Identification of Conditions for Successful Aphid Control by Ladybirds in Greenhouses
Abstract
:1. Introduction
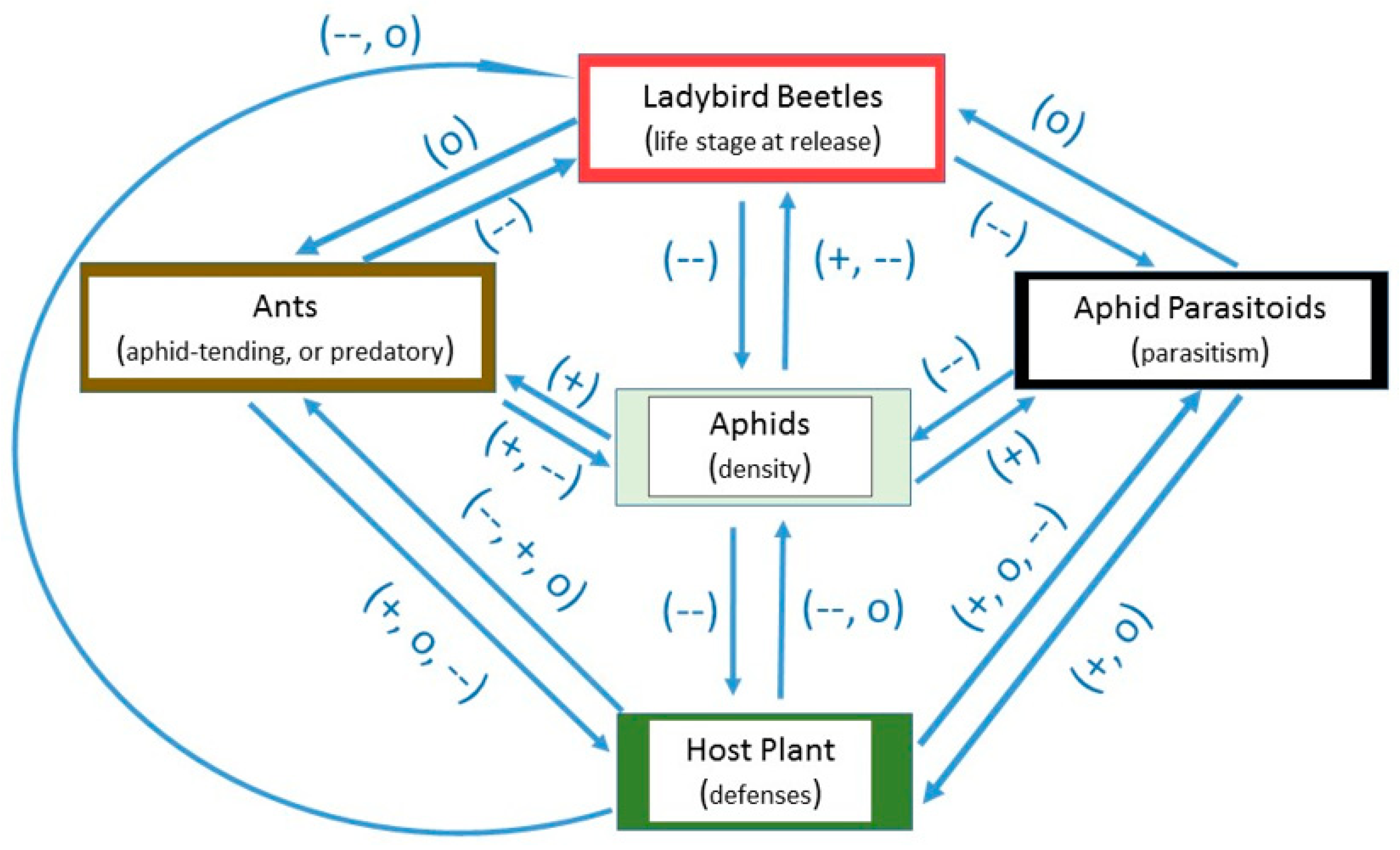
2. Factors Potentially Affecting Ladybird Success in Greenhouses
2.1. Host Plant Defenses
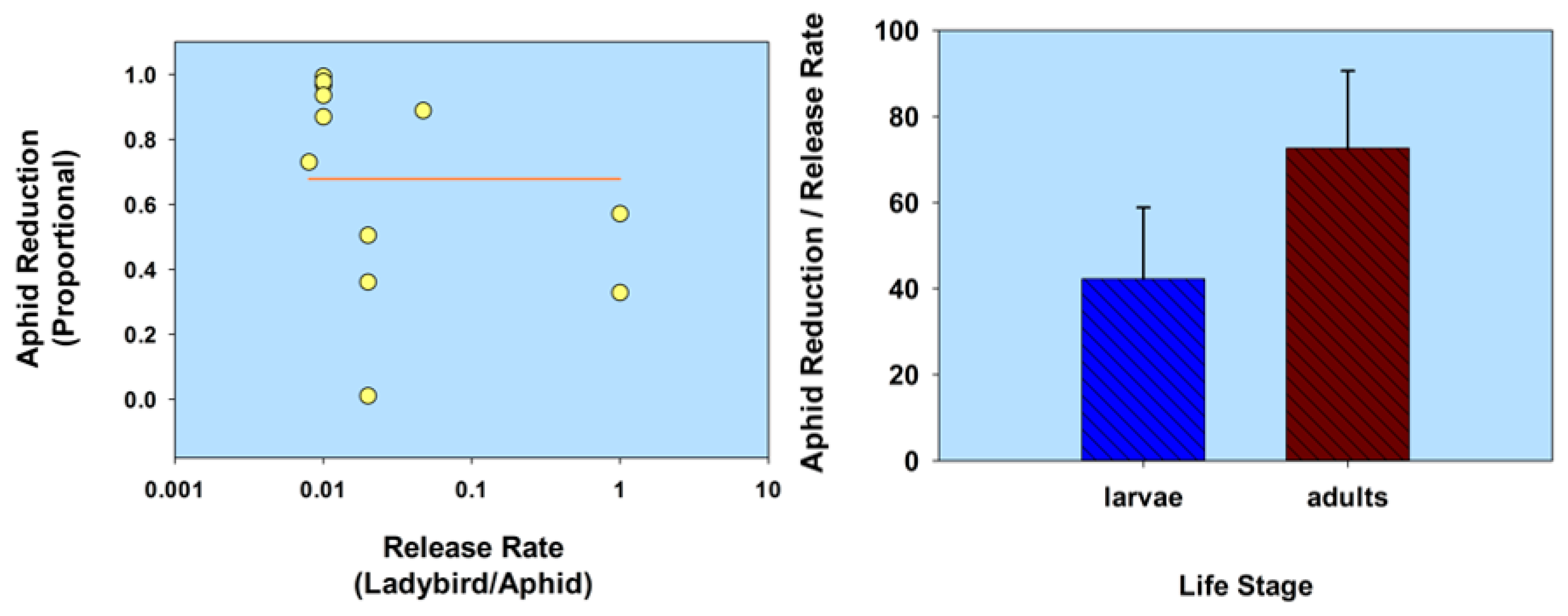
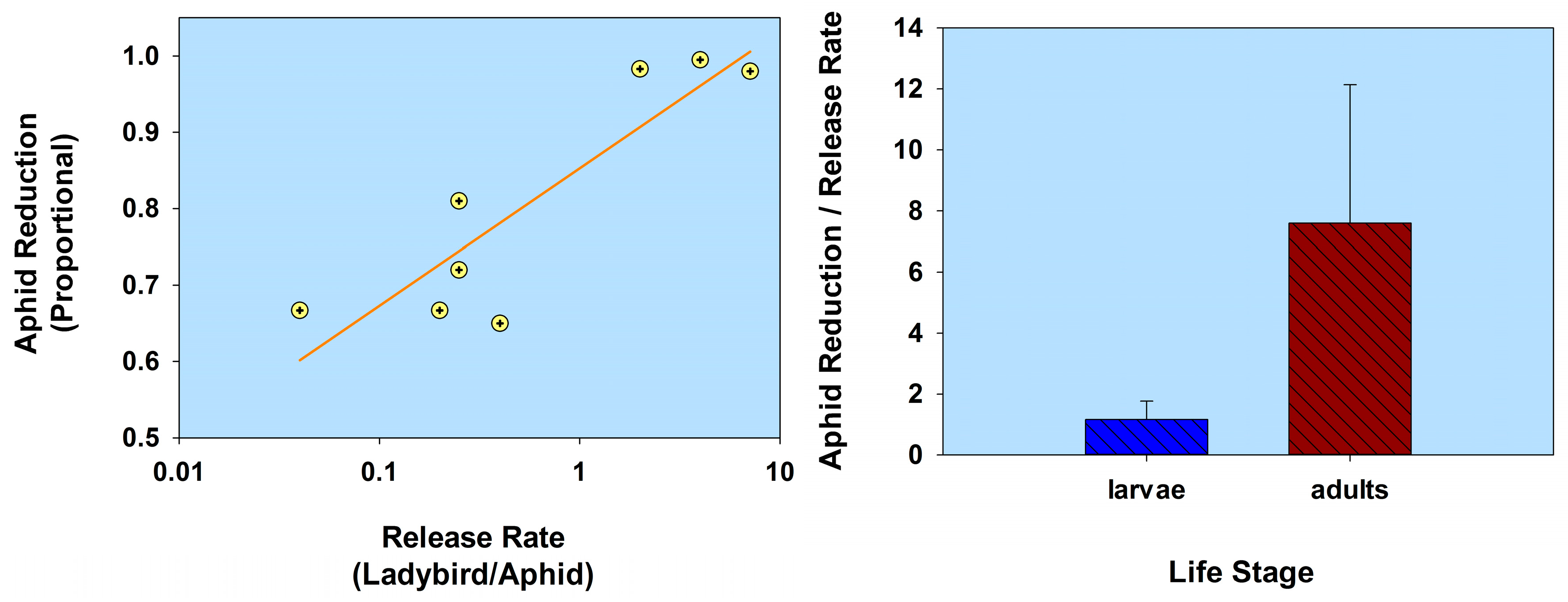
2.2. Life Stage
2.2.1. Normal Strain
2.2.2. Flightless Strain
2.3. Other Aphid Predators
2.4. Aphid Parasitoids
2.5. Foraging and Aphid-Tending Ants
3. Concluding Remarks
Acknowledgments
Conflicts of Interest
References
- Whitwer, S.H.; Castilla, N. Protected cultivation of horticultural crops worldwide. HortTechnology 1995, 5, 6–23. [Google Scholar]
- Gullino, M.L.; Albajes, R.; van Lenteren, J.C. Setting the stage: Characteristics of protected cultivation and tools for sustainable crop protection, Chapter 1. In Integrated Pest and Disease Management in Greenhouse Crops; Albajes, R., Gullino, M.L., van Lenteren, J.C., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1999; pp. 1–15. [Google Scholar]
- Pilkington, L.J.; Messelink, G.; van Lenteren, J.C.; Le Mottee, K. “Protected Biological Control”—Biological pest management in the greenhouse industry. Biol. Control 2010, 52, 216–220. [Google Scholar] [CrossRef]
- Messelink, G.J.; Bennison, J.; Alomar, O.; Ingegno, B.L.; Tavella, L.; Shipp, L.; Palevsky, E.; Wäckers, F.L. Approaches to conserving natural enemy populations in greenhouse crops: Current methods and future prospects. BioControl 2014, 59, 377–393. [Google Scholar] [CrossRef]
- Blackman, R.L.; Eastop, V.F. Taxonomic issues, Chapter 1. In Aphids as Crop Pests; van Emden, H.F., Harrington, R., Eds.; CAB International: Oxfordshire, UK, 2007; pp. 1–29. [Google Scholar]
- Blümel, S. Biological control of aphids on vegetable crops. In Biological Control in Protected Culture; Heinz, K.M., Van Driesche, R.G., Parrella, M.P., Eds.; Ball Publ.: Batavia, IL, USA, 2004; pp. 297–312. [Google Scholar]
- Chau, A.; Heinz, K.M. Biological control of aphids on ornamental crops. In Biological Control in Protected Culture; Heinz, K.M., Van Driesche, R.G., Parrella, M.P., Eds.; Ball Publ.: Batavia, IL, USA, 2004; pp. 277–295. [Google Scholar]
- Freeman, S.; Nicoli, G. Strawberries, Chapter 32. In Integrated Pest and Disease Management in Greenhouse Crops; Albajes, R., Gullino, M.L., van Lenteren, J.C., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1999; pp. 454–472. [Google Scholar]
- Sewell, G.H.; Storch, R.H.; Manzer, F.E.; Forsythe, H.Y., Jr. The relationship between coccinellids and aphids in the spread of potato leafroll virus in a greenhouse. Am. Potato J. 1990, 67, 865–868. [Google Scholar] [CrossRef]
- Quisenberry, S.S.; Ni, X. Feeding injury, Chapter 13. In Aphids as Crop Pests; van Emden, H.F., Harrington, R., Eds.; CAB International: Oxfordshire, UK, 2007; pp. 331–352. [Google Scholar]
- Katis, N.I.; Tsitsipis, J.A.; Stevens, M.; Powell, G. Transmission of plant viruses, Chapter 14. In Aphids as Crop Pests; van Emden, H.F., Harrington, R., Eds.; CAB International: Oxfordshire, UK, 2007; pp. 353–390. [Google Scholar]
