A Molecular Method for the Identification of Honey Bee Subspecies Used by Beekeepers in Russia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bees Collection and Morphological Analysis
2.2. DNA Barcoding
2.3. PCR-RFLP
3. Results
3.1. DNA Barcoding
3.2. Development PCR-RFLP
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Robinson, W.S.; Nowogrodzki, R.; Morse, R.A. The value of honey bees as pollinators of U.S. crops. Am. Bee J. 1989, 129, 477–487. [Google Scholar]
- Tarpy, D.R.; Summers, J.; Keller, J.J. Comparison of parasitic mites in Russian-hybrid and Italian honey bee (Hymenoptera: Apidae) colonies across three different locations in North Carolina. J. Econ. Entomol. 2007, 100, 258–266. [Google Scholar] [CrossRef] [PubMed]
- De Guzmana, L.I.; Rinderera, T.E.; Delattea, G.T.; Stelzera, J.A.; Beamana, L.; Kuznetsov, V. Resistance to Acarapis woodi by honey bees from far-eastern Russia. Apidologie 2002, 33, 411–415. [Google Scholar] [CrossRef]
- Gruber, K.; Schöning, C.; Otte, M.; Kinuthia, W.; Hasselmann, M. Distinct subspecies or phenotypic plasticity? Genetic and morphological differentiation of mountain honey bees in East Africa. Ecol. Evol. 2013, 3, 3204–3218. [Google Scholar] [CrossRef] [PubMed]
- Tofilski, A. Using geometric morphometrics and standard morphometry to discriminate three honeybee subspecies. Apidologie 2008, 39, 558–563. [Google Scholar] [CrossRef]
- Silva, F.L.; Sella, M.L.G.; Francoy, T.M.; Costa, A.H.R. Evaluating classification and feature selection techniques for honeybee subspecies identification using wing images. Comput. Electron. Agric. 2015, 115, 68–77. [Google Scholar] [CrossRef]
