.; Martínez-Hernández, F.; Pérez-García, F.; Garrido-Becerra, J.; Benito, B.; Salmerón-Sánchez, E.; Guirado, J.; Merlo, M.; Mota, J. Extreme habitat loss in a Mediterranean habitat: Maytenus senegalensis subsp. europaea. Plant Biosyst. 2015, 149, 503–511. [Google Scholar] [CrossRef]
- Gilbertson, R.L.; Batuman, O.; Webster, C.G.; Adkins, S. Role of the insect supervectors Bemisia tabaci and Frankliniella occidentalis in the emergence and global spread of plant viruses. Annu. Rev. Virol. 2015, 2, 67–93. [Google Scholar] [CrossRef] [PubMed]
- Lozano, R.; Diánez, F.; Camacho, F. Evolution of the phytosanitary control system in the intensive horticulture model of high yield in Almería (2005-2008). J. Food Agric. Environ. 2010, 8, 330–338. [Google Scholar]
- Van Lenteren, J.C. The state of commercial augmentative biological control: Plenty of natural enemies, but a frustrating lack of uptake. Biocontrol 2012, 57, 1–20. [Google Scholar] [CrossRef]
- Glass, R. Biological control in the greenhouses of Almeria and challenges for a sustainable intensive production. Outlooks Pest Manag. 2012, 23, 276–279. [Google Scholar] [CrossRef]
- Perdikis, D.; Kapaxidi, E.; Papadoulis, G. Biological control of insect and mite pests in greenhouse solanaceous crops. Eur. J. Plant Sci. Biotechnol. 2008, 2, 125–144. [Google Scholar]
- Rodríguez, E.; Schwarzer, V.; Van der Blom, J.; Cabello, T.; González, M. The selection of native insectary plants for landscaping in greenhouse areas of SE Spain. Landsc. Manag. Funct. Biodivers. IOBC/WPRS Bull 2012, 75, 73–76. [Google Scholar]
- Rodríguez, E.; van der Blom, J.; González, M.; Sánchez, E.; Janssen, D.; Ruiz, L.; Elorrieta, M.A. Plant viruses and native vegetation in Mediterranean greenhouse areas. Sci. Hortic. 2014, 165, 171–174. [Google Scholar] [CrossRef]
- Fiedler, A.K.; Landis, D. Attractiveness of Michigan native plants to arthropod natural enemies and herbivores. Environ. Entomol. 2007, 36, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, R.; Tuell, J.; Fiedler, A.; Gardiner, M.; Landis, D. Maximizing arthropod-mediated ecosystem services in agricultural landscapes: The role of native plants. Front. Ecol. Environ. 2009, 7, 196–203. [Google Scholar] [CrossRef]
- James, D.G.; Seymour, L.; Lauby, G.; Buckley, K. Beneficial insects attracted to native flowering buckwheats (Eriogonum michx) in central Washington. Environ. Entomol. 2014, 43, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Morandin, L.A.; Long, R.F.; Kremen, C. Hedgerows enhance beneficial insects on adjacent tomato fields in an intensive agricultural landscape. Agric. Ecosyst. Environ. 2014, 189, 164–170. [Google Scholar] [CrossRef]
- Rodríguez, E.; González, M.; Paredes, D.; Campos, M.; Benítez, E. Selecting native perennial plants for ecological intensification in Mediterranean greenhouse horticulture. Bull. Entomol. Res. 2017, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, A.L. Ecology of the true spiders (Araneomorphae). Annu. Rev. Entomol. 1973, 18, 305–348. [Google Scholar] [CrossRef]
- Wise, D. Spiders in Ecological Webs; Cambridge University: New York, NY, USA, 1993; pp. 48–50, ISBN-13 978-0521310611. [Google Scholar]
