1. Introduction
Increased use of insecticide sprays for the targeted control of tarnished plant bug (
Lygus lineolaris (Palisot de Beauvois)) in the Midsouth has been widely discussed and highlighted by numerous authors over the last two decades [
1,
2]. The current number of sprays made for tarnished plant bug is increasing and is somewhat reminiscent of scheduled “calendar day” approaches to pest management from the 1950s and 1960s that led to adverse effects of over-reliance on chemical control, resulting environmental problems, outbreaks of secondary pests, and a pesticide treadmill that evolved around cycles of new insecticide chemistry [
3]. While current insecticides are generally considered to be less harmful to the environment, heavy use of insecticides for plant bugs greatly diminishes the potential economic and environmental advantages of the selective action of transgenic Bt cottons for lepidopteran pests and the successful removal of boll weevil (
Anthonomus grandis Boheman) as a key pest of southern cotton [
4].
Lygus spp. have long been a pest of cotton [
5], and efforts to manage the pest are critical to profitable cotton production.
Scott and Snodgrass [
6], Snodgrass [
7], and Luttrell and King [
8] provide historical overviews of USDA research and efforts to study the chemical control of tarnished plant bug in the Mississippi Delta over the last 30 years. While the chemical control of tarnished plant bug continues to be a challenge and an economic and ecological problem [
9], significant research has been devoted to the topic by federal and state researchers (multiple review articles in Nordlund and Hardee [
10] and Edwards [
11]). Chemical control options have expanded over the last 30 years, and extension/research entomologists have made efforts to confirm and revise thresholds [
12,
13]. Researchers at the USDA ARS’ Southern Insect Management Research Unit have produced a wealth of information on tarnished plant bug response to insecticides in laboratory assays [
14,
15,
16,
17,
18,
19,
20,
21,
22], and examinations of resistance mechanisms associated with the variable response of tarnished plant bugs to insecticides [
23,
24,
25,
26,
27,
28]. Research at both the University of Arkansas [
29] and Mississippi State University [
1] has also addressed similar research topics. Notable published research on the field control of tarnished plant bugs with foliar applied insecticides include Scott et al. [
30] and Reed, Jackson, and Harris [
2].
Fleming et al. [
1] indicated that insecticide use for
L. lineolaris in the Delta region of Mississippi has increased at a rate of ~0.2 applications per year since 1999, reaching an average of five or more applications per year since 2008. Snodgrass et al. [
20,
21,
31] provided assessments of tarnished plant bug resistance levels to acephate, permethrin, imidacloprid, and thiamethoxam up to 2007. This paper reports the results of continued assays and follow-up information for 2008 through 2015, and possible linkages are explored between measured levels of susceptibility, recorded insecticide use, estimated crop loss, and control costs for the eight-year period.
4. Discussion
This paper continues a history of the reporting of assay responses of tarnished plant bug to the major classes of insecticide used in the Delta. There is a wealth of previous information on tarnished plant bug response to insecticides in this area, including early work [
43,
44], numerous papers from the Snodgrass laboratory [
7,
14,
15,
16,
17,
19,
20,
21,
22,
45], studies in Arkansas [
29,
33], studies in Louisiana [
46,
47], a summary of small-plot field experiments in Mississippi [
2], and a number of efforts to understand tarnished plant bug resistance mechanisms [
1,
23,
25,
26,
27,
28]. Understanding the changes in susceptibility over time are important, but understanding how these changes evolve and how management practices can be refined with this information is particularly relevant to managing tarnished plant bugs in the future. Snodgrass [
7] concluded a summary of 30 years of research with the tarnished plant bug in the Mississippi Delta by writing “as long as insecticides are the main method for controlling TPB (tarnished plant bug) in cotton, the TPB will remain a serious pest”.