- Santana, F.S.; Costa, A.H.R.; Truzzi, F.S.; Silva, F.L.; Santos, S.L.; Francoy, T.M.; Saraiva, A.M. A reference process for automating bee species identification based on wing images and digital image processing. Ecol. Inform. 2014, 24, 248–260. [Google Scholar] [CrossRef]
- Koca, A.O.; Kandemir, I. Comparison of two morphometric methods for discriminating honey bee (Apis mellifera L.) populations in Turkey. Turk. J. Zool. 2013, 37, 205–210. [Google Scholar]
- Meulemeester, T.D.; Michez, D.; Aytekin, A.M.; Danforth, B.N. Taxonomic affinity of halictid bee fossils (Hymenoptera: Anthophila) based on geometric morphometrics analyses of wing shape. J. Syst. Palaeontol. 2012, 10, 755–764. [Google Scholar] [CrossRef]
- Nedić, N.; Jevtić, G.; Jež, G.; Anđelković, B.; Milosavljević, S.; Kostić, M. Forewing differentiation of the honey bees from Serbia. Biotechnol. Anim. Husb. 2011, 27, 1387–1394. [Google Scholar] [CrossRef]
- Kulici, M.; Bajrami, Z.; Kume, K. Genetic Local Differentiation of A.m.carnica Population as well as Subspecies A.m.macedonica, A.m.ligustica, A.m.mellifera, A.m.caucasica, in Germany, Alpine Region, Austria, Croatia, Serbia, Northern Kosovo, Albania and Macedonia. J. Nat. Sci. Res. 2014, 4, 53–60. [Google Scholar]
- Francoy, T.M.; Wittmann, D.; Drauschke, M.; Muller, S.; Steinhage, V.; Bezerra-Laure, M.A.F.; De Jong, D.; Goncalves, L.S. Identification of africanized honey bees through wing morphometrics: two fast and efficient procedures. Apidologie 2008, 39, 488–494. [Google Scholar] [CrossRef]
- Dla Rúa, P.; Galián, J.; Serrano, J.; Moritz, R.F. Genetic structure of Balearic honeybee populations based on microsatellite polymorphism. Genet. Sel. Evol. 2003, 35, 339–350. [Google Scholar] [CrossRef]
- Oleksa, A.; Tolski, A. Wing geometric morphometrics and microsatellite analysis provide similar discrimination of honey bee subspecies. Apidologie 2015, 46, 49–60. [Google Scholar] [CrossRef]