- Marc, P.; Canard, A.; Ysnel, F. Spiders (Araneae) useful for pest limitation and bioindication. Agric. Ecosyst. Environ. 1999, 74, 229–273. [Google Scholar] [CrossRef]
- Nyffeler, M.; Sunderland, K.D. Composition, abundance and pest control potential of spider communities in agroecosystems: A comparison of European and us studies. Agric. Ecosyst. Environ. 2003, 95, 579–612. [Google Scholar] [CrossRef]
- Riechert, S.E.; Lockley, T. Spiders as biological control agents. Annu. Rev. Entomol. 1984, 29, 299–320. [Google Scholar] [CrossRef]
- Schmitz, O.J. Effects of predator hunting mode on grassland ecosystem function. Science 2008, 319, 952–954. [Google Scholar] [CrossRef] [PubMed]
- Bucher, R.; Menzel, F.; Entling, M.H. Risk of spider predation alters food web structure and reduces local herbivory in the field. Oecologia 2015, 178, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Finke, D.L.; Denno, R.F. Predator diversity dampens trophic cascades. Nature 2004, 429, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Hodge, M.A. The implications of intraguild predation for the role of spiders in biological control. J. Arachnol. 1999, 351–362. [Google Scholar]
- Ghavami, S. The potential of predatory spiders as biological control agents of cotton pests in Tehran provinces of Iran. Asian J. Exp. Sci. 2008, 22, 303–306. [Google Scholar]
- Hagler, J.R.; Naranjo, S.E. Use of a gut content ELISA to detect whitefly predator feeding activity after field exposure to different insecticide treatments. Biocontrol Sci. Technol. 2005, 15, 321–339. [Google Scholar] [CrossRef]
- Nyffeler, M. Prey selection of spiders in the field. J. Arachnol. 1999, 317–324. [Google Scholar]
- Nyffeler, M.; Sterling, W.; Dean, D. How spiders make a living. Environ. Entomol. 1994, 23, 1357–1367. [Google Scholar] [CrossRef]
- Zhang, G.F.; Lü, Z.C.; Wan, F.H.; Lövei, G.L. Real-time PCR quantification of Bemisia tabaci (Homoptera: Aleyrodidae) b-biotype remains in predator guts. Mol. Ecol. Resour. 2007, 7, 947–954. [Google Scholar] [CrossRef]
- Zrubecz, P.; Toth, F.; Nagy, A. Is Xysticus kochi (Araneae: Thomisidae) an efficient indigenous biocontrol agent of Frankliniella occidentalis (Thysanoptera: Thripidae)? Biocontrol 2008, 53, 615–624. [Google Scholar] [CrossRef]
- Piñero, F.; Tinaut, A.; Aguirre-Segura, A.; Miñano, J.; Lencina, J.; Ortiz-Sánchez, F.; Pérez-López, F. Terrestrial arthropod fauna of arid areas of SE Spain: Diversity, biogeography, and conservation. J. Arid Environ. 2011, 75, 1321–1332. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Yee, T.W.; Mitchell, N.D. Generalized additive models in plant ecology. J. Veg. Sci. 1991, 2, 587–602. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Wood, S. Generalized Additive Models: An Introduction with R; CRC Press: Boca Raton, FL, USA, 2006; ISBN 9781584884743. [Google Scholar]
- Nobre, T.; Meierrose, C. In The species composition, within-plant distribution, and possible predatory role of spiders (Araneae) in a vineyard in southern Portugal. In Proceedings of the 18th European Colloquium of Arachnology; Gajdos, P., Pékar, S., Eds.; Ekologia: Bratislava, Slovakia, 2000; pp. 193–200. [Google Scholar]
