Behavioral Responses of the Common Bed Bug, Cimex lectularius, to Insecticide Dusts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Insecticide Dusts
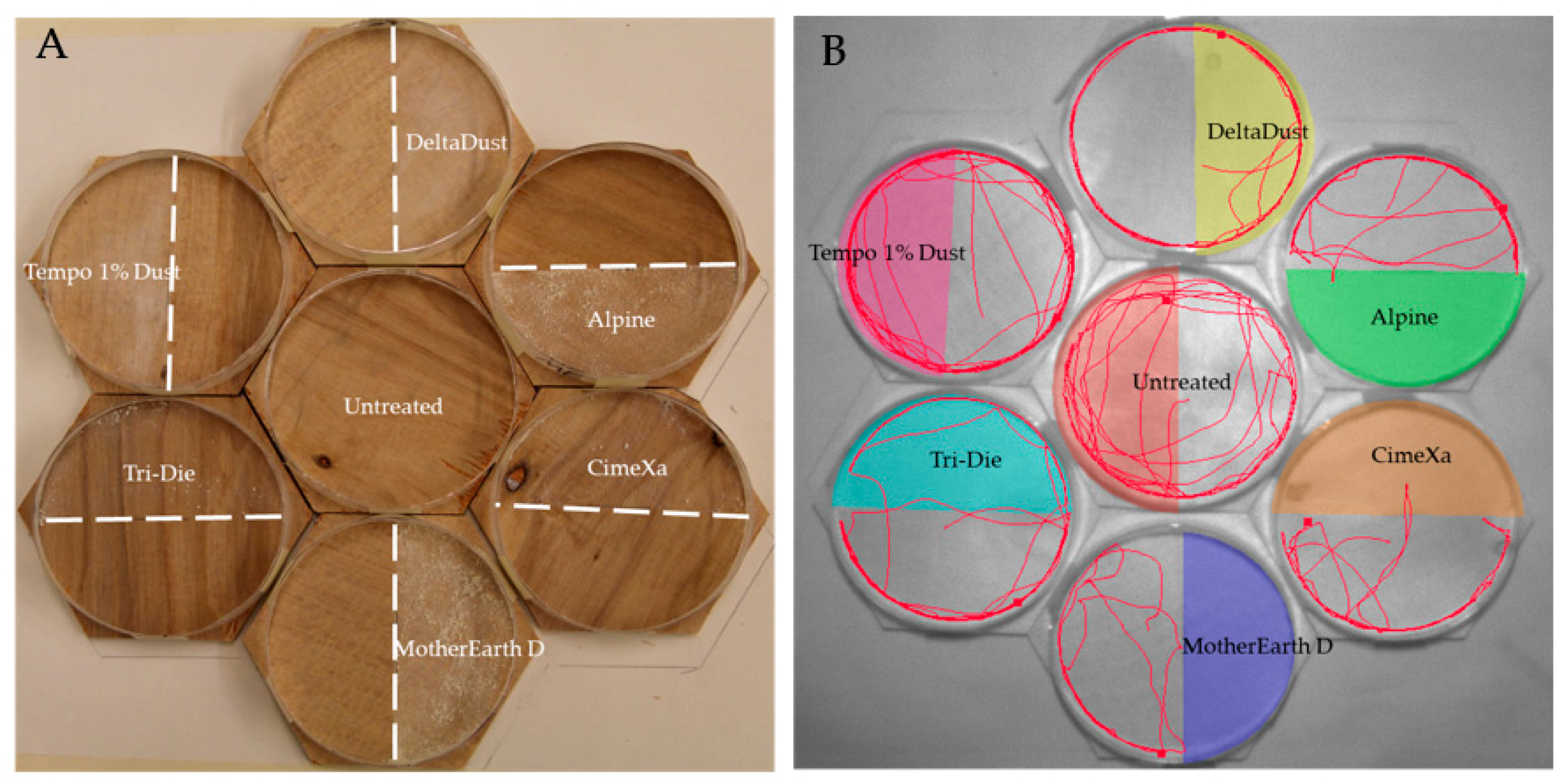
2.3. Arenas
2.4. Tracking Activity
2.5. Forced Exposure Assays
2.6. Data Analysis
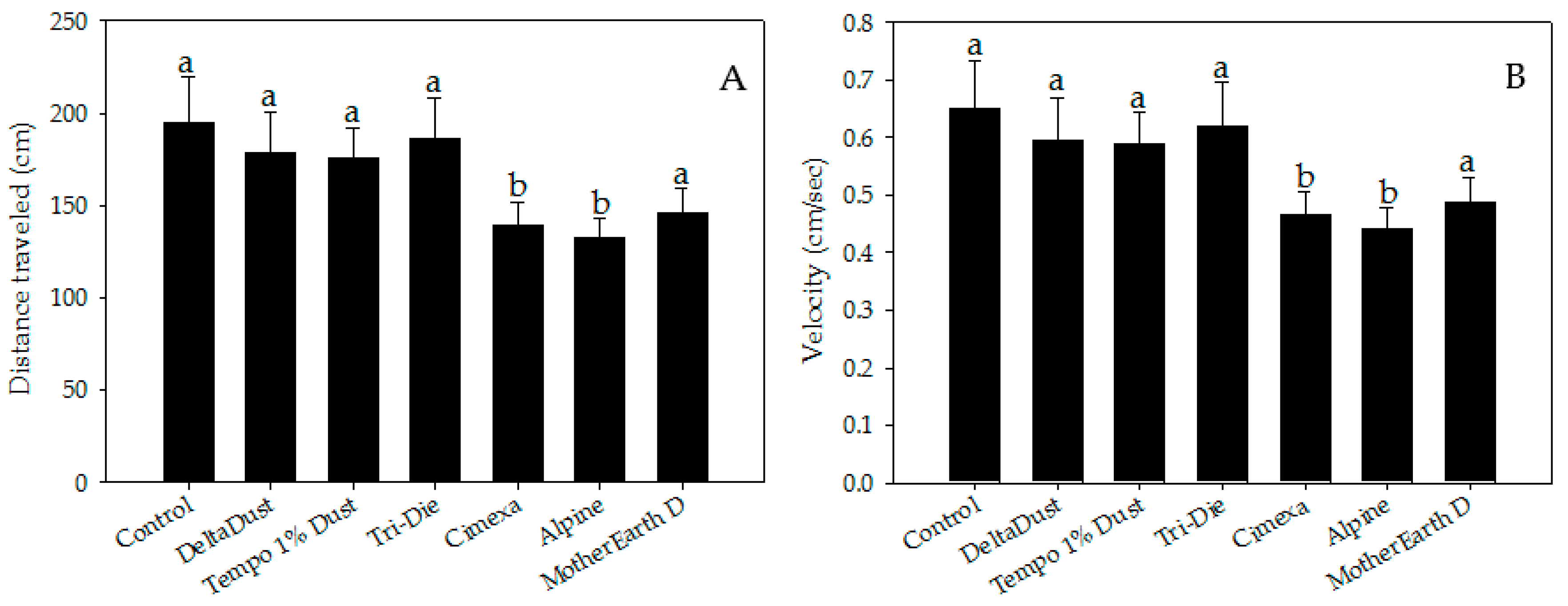
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Potter, M.F.; Haynes, K.F.; Fredericks, J. Bed bugs across America. Pestworld. November/December 2015, pp. 5–14. Available online: https://www.npmapestworld.org/default/assets/File/newsroom/magazine/2015/nov-dec_2015.pdf (accessed on 8 August 2017).
- Doggett, S.L. Bed bug survey—Are we biting back? Professional Pest Magazine. August/September 2016, pp. 28–30. Available online: http://www.ppmmagazine.com.au/magazine/enterId/forward_action/read/id/290 (accessed on 8 August 2017).