- Zinovyeva, N.A.; Krivtsov, N.I.; Borodachev, A.V.; Lebedev, V.I.; Fornara, M.S. Differentiation of the basic bees breeds with the help of micro-satellites. V. Ryazan State Agrotechnol. Univ. 2011, 4, 23–27. (In Russian) [Google Scholar]
- Alattal, Y.; Alsharhi, M.; Al-Ghamdi, A.; Alfaify, S.; Migdadi, H.; Ansari, M. Characterization of the native honey bee subspecies in Saudi Arabia using the mtDNA COI–COII intergenic region and morphometric characteristics. Bull. Insectol. 2014, 67, 31–37. [Google Scholar]
- Chávez-Galarza, J.; Garnery, L.; Henriques, D.; Neves, C.J.; Loucif-Ayad, W.; Jonhston, J.S.; Pinto, M.A. Mitochondrial DNA variation of Apis mellifera iberiensis: further insights from a large-scale study using sequence data of the tRNAleu-cox2 intergenic region. Apidologie 2017, 48, 533–544. [Google Scholar] [CrossRef]
- Abrahamovich, A.H.; Atela, O.; De la Rúa, P.; Galián, J. Assessment of the mitochondrial origin of honey bees from Argentina. J. Apic. Res. Bee World 2007, 46, 191–194. [Google Scholar] [CrossRef]
- Cornuet, J.M.; Garnery, L.; Solignac, M. Putative origin and function of the intergenic region between COI and COII of Apis mellifera L. mitochondrial DNA. Genetics 1991, 128, 393–403. [Google Scholar] [PubMed]
- Ostroverkhova, N.V.; Konusova, O.L.; Kucher, A.N.; Kireeva, T.N.; Vorotov, A.A.; Belykh, E.A. Genetic diversity of the locus COI-COII of mitochondrial DNA in honeybee populations (Apis mellifera L.) from the Tomsk region. Genetika 2015, 51, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.; Freitas, A.I.; Jesus, J.; De la Rua, P.; Brehm, A. Structure and genetic variation of the mitochondrial control region in the honey bee Apis mellifera. Apidologie 2015, 46, 515–526. [Google Scholar] [CrossRef]