- Scopes, N.E.A. The evaluation and use of predators for protected cropping. Ann. Appl. Biol. 1975, 80, 123–124. [Google Scholar] [CrossRef]
- Yang, N.-W.; Zang, L.-S.; Wang, S.; Guo, J.-Y.; Xu, H.-X.; Zhang, F.; Wan, F.-H. Biological pest management by predators and parasitoids in the greenhouse vegetables in China. Biol. Control 2014, 68, 92–102. [Google Scholar] [CrossRef]
- Boivin, G.; Hance, T.; Brodeur, J. Aphid parasitoids in biological control. Can. J. Plant Sci. 2012, 92, 1–12. [Google Scholar] [CrossRef]
- Rabasse, J.-M.; van Steenis, M.J. Biological control of aphids, Chapter 16. In Integrated Pest and Disease Management in Greenhouse Crops; Albajes, R., Gullino, M.L., van Lenteren, J.C., Elad, Y., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1999; pp. 235–253. [Google Scholar]
- Prado, S.G.; Jandricic, S.E.; Frank, S.D. Ecological interactions affecting the efficacy of Aphidius colemani in greenhouse crops. Insects 2015, 6, 538–575. [Google Scholar] [CrossRef] [PubMed]
- Gamarra, D.C.; Bueno, H.P.; Auad, A.M. Efecto de los tricomas glandulares de Solanum berthaultii en el parasitismo de Aphidius colemani (Hymenoptera: Aphidiidae) sobre Myzus persicae (Homoptera: Aphididae). Vedalia 1997, 4, 21–24. (In Spanish) [Google Scholar]
- Colfer, R.G.; Rosenheim, J.A. Predation on immature parasitoids and its impact on aphid suppression. Oecologia 2001, 126, 292–304. [Google Scholar] [CrossRef]
- Hagen, K.S. Biology and ecology of predaceous Coccinellidae. Annu. Rev. Entomol. 1962, 7, 289–326. [Google Scholar] [CrossRef]
- Hagen, K.S.; van den Bosch, R. Impact of pathogens, parasites, and predators on aphids. Annu. Rev. Entomol. 1968, 13, 325–384. [Google Scholar] [CrossRef]
- Hodek, I. Food relationships, Chapter 6. In Ecology of Coccinellidae; Hodek, I., Honĕk, A., Eds.; Kluwer Academic Publishers: Boston, MA, USA, 1996; pp. 143–248. [Google Scholar]
- Obrycki, J.J.; Kring, T.J. Predaceous Coccinellidae in biological control. Annu. Rev. Entomol. 1998, 43, 295–321. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.W. Role of aphid predator guild in controlling spirea aphid populations on apple in West Virginia, USA. Biol. Control 2004, 29, 189–198. [Google Scholar] [CrossRef]
- Mignault, M.-P.; Roy, M.; Brodeur, J. Soybean aphid predators in Québec and the suitability of Aphis glycines as prey for three Coccinellidae. BioControl 2006, 51, 89–106. [Google Scholar] [CrossRef]
- Obrycki, J.J.; Harwood, J.D.; Kring, T.J.; O’Neil, R.J. Aphidophagy by Coccinellidae: Application of biological control in agroecosystems. Biol. Control 2009, 51, 244–254. [Google Scholar] [CrossRef]
- Ragsdale, D.W.; Landis, D.A.; Brodeur, J.; Heimpel, G.E.; Desneux, N. Ecology and management of the soybean aphid in North America. Annu. Rev. Entomol. 2011, 56, 375–399. [Google Scholar] [CrossRef] [PubMed]
- Dutcher, J.D.; Karar, H.; Abbas, G. Seasonal abundance of aphids and aphidophagous insects in pecan. Insects 2012, 3, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- Long, E.Y.; Finke, D.L. Contribution of predator identity to the suppression of herbivores by a diverse predator assemblage. Environ. Entomol. 2014, 43, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Mills, N.J. Satiation and the functional response: A test of a new model. Ecol. Entomol. 1982, 7, 305–315. [Google Scholar] [CrossRef]