- Eddy, C.; Jones, S.C. Bed bugs, public health, and social justice: Part 2, an opinion survey. J. Environ. Health 2011, 73, 15–17. [Google Scholar] [PubMed]
- Aultman, J.M. Don’t let the bedbugs bite: The Cimicidae debacle and the denial of healthcare and social justice. Med. Health Care Philos. 2013, 16, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Penn, J.M.; Penn, H.J.; Potter, M.F.; Hu, W. Bed bugs and hotels Traveler insights and implications for the industry. Am. Entomol. 2017, 63, 79–88. [Google Scholar] [CrossRef]
- Usinger, R.L. Monograph of Cimicidae (Hemiptera-Heteroptera); Thomas Say Foundation: College Park, MD, USA, 1966. [Google Scholar]
- Potter, M.F. The perfect storm: An extension view on bed bugs. Am. Entomol. 2006, 52, 102–104. [Google Scholar] [CrossRef]
- Bennett, G.W.; Gondhalekar, A.D.; Wang, C.; Buczkowski, G.; Gibb, T.J. Using research and education to implement practical bed bug control programs in multifamily housing. Pest Manag. Sci. 2015, 72, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.; Wang, C.; Singh, N. Evaluation of a model communitywide bed bug management program in affordable housing. Pest Manag. Sci. 2015, 72, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.; Sutherland, A.M.; Gouge, D.H.; Spafford, H.; Nair, S.; Lewis, V.; Choe, D.-H.; Li, C.; Young, D. Pest management strategies for bed bugs (Hemiptera: Cimicidae) in multiunit housing: A literature review on field studies. J. Integr. Pest Manag. 2017, 8, 1–10. [Google Scholar] [CrossRef]
- Sutherland, A.; Choe, D.H.; Lewis, V.; Young, D.; Romero, A.; Spafford, H.; Gouge, D. Survey sheds light on bed bugs in multi-unit housing. Pest Control Technol. 2015, 43, 26–36. [Google Scholar]
- Pinto, L.J.; Cooper, R.; Kraft, S.K. Bed Bugs Handbook: The Complete Guide to Bed Bugs and Their Control; Pinto & Associates, Inc.: Mechanicsville, MD, USA, 2007. [Google Scholar]
- Romero, A.; Potter, M.F.; Potter, D.A.; Haynes, K.F. Insecticide resistance in the bed bug: A factor in the pest’s sudden resurgence? J. Med. Entomol. 2007, 44, 175–178. [Google Scholar] [PubMed]
- Zhu, F.; Wigginton, J.; Romero, A.; Moore, A.; Ferguson, K.; Palli, R.; Potter, M.F.; Haynes, K.F.; Palli, S.R. Widespread distribution of knockdown resistance mutations in the bed bug, Cimex lectularius (Hemiptera: Cimicidae), populations in the United States. Arch. Insect Biochem. 2010, 73, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.; Anderson, T.D. High levels of resistance in the common bed bug, Cimex lectularius (Hemiptera: Cimicidae), to neonicotinoid insecticides. J. Med. Entomol. 2016, 53, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Dang, K.; Doggett, S.L.; Singham, G.V.; Lee, C.Y. Insecticide resistance and resistance mechanisms in bed bugs, Cimex spp. (Hemiptera: Cimicidae). Paras Vectors 2017, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Ebeling, W. Sorptive dusts for pest control. Annu. Rev. Entomol. 1971, 16, 123–158. [Google Scholar] [CrossRef] [PubMed]
- Korunic, Z. Diatomaceous earths, a group of natural insecticides. J. Stored Prod. Res. 1998, 34, 87–97. [Google Scholar] [CrossRef]
- Benoit, J.B.; Phillips, S.A.; Croxall, T.J.; Christensen, B.S.; Yoder, J.A.; Denlinger, D.L. Addition of alarm pheromone components improves the effectiveness of desiccant dusts against Cimex lectularius. J. Med. Entomol. 2009, 46, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Stedfast, M.L.; Miller, D.M. Development and evaluation of a proactive bed bug (Hemiptera: Cimicidae) suppression program for low income multi-unit housing facilities. J. Integr. Pest Manag. 2014, 5, E1–E7. [Google Scholar] [CrossRef]
- Wang, C.L.; Gibb, T.; Bennett, G.W. Evaluation of two least toxic integrated pest management programs for managing bed bugs (Heteroptera: Cimicidae) with discussion of a bed bug intercepting device. J. Med. Entomol. 2009, 46, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Potter, M.F.; Romero, A.; Haynes, K.F.; Wickemeyer, W. Battling bed bugs in apartments. Pest Control Technol. 2006, 34, 45–52. [Google Scholar]