- Cho, S.; Huang, Z.Y.; Green, D.R.; Smith, D.R.; Zhang, J. Evolution of the complementary sex-determination gene of honey bees: Balancing selection and trans-species polymorphisms. Genome Res. 2006, 16, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.A.; Henriques, D.; Chávez-Galarza, J.; Kryger, P.; Garnery, L.; van der Zee, R.; Dahle, B.; Soland-Reckeweg, G.; de la Rua, P.; Dall’ Olio, R.; et al. Genetic integrity of the Dark European honey bee (Apis mellifera mellifera) from protected populations: A genome-wide assessment using SNPs and mtDNA sequence data. J. Apic. Res. 2014, 53, 269–278. [Google Scholar] [CrossRef]
- Johnston, J.S.; Chávez-Galarza, J.; De La Rua, P.; Pinto, M.A. SNPs selected by information content outperform randomly selected microsatellite loci for delineating genetic identification and introgression in the endangered dark European honeybee (Apis mellifera mellifera). Mol. Ecol. Res. 2016, 17, 783–795. [Google Scholar]
- Chávez-Galarza, J.; Henriques, D.; Johnston, J.S.; Azevedo, J.C.; Patton, J.C.; Muñoz, I.; De la Rua, P.; Pinto, M.A. Signatures of selection in the Iberian honey bee (Apis mellifera iberiensis) revealed by a genome scan analysis of single nucleotide polymorphisms. Mol. Ecol. 2013, 22, 5890–5907. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.D.; Gregory, T.R. The promise of DNA Barcoding for Taxonomy. Syst. Biol. 2005, 54, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.D.; Cywinska, A.; Ball, S.L.; de Waard, J.R. Biological identifications through DNA barcodes. Proc. Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Ferri, G.; Alu, M.; Corradini, B.; Licata, M.; Beduschi, G. Species identification through DNA “barcodes”. Genet. Test. Mol. Biomark. 