- Dixon, A.F.G.; Hemptinne, J.-L.; Kindlmann, P. Effectiveness of ladybirds as biological control agents: Patterns and processes. Entomophaga 1997, 42, 71–83. [Google Scholar] [CrossRef]
- Dixon, A.F.G. Insect Predator-Prey Dynamics: Ladybird Beetles & Biological Control; Cambridge Univ. Press: Cambridge, UK, 2000. [Google Scholar]
- Magro, A.; Hemptinne, J.L.; Codreanu, P.; Grosjean, S.; Dixon, A.F.G. Does the satiation hypothesis account for the differences in efficacy of coccidophagous and aphidophagous ladybird beetles in biological control? A test with Adalia bipunctata and Cryptolaemus montrouzieri. BioControl 2002, 47, 537–543. [Google Scholar] [CrossRef]
- Kindlmann, P.; Yasuda, H.; Kajita, Y.; Sato, S.; Dixon, A.F.G. Predator efficiency reconsidered for a ladybird-aphid system. Front. Ecol. Evol. 2015. [Google Scholar] [CrossRef]
- Wyss, E.; Villiger, M.; Hemptinne, J.L.; Muller-Scharer, H. Effects of augmentative releases of eggs and larvae of the ladybird beetle, Adalia bipunctata, on the abundance of the rosy apple aphid, Dysaphis plantaginea, in organic apple orchards. Entomol. Exp. Appl. 1999, 90, 167–173. [Google Scholar] [CrossRef]
- Kehrli, P.; Wyss, E. Effects of augmentative releases of the coccinellid, Adalia bipunctata, and of insecticide treatments in autumn on the spring population of aphids of the genus Dysaphis in apple orchards. Entomol. Exp. Appl. 2001, 99, 245–252. [Google Scholar] [CrossRef]
- Kirby, W.; Spence, W. An Introduction to Entomology: Or Elements of the Natural History of Insects, 3rd ed.; Longman, Hurst, Rees, Orme, and Brown: London, UK, 1818; Volume 1. [Google Scholar]
- Van Driesche, R.G.; Heinz, K.M. An overview of biological control in protected culture. In Biological Control in Protected Culture; Heinz, K.M., Van Driesche, R.G., Parrella, M.P., Eds.; Ball Publ.: Batavia, IL, USA, 2004; pp. 1–24. [Google Scholar]
- Frazer, B.D. Coccinellidae. In Aphids: Their Biology, Natural Enemies, and Control, Vol. B; Minks, A.K., Harrewijn, P., Eds.; Elsevier: New York, NY, USA, 1988; pp. 231–247. [Google Scholar]
- Yano, E. Ecological considerations for biological control of aphids in protected culture. Popul. Ecol. 2006, 48, 333–339. [Google Scholar] [CrossRef]
- Powell, W.; Pell, J.K. Biological control, Chapter 18. In Aphids as Crop Pests; van Emden, H.F., Harrington, R., Eds.; CAB International: Oxfordshire, UK, 2007; pp. 469–513. [Google Scholar]
- Riddick, E.W.; Simmons, A.M. Do plant trichomes cause more harm than good to predatory insects? Pest Manag. Sci. 2014, 70, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Way, M.J. Mutualism between ants and honeydew-producing Homoptera. Annu. Rev. Entomol. 1963, 8, 307–344. [Google Scholar] [CrossRef]
- Majerus, M.E.N.; Sloggett, J.J.; Godeau, J.-F.; Hemptinne, J.L. Interactions between ants and aphidophagous and coccidophagous ladybirds. Popul. Ecol. 2007, 49, 15–27. [Google Scholar] [CrossRef]