- Michalko, R.; Pekár, S. The biocontrol potential of Philodromus (Araneae, Philodromidae) spiders for the suppression of pome fruit orchard pests. Biol. Control 2015, 82, 13–20. [Google Scholar] [CrossRef]
- Riechert, S. The use of behavioral ecotypes in the study of evolutionary processes. In Geographic Variation in Behavior: Perspectives on Evolutionary Mechanisms; Foster, S.A., Endler, J.A., Eds.; Oxford University Press: Oxford, UK, 1999; pp. 3–32. [Google Scholar]
- Sunderland, K. Mechanisms underlying the effects of spiders on pest populations. J. Arachnol. 1999, 27, 308–316. [Google Scholar]
- Traugott, M.; Bell, J.; Raso, L.; Sint, D.; Symondson, W.O. Generalist predators disrupt parasitoid aphid control by direct and coincidental intraguild predation. Bull. Entomol. Res. 2012, 102, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Rypstra, A.N.; Carter, P.E.; Balfaour, R.A.; Marshall, S.D. Architectural features of agricultural habitats and their impact on the spiders inhabitants. J. Arachnol. 1999, 27, 371–377. [Google Scholar]
- Greenstone, M.H. Determinats of web spiders species diversity: Vegetation structural diversity vs. prey availability. Oecologia 1984, 62, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Nentwig, W. Weedy plant species and their beneficial arthropods: Potential for manipulations in field crops. In Enhancing Biological Control: Habitat Management to Promote Natural Enemies of Agricultural Pests; Pickett, C.H., Bugg, R.L., Eds.; UC Press: Berkeley, CA, USA, 1998; pp. 49–72. [Google Scholar]
- Romero, G.Q.; Vasconcellos-Neto, J. The effects of plant structure on the spatial and microspatial distribution of a bromeliad-living jumping spider (Salticidae). J. Anim. Ecol. 2005, 74, 12–21. [Google Scholar] [CrossRef]
- Souza, A.L.T.D.; Martins, R.P. Distribution of plant-dwelling spiders: Inflorescences vs. vegetative branches. Austral Ecol. 2004, 29, 342–349. [Google Scholar] [CrossRef]
- Sharma, S.R.; Verma, A. Effect of cultural practices on virus infection in cowpea. Z. Acker Planzenbau 1984, 153, 23–31. [Google Scholar]
- Smith, H.A.; McSorley, R. Potential of field corn as a barrier cropland eggplant as a trap crop for management of Bemisia argentifolii (Homoptera: Aleyrodidae) on common bean in North Florida. Fla. Entomol. 2000, 83, 145–158. [Google Scholar] [CrossRef]
- Rataul, H.S.; Gill, C.K.; Brar, S. Use of barrier crop and some cultural measures in the management of yellow mosaic virus on soybean. J. Res. Punjab Agric. Univ. 1989, 26, 227–230. [Google Scholar]
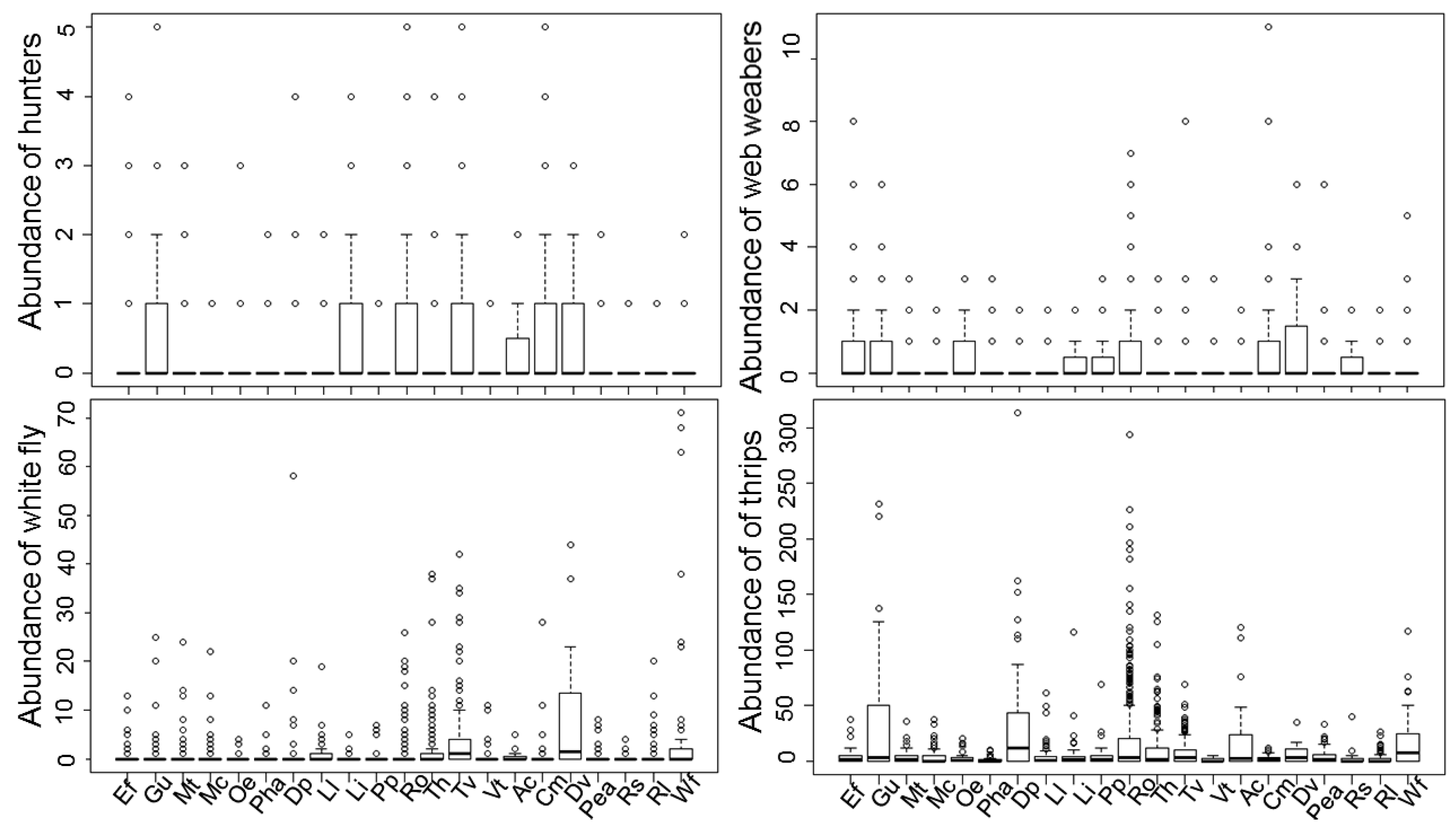

| Species Assayed | Common Name | Family | Plant Code | Number Assayed |
|---|---|---|---|---|
| Ephedra fragilis Desf. | joint pine | Ephedraceae | Ef | 7 |
| Genista umbellata Poir. | bolina | Fabaceae | Gu | 7 |
| Macrochloa tenacissima (L.) Kunth | alfa grass | Poaceae | Mt | 9 |
| Myrtus communis L. | myrtle | Myrtaceae | Mc | 7 |
| Olea europaea var. sylvestris L. | wild olive tree | Oleaceae | Oe | 3 |
| Phillyrea angustifolia L. | false olive | Oleaceae | Pha | 10 |