2009, 13, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.D.; Ratnasingham, S.; de Waard, J.R. Barcoding animal life: Cytochrome c oxidase subunit 1 divergences among closely related species. Proc. Biol. Sci. 2003, 270, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Bouga, M.; Alaux, C.; Bienkowska, M.; Büchler, R.; Carreck, N.L.; Cauia, E.; Chlebo, R.; Dahle, B.; Dall’Olio, R.; De la Rúa, P.; et al. A review of methods for discrimination of honey bee populations as applied to European beekeeping. J. Apic. Res. 2011, 50, 51–84. [Google Scholar] [CrossRef]
- Hebert, P.D.; Penton, E.H.; Burns, J.M.; Janzen, D.H.; Hallwachs, W. Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator. PNAS 2004, 101, 14812–14817. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.J. DNA barcodes for insects. Methods Mol. Biol. 2012, 858, 17–46. [Google Scholar] [PubMed]
- Kimura, M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Packer, L.; Ruz, L. DNA barcoding the bees (Hymenoptera: Apoidea) of Chile: Species discovery in a reasonably well known bee fauna with the description of a new species of Lonchopria (Colletidae). Genome 2017, 60, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Kevan, P.G.; Packer, L. DNA barcoding a regional bee (Hymenoptera: Apoidea) fauna and its potential for ecological studies. Mol. Ecol. Res. 2009, 1, 196–207. [Google Scholar]
- Ozdil, F.; Ilhan, F. Phylogenetic relationship of Turkish Apis mellifera subspecies based on sequencing of mitochondrial cytochrome C oxidase I region. Genet. Mol. Res. 2012, 11, 1130–1141. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.A.; Rubink, W.L.; Patton, J.C.; Coulson, R.N.; Johnston, J.S. Africanization in the United States: Replacement of Feral European Honey Bees (Apis mellifera L.) by an African Hybrid Swarm. Genetics 2005, 170, 1653–1665. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, C.W.; Behura, S.K.; Berlocher, S.H.; Clark, A.G.; Johnston, J.S.; Sheppard, W.S.; Smith, D.R.; Suarez, A.V.; Weaver, D.; Tsutsui, N.D. Thrice out of Africa: Ancient and recent expansions of the honey bee, Apis mellifera. Science 2006, 314, 642–645. [Google Scholar] [CrossRef] [PubMed]
- Syromyatnikov, M.Y.; Borodachev, A.V.; Kokina, A.V.; Popov, V.N. Voronezh State University: Voronezh, Russia, Unpublished data. 2017.

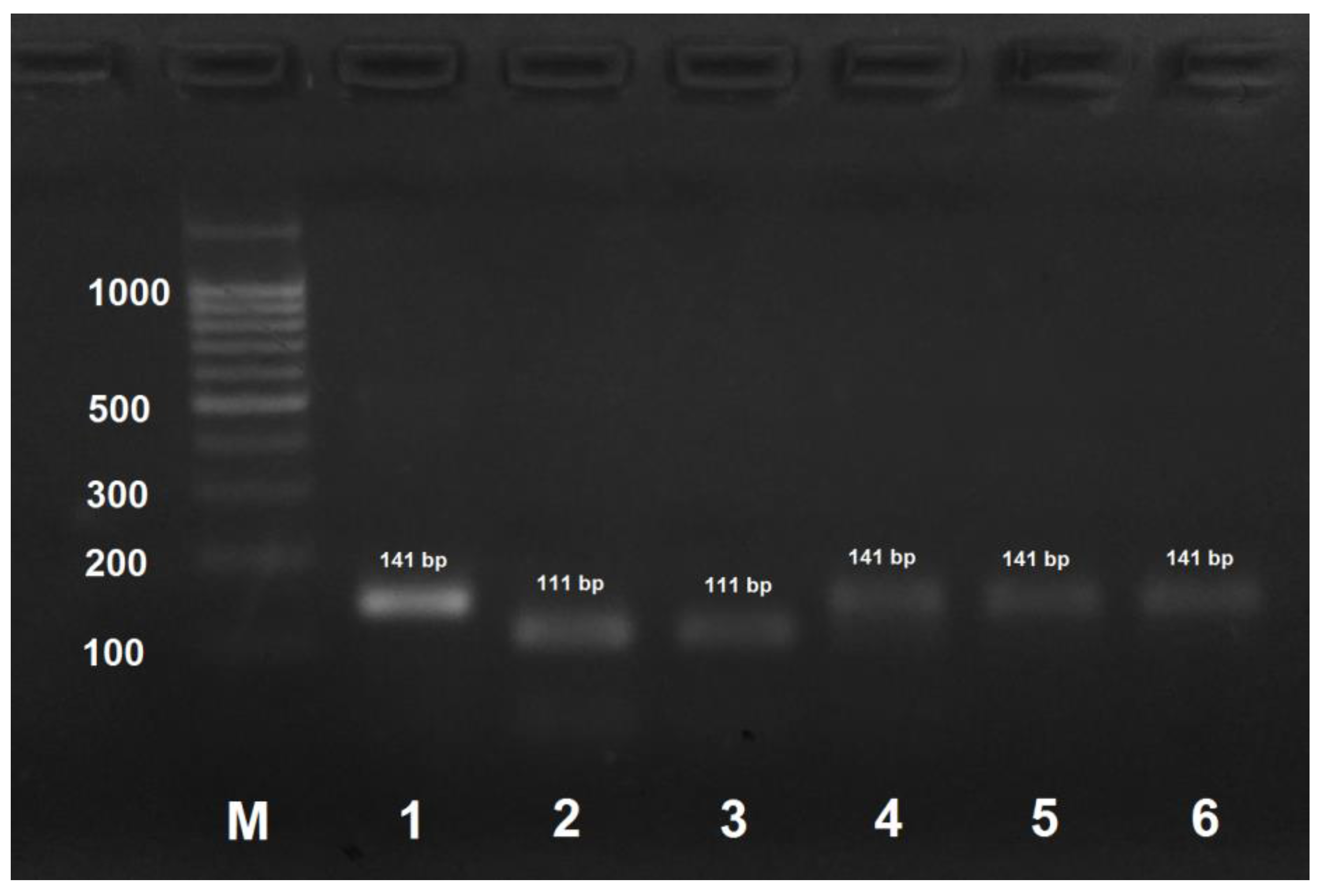
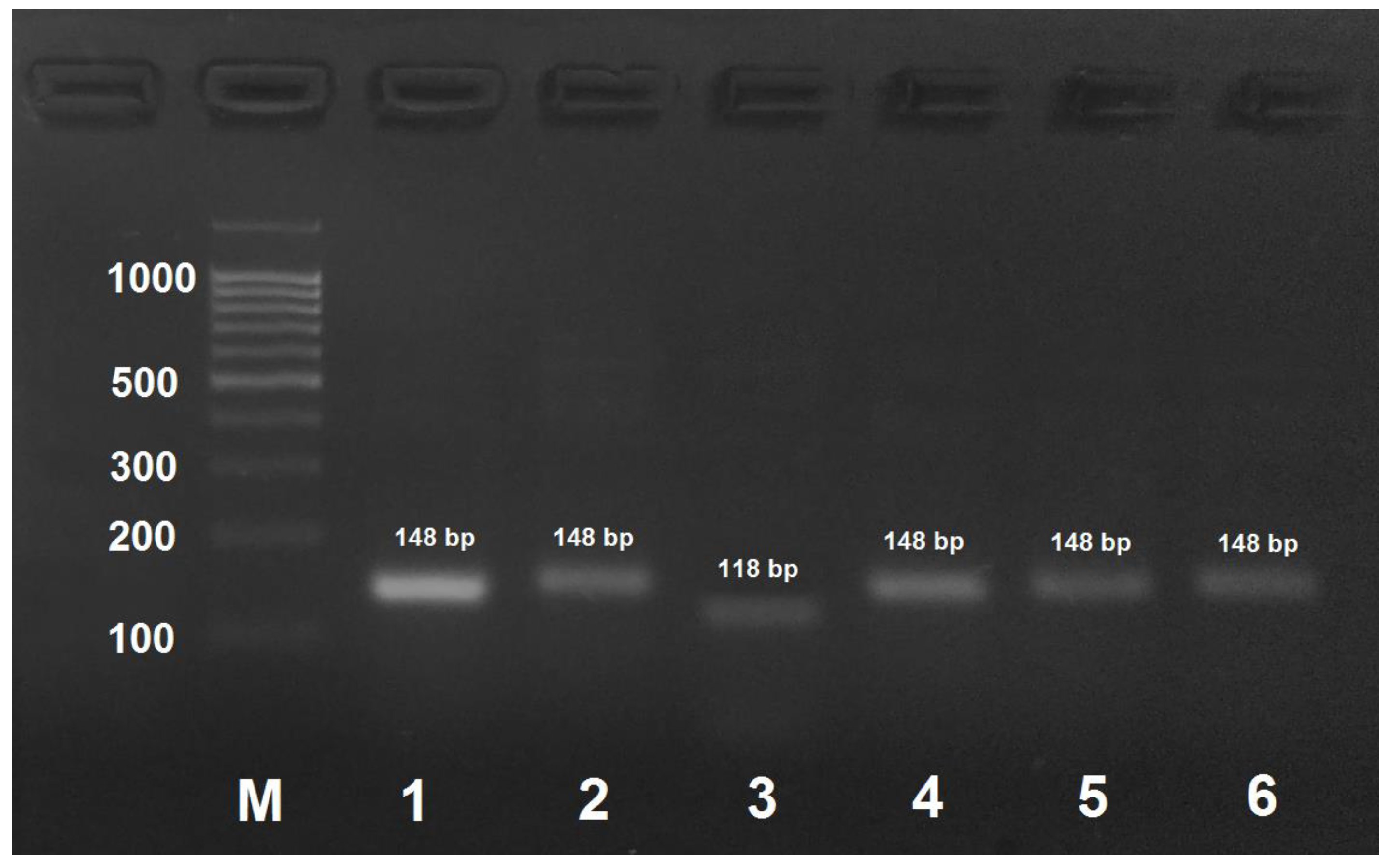
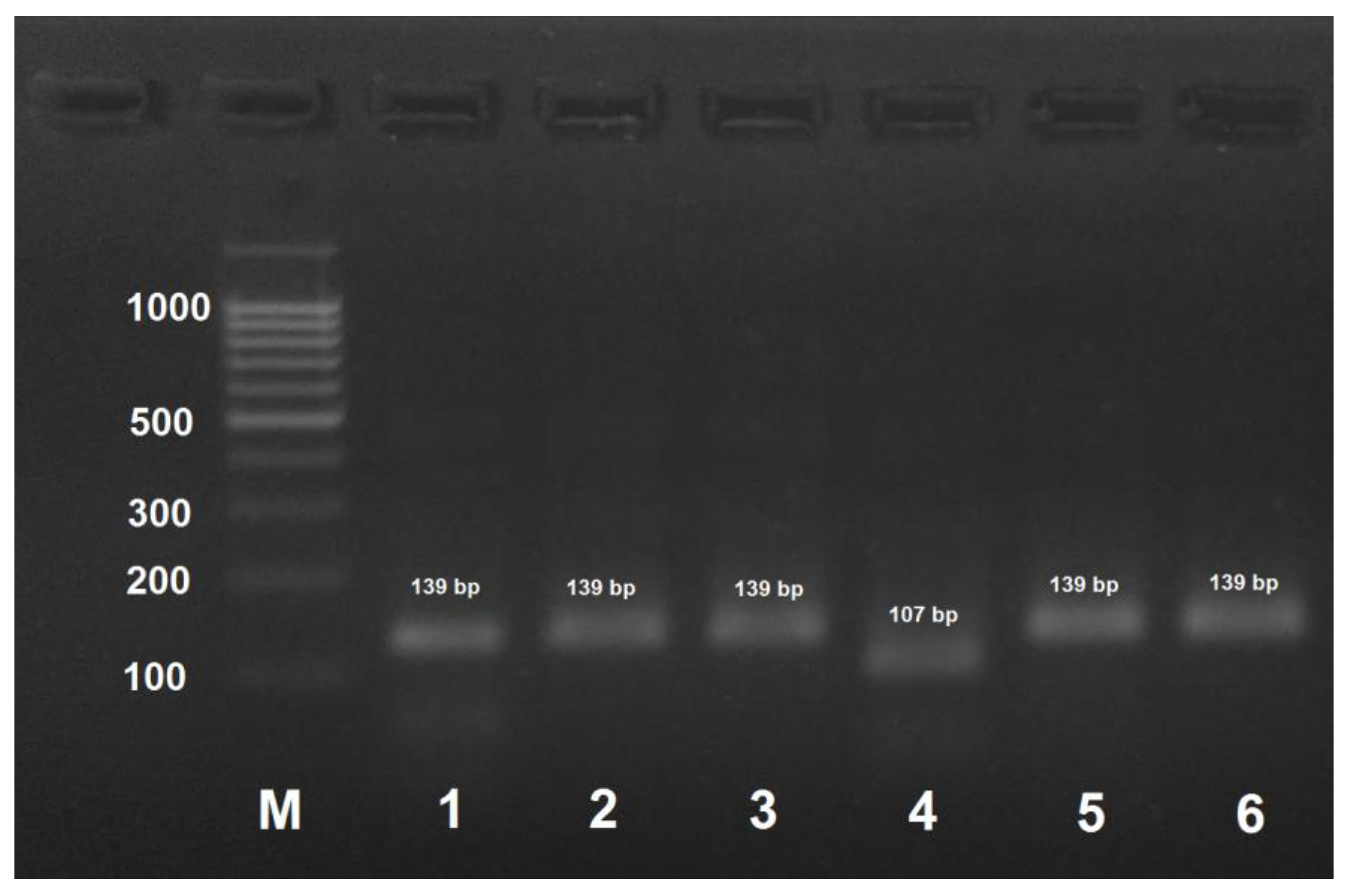
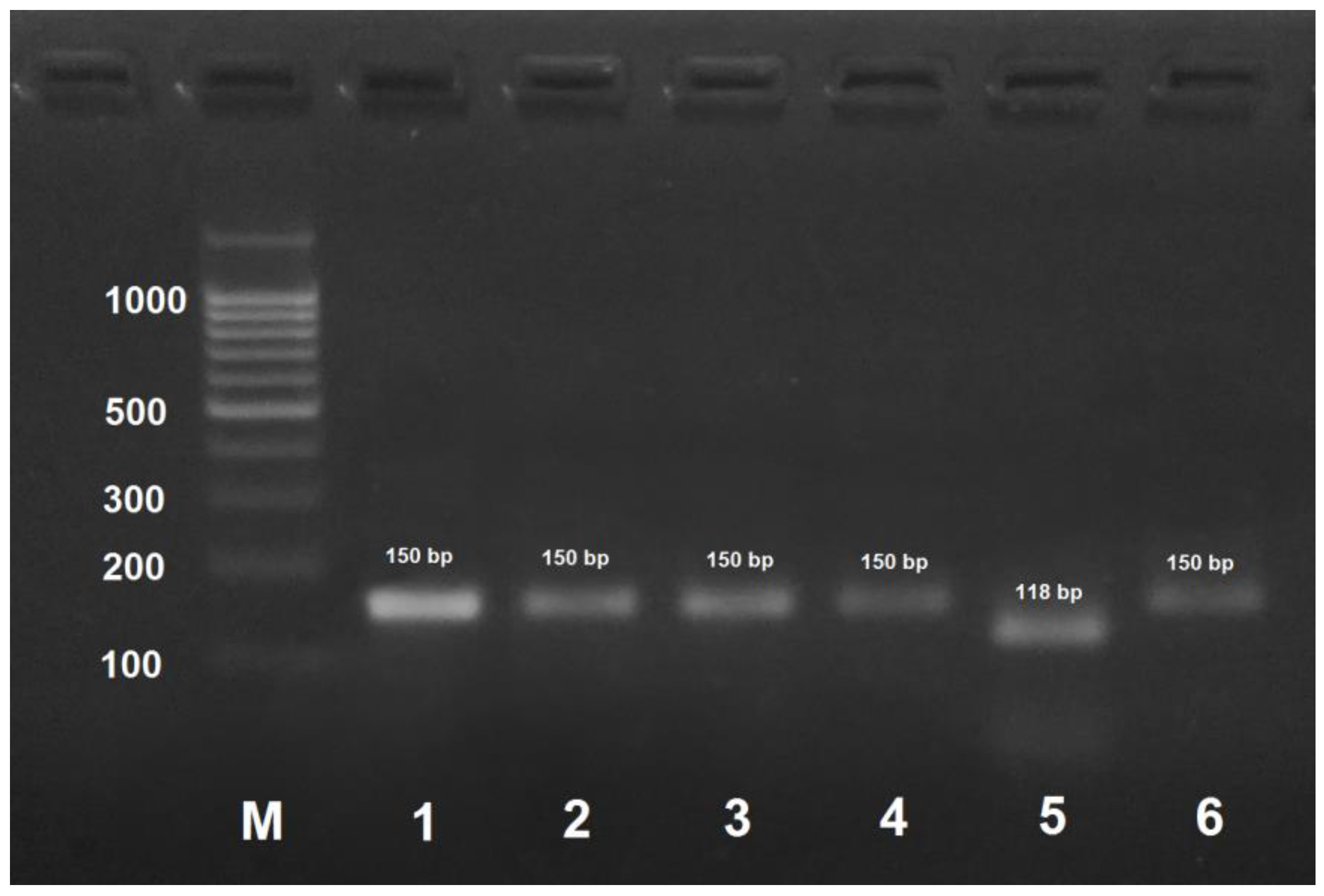
| Breed | Proboscis Length, mm | Third Tergit Width, mm | Cubital Index, % | Tarsal Index, % | ||||
|---|---|---|---|---|---|---|---|---|
| M ± m | Cv, % | M ± m | Cv, % | M ± m | Cv | M ± m | Cv | |
| A. m. mellifera | 6.2 ± 0.02 | 1.8 | 5.0 ± 0.04 | 1.3 | 62.3 ± 1.5 | 6.2 | 55.6 ± 0.2 | 4.0 |
| A. m. carpatica | 6.7 ± 0.02 | 2.6 | 4.7 ± 0.01 | 2.2 | 43.1 ± 0.40 | 5.5 | 52.0 ± 0.6 | 2.5 |
| A. m. caucasica | 6.9 ± 0.01 | 1.2 | 4.7 ± 0.01 | 1.4 | 51.2 ± 0.20 | 3.2 | 55.0 ± 0.2 | 4.1 |
| A. m. carnica | 6.7 ± 0.02 | 2.2 | 4.9 ± 0.02 | 2.3 | 37.9 ± 0.3 | 5.0 | 54.0 ± 0.4 | 2.5 |
| Primer Name | Primer Direction | Primer Sequence |
|---|---|---|
| LepF1 | forward | ATTCAACCAATCATAAAGATATTGG |
| LepR1 | reverse | TAAACTTCTGGATGTCCAAAAAATCA |
| AmCarp-f | forward | GAATATGAGCCGGAATAGTAGGA |
| AmCar-r | reverse | ATGTGTTGAAGTTACGGTCA |
| CYTB-f | forward | TATGTACTACCATGAGGACAAATATC |
| CYTB-r | reverse | ATTACACCTCCTAATTTATTAGGAAT |
| Subspecies | Primers | 5′–3′Sequence | |
|---|---|---|---|