- Kaplan, I.; Eubanks, M.D. Aphids alter the community-wide impact of fire ants. Ecology 2005, 86, 1640–1649. [Google Scholar] [CrossRef]
- Shannag, H.K.; Obeidat, W.M. Interaction between plant resistance and predation of Aphis fabae (Homoptera: Aphididae) by Coccinella septempunctata (Coleoptera: Coccinellidae). Ann. Appl. Biol. 2008, 152, 331–337. [Google Scholar] [CrossRef]
- Chang, G.C.; Eigenbrode, S.D. Delineating the effects of a plant trait on interactions among associated insects. Oecologia 2004, 139, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Obrycki, J.J.; Tauber, M.J. Natural enemy activity on glandular pubescent potato plants in the greenhouse: An unreliable predictor of effects in the field. Environ. Entomol. 1984, 13, 679–683. [Google Scholar] [CrossRef]
- Gurney, B.; Hussey, N.W. Evaluation of some coccinellid species for the biological control of aphids in protected cropping. Ann. Appl. Biol. 1970, 65, 451–458. [Google Scholar] [CrossRef]
- Boiça, J.A.; Santos, T.M.; Kuranishi, A.K. Desenvolvimento larval e capacidade predatória de Cycloneda sanguinea (L.) e Hippodamia convergens Guérin-Men. alimentadas com Aphis gossypii Glover sobre cultivars de algodoeiro. Acta Sci. Agron. 2004, 26, 239–244. (In Portuguese) [Google Scholar]
- Hämäläinen, M. Control of aphids on sweet peppers, chrysanthemums and roses in small greenhouses using the ladybeetles Coccinella septempunctata and Adalia bipunctata (Col., Coccinellidae). Ann. Agric. Fenn. 1977, 16, 117–131. [Google Scholar]
- Kuznetsov, V.N.; Hong, P. Employment of Chinese coccinellids in biological control of aphids in greenhouse in Primorye. Far Eastern Entomol. 2002, 119, 1–5. [Google Scholar]
- Seo, M.J.; Youn, Y.N. Effective preservation methods of the Asian ladybird, Harmonia axyridis (Coleoptera: Coccinellidae), as an application strategy for the biological control of aphids. J. Asia-Pac. Entomol. 2002, 5, 209–214. [Google Scholar] [CrossRef]
- Rondon, S.I.; Cantliffe, D.J.; Price, J.F. Population dynamics of the cotton aphid, Aphis gossypii (Homoptera: Aphididae), on strawberries grown under protected culture. Fla. Entomol. 2005, 88, 152–158. [Google Scholar] [CrossRef]
- LaRock, D.R.; Mirdad, Z.; Ellington, J.J.; Carillo, T.; Southward, M. Control of green peach aphids Myzus persicae with lady beetles Harmonia axyridis on Chile Capsicum annum in the greenhouse. Southwest. Entomol. 2003, 28, 249–253. [Google Scholar]
- Kuroda, T.; Miura, K. Comparison of the effectiveness of two methods for releasing Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae) against Aphis gossypii Glover (Homoptera: Aphididae) on cucumbers in a greenhouse. Appl. Entomol. Zool. 2003, 38, 271–274. [Google Scholar] [CrossRef]
- Adachi-Hagimori, T.; Shibao, M.; Tanaka, H.; Seko, T.; Miura, K. Control of Myzus persicae and Lipaphis erysimi (Hemiptera: Aphididae) by adults and larvae of a flightless strain of Harmonia axyridis (Coleoptera: Coccinellidae) on non-heading Brassica cultivars in the greenhouse. BioControl 2011, 56, 207–213. [Google Scholar] [CrossRef]