- Reinhardt, K.; Isaac, D.; Naylor, R. Estimating the feeding rate of the bedbug Cimex lectularius in an infested room: An inexpensive method and a case study. Med. Vet. Entomol. 2010, 24, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Haynes, K.F. Sublethal effects of insecticides on the behavioral responses of insects. Annu. Rev. Entomol. 1988, 33, 149–168. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.; Potter, M.F.; Haynes, K.F. Behavioral responses of the bed bug to insecticide residues. J. Med. Entomol. 2009, 46, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.C.; Bryant, J.L.; Sivakoff, F.S. Sublethal effects of ActiveGuard exposure on feeding behavior and fecundity of the bed bug (Hemiptera: Cimicidae). J. Med. Entomol. 2015, 52, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Crawley, S.E.; Kowles, K.A.; Gordon, J.R.; Potter, M.F.; Haynes, K.F. Impact of sublethal exposure to a pyrethroid-neonicotinoid insecticide on mating, fecundity and development in the bed bug Cimex lectularius L. (Hemiptera: Cimicidae). PLoS ONE 2017, 12, e0177410. [Google Scholar] [CrossRef] [PubMed]
- Crawley, S.E.; Kowles, K.A.; Gordon, J.R.; Potter, M.F.; Haynes, K.F. Behavioral effects of sublethal exposure to a combination of β-cyfluthrin and imidacloprid in the bed bug, Cimex lectularius L. Pest Manag. Sci. 2017, 73, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Doggett, S.L.; Russell, R.C. The resurgence of bed bugs, Cimex spp. (Hemiptera: Cimicidae) in Australia. In Proceedings of the Sixth International Conference on Urban Pests, Budapest, Hungary, 13–16 July 2008; Robinson, W.H., Bajomi, D., Eds.; OOK-Press: Budapest, Hungary, 2008; pp. 407–425. [Google Scholar]
- Anderson, J.F.; Cowles, R.S. Susceptibility of Cimex lectularius (Hemiptera: Cimicidae) to pyrethroid insecticides and to insecticidal dusts with or without pyrethroid insecticides. J. Econ. Entomol. 2012, 105, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Wang, C.; Wang, D.; Cooper, R.; Zha, C. Comparative efficacy of selected dust insecticides for controlling Cimex lectularius (Hemiptera: Cimicidae). J. Econ. Entomol. 2016, 109, 1819–1826. [Google Scholar] [CrossRef] [PubMed]
- Eastin, J.L.; Burden, G.S. Test with five silica dusts against German cockroaches. Fla. Entomol. 1960, 43, 99–102. [Google Scholar] [CrossRef]
- Rigaux, M.; Haubruge, E.; Fields, P.G. Mechanisms for tolerance to diatomaceous earth between strains of Tribolium castaneum. Entomol. Exp. Appl. 2001, 101, 33–39. [Google Scholar] [CrossRef]
- Luz, C.; Rodrigues, J.; Rocha, L.F. Diatomaceous earth and oil enhance effectiveness of Metarhizium anisopliae against Triatoma infestans. Acta Trop. 2012, 122, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Montes, C.; Cuadrillero, C.; Vilella, D. Maintenance of a laboratory colony of Cimex lectularius (Hemiptera: Cimicidae) using an artificial feeding technique. J. Med. Entomol. 2002, 39, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.; Potter, M.F.; Haynes, K.F. Circadian rhythm of locomotor activity in the bed bug, Cimex lectularius L. J. Insect Physiol. 2010, 56, 1516–1522. [Google Scholar] [CrossRef] [PubMed]
- Noldus, L.P.J.J.; Spink, A.J.; Tegelenbosch, R.A.J. Computerised video tracking, movement analysis and behaviour recognition in insects. Comput. Electron. Agric. 2002, 35, 201–227. [Google Scholar] [CrossRef]
- Minitab. MINITAB Statistical Software, Release 14.20 for Windows; MINITAB: State College, PA, USA, 2005. [Google Scholar]
- Wang, D.; Wang, C.; Singh, N.; Eiden, A.; Cooper, R.; Zha, C. Effect of hunger level and time elapsed from field collection on the locomotion behavior and response to stimulation in the common bed bug, Cimex lectularius. J. Econ. Entomol. 2017. [CrossRef]
- Akhtar, Y.; Isman, M.B. Efficacy of diatomaceous earth and a DE-aerosol formulation against the common bed bug, Cimex lectularius Linnaeus in the laboratory. J. Pest Sci. 2016, 89, 1013–1021. [Google Scholar] [CrossRef]
- Lilly, D.G.; Webb, C.; Doggett, S.L. Evidence of tolerance to silica-based desiccant dusts in a pyrethroid-resistant strain of Cimex lectularius (Hemiptera: Cimicidae). Insects 2016, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.; Potter, M.F.; Haynes, K.F. Are dusts the bed bug bullet. Pest Manag. Prof. 2009, 77, 22–30. [Google Scholar]