While the data presented here include previously unpublished resistance information from the Snodgrass laboratory, it also attempts to transition the research to new approaches. Research conducted in 2014 and 2015 introduced the use of the USDA lab colony as an experimental control. The historical use of collections from Crossett, AR, as an index of susceptibility may not be sustainable (
Table 1), and the historical approach potentially confounds insect nutrition and age with measurements of insecticide susceptibility. Indirect comparisons back to previous published benchmarks are useful and important, but paired comparisons to a susceptible lab colony would strengthen experimental measurements and allow researchers to control experimental error. Both Zhu and Luttrell [
23] and Zhu et al. [
24] made comparisons between field-collected strains and a meridic diet-fed laboratory colony of tarnished plant bugs, and Fleming et al. [
1] discussed their omission of a laboratory-susceptible strain and the possibility that all of their strains may have had some level of insecticide resistance. The high tolerance and variability of the USDA lab colony to acephate, permethrin, and thiamethoxam needs additional research if it is to be used as an experimental control. Regardless, there needs to be more experimental consistency and more understanding of the relationships between laboratory susceptibility and control in the field. Because of the high tolerance and variability observed with the lab colony, comparisons of insecticide susceptibility were based on previously published benchmarks from the Snodgrass laboratory using insects from Crossett and our more recent direct measurements of response from field collections of insects from the Crossett location (
Table 1). Based on these traditional benchmark comparisons to the Crossett susceptible location, the USDA lab colony would be judged to be resistant to most of the insecticides tested. The variability of response in the USDA lab colony was greatest for acephate and permethrin assays, while the variability with the neonicotinoids and sulfoxaflor was less, perhaps suggesting the presence of resistance genes for acephate and pyrethroid resistance within the USDA lab colony. A more plausible explanation is likely linked to the differences in food between the lab colony’s meridic diet and the field-collected insects that have fed on a wide range of nutritionally variable native plants and crops. Nutrition and host plant development have been shown to affect measurements of insecticide susceptibility in several other insect species [
48,
49,
50,
51,
52].
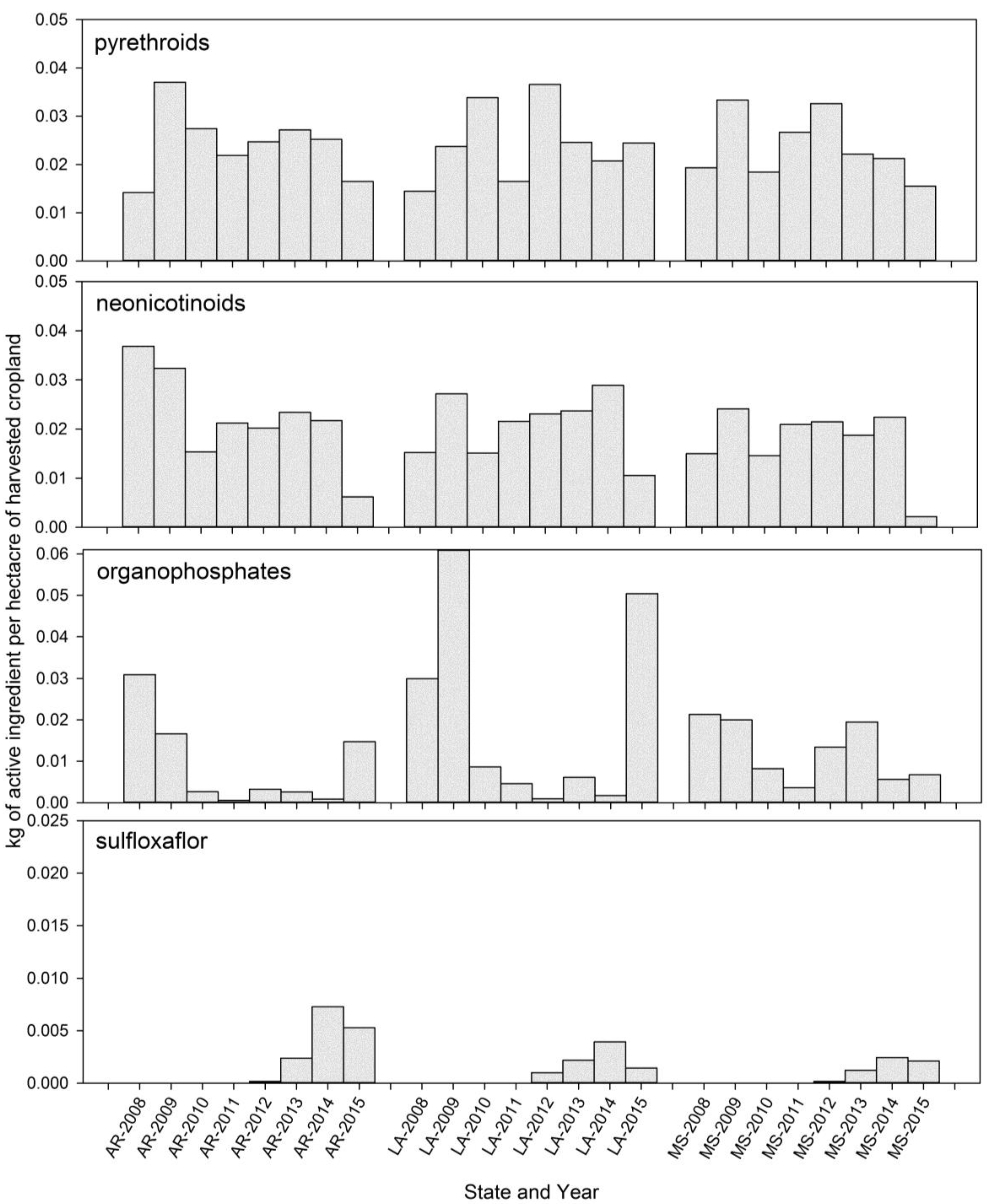
Based on the acephate assay results of this study (
Table 2 and
Table 3,