- Seko, T.; Sumi, A.; Nakano, A.; Kameshiro, M.; Kaneda, T.; Miura, K. Suppression of aphids by augmentative release of larvae of flightless Harmonia axyridis. J. Appl. Entomol. 2014, 138, 326–337. [Google Scholar] [CrossRef]
- Tamaki, G.; Weeks, R.E. Efficiency of three predators, Geocoris bullatus, Nabis americoferus, and Coccinella transversoguttata, used alone or in combination against three insect prey species, Myzus persicae, Ceramica picta, and Mamestra configurata, in a greenhouse study. Environ. Entomol. 1972, 1, 258–263. [Google Scholar] [CrossRef]
- Valério, E.; Cecílio, A.; Mexia, A. Population dynamics of aphids (Homoptera: Aphididae) and beneficial organisms on protected strawberry crops. Boletín Sanidad Vegetal Plagas 2007, 33, 153–161. [Google Scholar]
- Valério, E.; Cecílio, A.; Mexia, A. Interactions between aphid species and beneficial organisms in sweet pepper protected crop. Bol. Sanid. Veg. Plagas 2007b, 33, 143–152. [Google Scholar]
- Lucas, É. Intraguild interactions, Chapter 7. In Ecology and Behaviour of the Ladybird Beetles (Coccinellidae), 1st ed.; Hodek, I., van Emden, H.F., Honĕk, A., Eds.; Blackwell Publishing Ltd.: West Sussex, UK, 2012; pp. 343–374. [Google Scholar]
- Yang, F.; Wang, Q.; Wang, D.; Xu, B.; Xu, J.; Lu, Y.; Harwood, J.D. Intraguild predation among three common coccinellids (Coleoptera: Coccinellidae) in China: Detection using DNA-based gut-content analysis. Environ. Entomol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Ingels, B.; De Clercq, P. Effect of size, extraguild prey and habitat complexity on intraguild interactions: A case study with the invasive ladybird Harmonia axyridis and the hoverfly Episyrphus balteatus. BioControl 2011, 56, 871–882. [Google Scholar] [CrossRef]
- Nedved, O.; Fois, X.; Ungerova, D.; Kalushkov, P. Alien vs. predator—The native lacewing Chrysoperla carnea is the superior intraguild predator in trials against the invasive ladybird Harmonia axyridis. Bull. Insectol. 2013, 66, 73–78. [Google Scholar]
- Noppe, C.; Michaud, J.P.; De Clercq, P. Intraguild predation between lady beetles and lacewings: Outcomes and consequences vary with focal prey and arena of interaction. Ann. Entomol. Soc. Am. 2012, 105, 562–571. [Google Scholar] [CrossRef]
- Janssen, A.; Montserrat, M.; HilleRisLambers, R.; de Roos, A.M.; Pallini, A.; Sabelis, M.W. Intraguild predation usually does not disrupt biological control. In Trophic and Guild Interactions in Biological Control; Brodeur, J., Boivin, G., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 21–44. [Google Scholar]
- Xue, Y.; Bahlai, C.A.; Frewin, A.; McCreary, C.M.; Des Marteaux, L.E.; Schaafsma, A.W.; Hallett, R.H. Intraguild predation of the aphid parasitoid Aphelinus certus by Coccinella septempunctata and Harmonia axyridis. BioControl 2012, 57, 627–634. [Google Scholar] [CrossRef]
- Fu, W.; Yu, X.; Ahmed, N.; Zhang, S.; Liu, T. Intraguild predation on the aphid parasitoid Aphelinus asychis by the ladybird Harmonia axyridis. BioControl 2017, 62, 61–70. [Google Scholar] [CrossRef]
- Sterk, G.; Meesters, P. IPM on strawberries in glasshouses and plastic tunnels in Belgium, new possibilities. In Proceedings of the Third International Strawberry Symposium, Veldhoven, The Netherlands, 29 April–4 May 1997; pp. 905–911. [Google Scholar]