| Dorycnium pentaphyllum Scop. | prostrate canary clover | Fabaceae | Dp | 6 |
| Lavandula latifolia Medik. | spike lavender | Lamiaceae | Li | 6 |
| Lycium intricatum Boiss. | cambrón | Solanaceae | Li | 4 |
| Phlomis purpurea L. | purple phlomis | Lamiaceae | Pp | 2 |
| Rosmarinus officinalis L. | rosemary | Lamiaceae | Ro | 25 |
| Thymus hyemalis Lange. | winter thyme | Lamiaceae | Th | 17 |
| Thymus vulgaris L. | thyme | Lamiaceae | Tv | 19 |
| Viburnum tinus L. | laurustinus | Adoxaceae | Vt | 4 |
| Anthyllis cytisoides L. | albaida | Fabaceae | Ac | 2 |
| Crithmum maritimum L. | rock samphire | Apiaceae | Cm | 6 |
| Dittrichia viscosa (L.) Greuter | false yellowhead | Asteraceae | Dv | 2 |
| Periploca angustifolia Labill. | cornical | Asclepiadaceae | Pea | 6 |
| Retama sphaerocarpa (L.) Boiss. | yellow broom | Fabaceae | Rs | 3 |
| Rhamnus lycioides subsp. lycioides L. | Mediterranean buckthorn | Rhamnaceae | Rl | 10 |
| Whitania frutescens (L.) Pauquy. | oroval | Solanaceae | Wf | 6 |
| Taxa | Frequency (%) |
|---|---|
| Web-weavers | 50.6 |
| Juveniles | 13.1 |
| Araneidae [Neoscona subfusca] | 78.7 |
| Araneidae (sp. 1 sp. 2 sp. 3) | 4.7 |
| Theridiidae [Anelosimus aulicus] | 1.5 |
| Linyphiidae/Tetragnatidae/Pholcidae | 2.0 |
| Hunting spiders | 49.4 |
| Salticidae [Thyene imperialis] | 23.2 |
| Salticidae [Heliophanus aeneus] | 13.7 |
| Thomisidae [Xysticus kochi] | 20.8 |
| Thomisidae [Thomisus onustus] | 4.7 |
| Thomisidae [Xysticus bufo] | 0.6 |
| Oxyopidae [Oxyopes spp.] | 10.0 |
| Oxyopidae [Peucetia viridans] | 0.5 |
| Philodromidae [Pulchellodromus spp.] | 21.1 |
| Philodromidae [Philodromus dispar] | 4.5 |
| Liocranidae/Lycosidae | 0.9 |
| Model | Types of Variables | Variables | Estimate | SE | χ2 | df | p Value |
|---|---|---|---|---|---|---|---|
| Hunter spiders | Numerical | Whitefly abundance | 0.015 | 0.005 | 18.771 | 1 | <0.001 |
| Numerical | Thrips abundance | −0.005 | 0.004 | 7.943 | 1 | <0.01 | |
| Categorical | Plant species | 193.792 | 20 | <0.001 | |||
| Smoothed | Month of sampling | 113.1 | 8.35 | <0.001 | |||
| Web-weavers | Numerical | Whitefly abundance | 0.014 | 0.007 | 4.0544 | 1 | <0.05 |
| Numerical | Thrips abundance | −0.004 | 0.004 | 0.782 | 1 | 0.3765 | |
| Categorical | Plant species | 164.204 | 20 | <0.001 | |||
| Smoothed | Month of sampling | 336 | 8.89 | <0.001 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cotes, B.; González, M.; Benítez, E.; De Mas, E.; Clemente-Orta, G.; Campos, M.; Rodríguez, E. Spider Communities and Biological Control in Native Habitats Surrounding Greenhouses. Insects 2018, 9, 33. https://doi.org/10.3390/insects9010033
Cotes B, González M, Benítez E, De Mas E, Clemente-Orta G, Campos M, Rodríguez E. Spider Communities and Biological Control in Native Habitats Surrounding Greenhouses. Insects. 2018; 9(1):33. https://doi.org/10.3390/insects9010033
Chicago/Turabian StyleCotes, Belén, Mónica González, Emilio Benítez, Eva De Mas, Gemma Clemente-Orta, Mercedes Campos, and Estefanía Rodríguez. 2018. "Spider Communities and Biological Control in Native Habitats Surrounding Greenhouses" Insects 9, no. 1: 33. https://doi.org/10.3390/insects9010033