Figure 2) and comparisons to declining levels of susceptibility reported by Snodgrass et al. [
21], tarnished plant bug populations in the Delta region of Arkansas, Louisiana, and Mississippi have high frequencies of resistance to this organophosphate insecticide. However, there is still wide variability among populations and some populations appear to be relatively susceptible (
Figure 2). Research by others [
1,
23,
24] has found similar results and elevated activities of metabolic enzymes in field populations collected from the Delta region. Both Zhu and Luttrell [
23] and Fleming et al. [
1] have reported elevated levels of esterase activity in Delta populations of tarnished plant bugs. Zhu and Luttrell [
23] associated these elevated levels with reduced susceptibility to acephate, while Fleming et al. [
1] observed differences in esterase activities in bugs from two different regions of Mississippi, but did not associate the differences with individual assay results. Both Zhu and Luttrell [
23] and Fleming et al. [
1] measured variable levels of glutathione S-transferase in the Delta populations of tarnished plant bug. Zhu and Luttrell [
23] indicated that inhibitors of glutathione S-transferase exhibited less suppression in 2010 as compared to 2006, and suggested that this may indicate a potential shift in the genetics of the pest populations. Recommended application rates of acephate were increased from 0.23–0.33 lb ai/acre to 0.5–1.0 lb ai/acre during this time period (
Table 10), indicating a growing concern for the level of field control being achieved. Reed et al. [
2] summarized results of replicated field experiments conducted to measure tarnished plant bug control in cotton with organophosphate insecticides, and reported that the average control measured from 1982 to 1997 was 57%. If this level of field control compares to the previous assay data of Snodgrass et al. [
21], and field control is even loosely related to changes in assay response, acephate applications alone do not adequately control tarnished plant bugs in the field. Bioassays were conducted with permethrin only in 2014 and 2015, as most of the previous systematic monitoring for pyrethroid resistance was based on the use of a diagnostic-dose assay [
18,
20]. Based on our 2014 and 2015 assays (
Figure 3 and
Table 4), tarnished plant bugs still express resistance to permethrin. Pyrethroid-resistant populations are common, but there is wide variability in response among populations and some are relatively susceptible. Perhaps, a return to careful monitoring of populations prior to spraying a pyrethroid would enhance the efficiency of insecticide selection decisions and allow growers to more carefully determine when to use pyrethroid insecticides [
18].