- Snyder, W.E.; Ballard, S.N.; Yang, S.; Clevenger, G.M.; Miller, T.D.; Ahn, J.J.; Hatten, T.D.; Berryman, A.A. Complementary biocontrol of aphids by the ladybird beetle Harmonia axyridis and the parasitoid Aphelinus asychis on greenhouse roses. Biol. Control 2004, 30, 229–235. [Google Scholar] [CrossRef]
- Meisner, M.; Harmon, J.P.; Harvey, C.T.; Ives, A.R. Intraguild predation on the parasitoid Aphidius ervi by the generalist predator Harmonia axyridis: The threat and its avoidance. Entomol. Exp. Appl. 2011, 138, 193–201. [Google Scholar] [CrossRef]
- Nakashima, Y.; Birkett, M.A.; Pye, B.J.; Powell, W. Chemically mediated intraguild predator avoidance by aphid parasitoids: Interspecific variability in sensitivity to semiochemical trails of ladybird predators. J. Chem. Ecol. 2006, 32, 1989–1998. [Google Scholar] [CrossRef] [PubMed]
- Oliver, T.H.; Jones, I.; Cook, J.M.; Leather, S.R. Avoidance responses of an aphidophagous ladybird, Adalia bipunctata, to aphid-tending ants. Ecol. Entomol. 2008, 33, 523–528. [Google Scholar] [CrossRef]
- Eubanks, M.D.; Blackwell, S.A.; Parrish, C.J.; Delamar, Z.D.; Hull-Sanders, H. Intraguild predation of beneficial arthropods by red imported fire ants in cotton. Environ. Entomol. 2002, 31, 1168–1174. [Google Scholar] [CrossRef]
- El-Ziady, S.; Kennedy, J.S. Beneficial effects of the common garden ant, Lasius niger L., on the black bean aphid, Aphis fabae Scopoli. Proc. R. Entomol. Soc. Lond. A 1956, 31, 61–65. [Google Scholar] [CrossRef]
- Powell, B.E.; Silverman, J. Impact of Linepithema humile and Tapinoma sessile (Hymenoptera: Formicidae) on three natural enemies of Aphis gossypii (Hemiptera: Aphididae). Biol. Control 2010, 54, 285–291. [Google Scholar] [CrossRef]
- Kaplan, I.; Eubanks, M.D. Disruption of cotton aphid (Homoptera: Aphididae)—Natural enemy dynamics by red imported fire ants (Hymenoptera: Formicidae). Environ. Entomol. 2002, 31, 1175–1183. [Google Scholar] [CrossRef]
- Piñol, J.; Espadaler, X.; Cañellas, N.; Pérez, N. Effects of the concurrent exclusion of ants and earwigs on aphid abundance in an organic citrus grove. BioControl 2009, 54, 515–527. [Google Scholar] [CrossRef]
- Romeu-Dalmau, C.; Espadaler, X.; Piñol, J. A simple method to differentially exclude ants from tree canopies based on ant body size. Methods Ecol. Evol. 2010, 1, 188–191. [Google Scholar] [CrossRef]
- Völkl, W.; Vohland, K. Wax covers in larvae of two Scymnus species: Do they enhance coccinellid larval survival? Oecologia 1996, 107, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, S. Aphid-attending ants increase the number of emerging adults of the aphid’s primary parasitoid and hyperparasitoids by repelling intraguild predators. Entomol. Sci. 2002, 5, 131–146. [Google Scholar]
- Schwartzberg, E.G.; Haynes, K.F.; Johnson, D.W.; Brown, G.C. Wax structures of Scymnus louisianae attenuate aggression from aphid-tending ants. Environ. Entomol. 2010, 39, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Pope, R.D. Wax production by coccinellid larvae (Coleoptera). Syst. Entomol. 1979, 4, 171–196. [Google Scholar] [CrossRef]