| A. mellifera carnica | AmCar-f | forward | ATTTCMTCAATTATAGGATCATTAAAYTTACC * |
| AmCar-r | reverse | CAGCTAATACAGGTAATGA | |
| A. mellifera carpatica | AmCarp-f | forward | AGATATTGGGATCTTGTA |
| AmCarp-r | reverse | CTAGTAACAATTGTATTATAAATTTGATCAGCG * | |
| A. mellifera mellifera H1 | AmEu1-f | forward | GGATGAACAGTATATCCACC |
| AmEu1-r | reverse | GTAACTATTAAGTTTAATGATCCTATAATAGC * | |
| A. mellifera mellifera H2 | AmEu2-f | forward | CTTTAATACTAGGATCACCTGATATAGCGAT * |
| AmEu2-r | reverse | CTGATAATGGTGGATATA | |
| Primers | Restriction Endonuclease | Product Length, Bp | ||||
|---|---|---|---|---|---|---|
| A. mellifera mellifera H1 | A. mellifera mellifera H2 | A. mellifera carpatica | A. mellifera carnica | A. mellifera caucasica | ||
| AmEu1-f, AmEu1-r | Alu I | 107, 32 | 139 | 139 | 139 | 139 |
| AmEu2-f, AmEu2-r. | Hinf I | 150 | 122, 28 | 150 | 150 | 150 |
| AmCarp-f, AmCarp-r | HspA I | 141 | 141 | 111, 30 | 111, 30 | 141 |
| AmCar-f, AmCar-r | Msp I | 148 | 148 | 148 | 118, 30 | 148 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syromyatnikov, M.Y.; Borodachev, A.V.; Kokina, A.V.; Popov, V.N. A Molecular Method for the Identification of Honey Bee Subspecies Used by Beekeepers in Russia. Insects 2018, 9, 10. https://doi.org/10.3390/insects9010010
Syromyatnikov MY, Borodachev AV, Kokina AV, Popov VN. A Molecular Method for the Identification of Honey Bee Subspecies Used by Beekeepers in Russia. Insects. 2018; 9(1):10. https://doi.org/10.3390/insects9010010
Chicago/Turabian StyleSyromyatnikov, Mikhail Y., Anatoly V. Borodachev, Anastasia V. Kokina, and Vasily N. Popov. 2018. "A Molecular Method for the Identification of Honey Bee Subspecies Used by Beekeepers in Russia" Insects 9, no. 1: 10. https://doi.org/10.3390/insects9010010