Snodgrass et al. [
21] reported variability in the response of tarnished plant bugs to both imidacloprid and thiamethoxam, and suggested that some imidacloprid resistance was present. At the time, it was also generally concluded that the neonicotinoids were the only widely used insecticides to which tarnished plant bug populations are still susceptible. In our studies (
Table 2 and
Table 5,
Figure 2), we found no evidence of increasing resistance to imidacloprid, although the populations commonly had higher LC
50s than the Crossett collections. With thiamethoxam, a few colonies collected from cotton fields experiencing control problems, in particular the colony collected from Tallahatchie County, MS, in 2014, were reason for concern, as was a population with an elevated response in 2010 (
Figure 2). Because of the importance of these insecticides to the effective control of tarnished plant bugs, and the possible selection for elevated esterase, P450, and glutathione S-transferase genes that may confer resistance to multiple classes of insecticides [
24], new approaches to tarnished plant bug control that lessen the current intensive and repeated use of insecticides of all classes are needed. As with the other insecticide classes, additional research is needed to associate variability in laboratory assays to insect survival and crop damage in the field.
Those bioassays conducted with sulfoxaflor may serve as future benchmarks, but these data were collected several years after the insecticide chemistry was commercially deployed and variability was observed in our 24 h glass vial assays (
Figure 3,
Table 2). Additional research is needed to refine the assay methods and perhaps extend the observation time, but as with the other insecticides, these studies need to be related to field observations of plant bug survival. The lack of benchmark information pre-commercial release of sulfoxaflor may hinder future resistance assessments.
Estimated insecticide use in Arkansas, Louisiana, and Mississippi varied considerably across years and states depending on the chemical class. Regressions between the estimated use of the four insecticide classes were all highly significant when all three states were pooled. Thiamethoxam LC
50s were a positive predictor of imidacloprid LC
50s, while the amount of pyrethroid insecticide applied per acre of harvested cropland was a negative predictor of imidacloprid LC
50. The subset data utilizing only counties and parishes with collection records revealed linkages between LC50 values and estimated use for several classes of insecticides (
Table 8). The observations of linkages between pyrethroids and neonicotinoids in measured LC
50s and the positive influence of estimated pyrethroid use on neonicotinoid susceptibility is concerning, especially in light of the research of Zhu and Luttrell [
23], Zhu et al. [
24], and Fleming et al. [
1] that report elevated levels of broad-based metabolic enzymes capable of detoxifying multiple classes of insecticides.
Thirty years of tarnished plant bug thresholds and insecticide recommendations from Mississippi State University’s Cooperative Extension Service provide snapshots of the evolution of thresholds, changes in control strategies, and availabilities of different classes of insecticides. Thresholds have been refined [
12,
13] with adjustments for relative critical densities among different sampling procedures, and treatment levels have generally decreased (i.e., sprays recommended at lower plant bug densities) as the recency of recommendations increased (
Table 10). For the first time since pyrethroids were introduced into Mississippi cotton production in 1979 [
53], specific pyrethroid insecticides are now listed in combination with other insecticides as recommended options for the control of plant bugs and fleahoppers [
54]. Additionally, pyrethroids are also no longer recommended for the control of bollworms and budworms for the first time since 1979. Correlations utilizing data from the annual loss estimates [
35] are not unexpected, with significant positive relationships observed between the total numbers of foliar applications, the number of applications made for tarnished plant bugs, and the cost of all foliar applications.