- Lohman, D.J.; Liao, Q.; Pierce, N.E. Convergence of chemical mimicry in a guild of aphid predators. Ecol. Entomol. 2006, 31, 41–51. [Google Scholar] [CrossRef]
- Peterson, J.A.; Ode, P.J.; Oliveira-Hofman, C.; Harwood, J.D. Integration of plant defense traits with biological control of arthropod pests: Challenges and opportunities. Front. Plant Sci. 2016. [Google Scholar] [CrossRef] [PubMed]
- Riddick, E.W.; Chen, H. Production of coleopteran predators, Chapter 2. In Mass Production of Beneficial Organisms: Invertebrates and Entomopathogens; Morales-Ramos, J.A., Rojas, M.G., Shapiro-Ilan, D.I., Eds.; Academic Press: London, UK, 2014; pp. 17–55. [Google Scholar]
- Riddick, E.W. Spotlight on the positive effects of the ladybird Harmonia axyridis on agriculture. BioControl 2016. [Google Scholar] [CrossRef]
- Seko, T.; Abe, J.; Miura, K.; Hikawa, M. The contribution of a beneficial insectary plant Scaevola aemula to survival and long-term establishment of flightless Harmonia axyridis in greenhouses. BioControl 2017, 62, 221–231. [Google Scholar] [CrossRef]
- Turgeon, J.; Tayeh, A.; Facon, B.; Lombaert, E.; De Clercq, P.; Berkvens, N.; Lundgren, J.G.; Estoup, A. Experimental evidence for the phenotypic impact of admixture between wild and biocontrol Asian ladybird (Harmonia axyridis) involved in the European invasion. J. Evol. Biol. 2011, 24, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Carnes, E.K. Collecting ladybirds (Coccinellidae) by the ton. Calif. State Comm. Hort. Mon. Bull. 1912, 1, 71–81. [Google Scholar]
- Flint, M.L.; Dreistadt, S.H. Interactions among convergent lady beetle (Hippodamia convergens) releases, aphid populations, and rose cultivar. Biol. Control 2005, 34, 38–46. [Google Scholar] [CrossRef]

| Factors | Ladybird | Aphid | Plant | Release Ratio (L:A) | Release Rate (L/A) | Aphid Reduction (%) 1 | Time Frame (Days) | Reference |
|---|---|---|---|---|---|---|---|---|
| Host plant defenses | Coccinella septempunctata (1st instars) | Aphis fabae | Vicia faba, c.v. major V. faba, c.v. 79S4 [in cages] | 1:1 | 1.0 | 32.8 | 9 | Shannag and Obeidat 2008 [45] |
| 1:1 | 1.0 | 57.1 | 9 | |||||
| Cycloneda sanguinea (2nd instars) Coleomegilla maculata (2nd instars) Adalia bipunctata (2nd instars) | Aphis gossypii | Cucumis sativus | 1:50 | 0.02 | 50.45 † | 11 | Gurney and Hussey 1970 [48] | |
| 1:50 | 0.02 | 0 † | 11 | |||||
| 1:50 | 0.02 | 36.02 † | 11 | |||||
| C. sanguinea (2nd instars) C. maculata (2nd instars) A. bipunctata (2nd instars) | Myzus persicae | Chrysanthemum indicum, c.v. BGA Tuneful | 1:100 | 0.01 | 99.36 † | 07 | Gurney and Hussey 1970 [48] | |
| 1:100 | 0.01 | 96.64 † | 07 | |||||
| 1:100 | 0.01 | 97.77 † | 07 | |||||
| A. bipunctata (adults) | M. persicae | C. indicum [in cages] | 1:117 | 0.008 | 73.02 † | 14 | Gurney and Hussey 1970 [48] | |
| 1:21 | 0.047 | 88.85 † | 14 | |||||
| C. sanguinea (adults) Hippodamia convergens (adults) | A. gossypii | Gossypium hirsutum | 1:100 | 0.01 | 93.5 † | 2 | Boiça et al. 2004 [49] | |
| 1:100 | 0.01 | 86.9 † | 2 | |||||