- Goddard, J. Long-term efficacy of various natural or “green” insecticides against bed bugs: A double-blind study. Insects 2014, 5, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Potter, M.F.; Haynes, K.F.; Christensen, C.; Neary, T.J.; Turner, C.R.; Washburn, L.; Washburn, M. Where do bed bugs stand when the dust settles? Pest Control Technol. 2013, 41, 72–74. [Google Scholar]
- Coon, B.F.; Wakeland, C. The repellency of pyrethrins dusts to the beet leafhopper on tomatoes. J. Econ. Entomol. 1940, 3, 389–393. [Google Scholar] [CrossRef]
- Potter, M.F.; Haynes, K.F.; Gordon, J.R.; Washburn, L.; Washburn, M.; Hardin, T. Silica gel: A better bed bug dessicant. Pest Control Technol. 2014, 42, 78–94. [Google Scholar]
- Dong, K.; Du, Y.Z.; Rinkevich, F.D.; Nomura, Y.; Xu, P.; Wang, L.; Silver, K.; Zhorov, B.S. Molecular biology of insect sodium channels and pyrethroid resistance. Insect Biochem. Mol. Biol. 2014, 50, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.L.; Singh, N.; Cooper, R.A.; Liu, C.F.; Buczkowski, G. Evaluation of an insecticide dust band treatment method for controlling bed bugs. J. Econ. Entomol. 2013, 106, 347–352. [Google Scholar] [CrossRef] [PubMed]
- El-Awami, I.O.; Dent, D.R. The interaction of surface and dust particle size on the pick-up and grooming behaviour of the German cockroach Blattella germanica. Entomol. Exp. Appl. 1995, 77, 81–87. [Google Scholar] [CrossRef]
- Gahlhoff, J.E.; Koehler, P.G. Penetration of the eastern subterranean termite into soil treated at various thicknesses and concentrations of Dursban TC and Premise 75. J. Econ. Entomol. 2001, 94, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Wang, C.; Cooper, R. Effectiveness of a reduced-risk insecticide based bed bug management program in low-income housing. Insects 2013, 4, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Kilpinen, O.; Steenberg, T. Repellent activity of desiccant dusts and conidia of the entomopathogenic fungus Beauveria bassiana when tested against poultry red mites (Dermanyssus gallinae) in laboratory experiments. Exp. Appl. Acarol. 2016, 70, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Faulde, M.K.; Scharninghausen, J.J.; Cavaljuga, S. Toxic and behavioural effects of different modified diatomaceous earths on the German cockroach (L.) (Orthoptera: Blattellidae) under simulated field conditions. J. Stored Prod. Res. 2016, 42, 253–263. [Google Scholar] [CrossRef]
- Zhu, J. Push and pull strategy in control of filth flies in urban settings. In Proceedings of the 2012 National Conference on Urban Entomology, Atlanta, GA, USA, 20–23 July 2012; Suiter, D., Ed.; USDA ARS: Washington, DC, USA, 2012; p. 168. [Google Scholar]
- Nalyanya, G.; Moore, C.B.; Schal, C. Integration of repellents, attractants, and insecticides in a “Push-Pull” strategy for managing German cockroach (Dictyoptera: Blattellidae) populations. J. Med. Entomol. 2000, 37, 427–434. [Google Scholar] [PubMed]
- Wang, C.; Lü, L.; Zhang, A.; Liu, C. Repellency of selected chemicals against bed bugs. J. Econ. Entomol. 2013, 106, 2522–2529. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.L.; Saltzmann, K.; Chin, E.; Bennett, G.W.; Gibb, T. Characteristics of Cimex lectularius (Hemiptera: Cimicidae), infestation and dispersal in a high-rise apartment building. J. Econ. Entomol. 2010, 103, 172–177. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agnew, J.L.; Romero, A. Behavioral Responses of the Common Bed Bug, Cimex lectularius, to Insecticide Dusts. Insects 2017, 8, 83. https://doi.org/10.3390/insects8030083
Agnew JL, Romero A. Behavioral Responses of the Common Bed Bug, Cimex lectularius, to Insecticide Dusts. Insects. 2017; 8(3):83. https://doi.org/10.3390/insects8030083
Chicago/Turabian StyleAgnew, John L., and Alvaro Romero. 2017. "Behavioral Responses of the Common Bed Bug, Cimex lectularius, to Insecticide Dusts" Insects 8, no. 3: 83. https://doi.org/10.3390/insects8030083