| Life stage (normal strain) | C. septempunctata (1st instars) A. bipunctata (1st instars) | M. persicae | Capsicum annuum | 1:10 | 0.10 | 93.7 † | 10 | Hämäläinen 1977 [50] |
| 1:20 | 0.05 | 35.1 † | 10 | |||||
| 1:10 | 0.10 | 97.5 † | 10 | |||||
| 1:20 | 0.05 | 85.7 † | 10 | |||||
| C. septempunctata (1st instars) A. bipunctata (1st instars) | M. persicae | Chrysanthemum morifolium | 1:43 | 0.023 | 90.7 | 08 | Hämäläinen 1977 [50] | |
| 1:16 | 0.062 | 91.5 | 08 | |||||
| 1:75 | 0.013 | 77.3 | 08 | |||||
| C. septempunctata (adults) A. bipunctata (adults) | M. persicae | C. morifolium | 1:12 | 0.083 | 86.95 | 08 | Hämäläinen 1977 [50] | |
| 1:50 | 0.02 | 94.0 | 08 | |||||
| 1:39 | 0.025 | 46.15 | 08 | |||||
| Leis (Harmonia) dimidiata (2nd instars) | A. gossypii | C. sativus | 1:10 | 0.10 | 85–90 | -- | Kuznetsov & Hong 2002 [51] | |
| 1:20 | 0.05 | 85–90 | -- | |||||
| Harmonia axyridis (3rd, 4th instars) | Chaetosiphon minor, Aphis forbesi | Fragaria × ananassa | 1:1.73 | 0.58 | 84.6 | 29 | Seo & Youn 2002 [52] | |
| C. maculata (3rd instars) | A. gossypii | F. × ananassa [in cages] | 1:15 | 0.07 | 46.3 | 14 | Rondon et al. 2005 [53] | |
| 1:5 | 0.20 | 87.3 | 14 | |||||
| 1:3 | 0.33 | 96.4 | 14 | |||||
| H. axyridis (adults) | M. persicae | C. annuum [in cages] | 1:20 | 0.05 | 99.2 † | 10 | LaRock et al. 2003 [54] | |
| 1:40 | 0.02 | 99.4 † | 10 | |||||
| 1:80 | 0.01 | 99.5 † | 10 | |||||
| 1:160 | 0.006 | 98.9 † | 10 | |||||
| 1:320 | 0.003 | 95.3 † | 10 | |||||
| 1:640 | 0.001 | 85.7 † | 10 | |||||
| Life stage (flightless strain) | H. axyridis (1st instars) (flightless strain) | A. gossypii | C. sativus | 1:2.5 | 0.4 | 65.0 † | 05 | Kuroda & Miura 2003 [55] |
| 1:0.5 | 2.0 | 98.3 † | 05 | |||||
| 1:0.25 | 4.0 | 99.5 † | 05 | |||||
| H. axyridis (2nd instars) (flightless strain) | Lipaphis erysimi | Brassica rapa | 1:0.14 | 7.1 | 98.0 | 21 | Adachi-Hagimori et al. 2011 [56] | |
| H. axyridis (adults) (flightless strain) | M. persicae L. erysimi | B. rapa | 1:3.9 | 0.25 | 72.0–98.0 | 21 | Adachi-Hagimori et al. 2011 [56] | |
| 1:3.9 | 0.25 | 81.0 | 21 | |||||
| H. axyridis (2nd instars) (flightless strain) H. axyridis (adults) (flightless strain) | Aulacorthum solani | Solanum melongena | 1:5 | 0.20 | 66.7 | 21 | Seko et al. 2014 [57] | |
| 1:25 | 0.04 | 66.7 | 21 | |||||
| Other predators | Coccinella transversoguttata (adults) C. transversoguttata (adults) plus one hemipteran C. transversoguttata (adults) plus two hemipterans | M. persicae | Beta vulgaris | 1:28.5 | 0.035 | 100 | 6 | Tamaki and Weeks 1972 [58] |
| 1:28.5 | 0.035 | 100 | 6 | |||||
| 1:28.5 | 0.035 | 100 | 6 |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riddick, E.W. Identification of Conditions for Successful Aphid Control by Ladybirds in Greenhouses. Insects 2017, 8, 38. https://doi.org/10.3390/insects8020038
Riddick EW. Identification of Conditions for Successful Aphid Control by Ladybirds in Greenhouses. Insects. 2017; 8(2):38. https://doi.org/10.3390/insects8020038
Chicago/Turabian StyleRiddick, Eric W. 2017. "Identification of Conditions for Successful Aphid Control by Ladybirds in Greenhouses" Insects 8, no. 2: 38. https://doi.org/10.3390/insects8020038






