Potential Hybridization between Two Invasive Termite Species, Coptotermes formosanus and C. gestroi (Isoptera: Rhinotermitidae), and Its Biological and Economic Implications
Abstract
:1. Introduction
2. Sympatric Areas
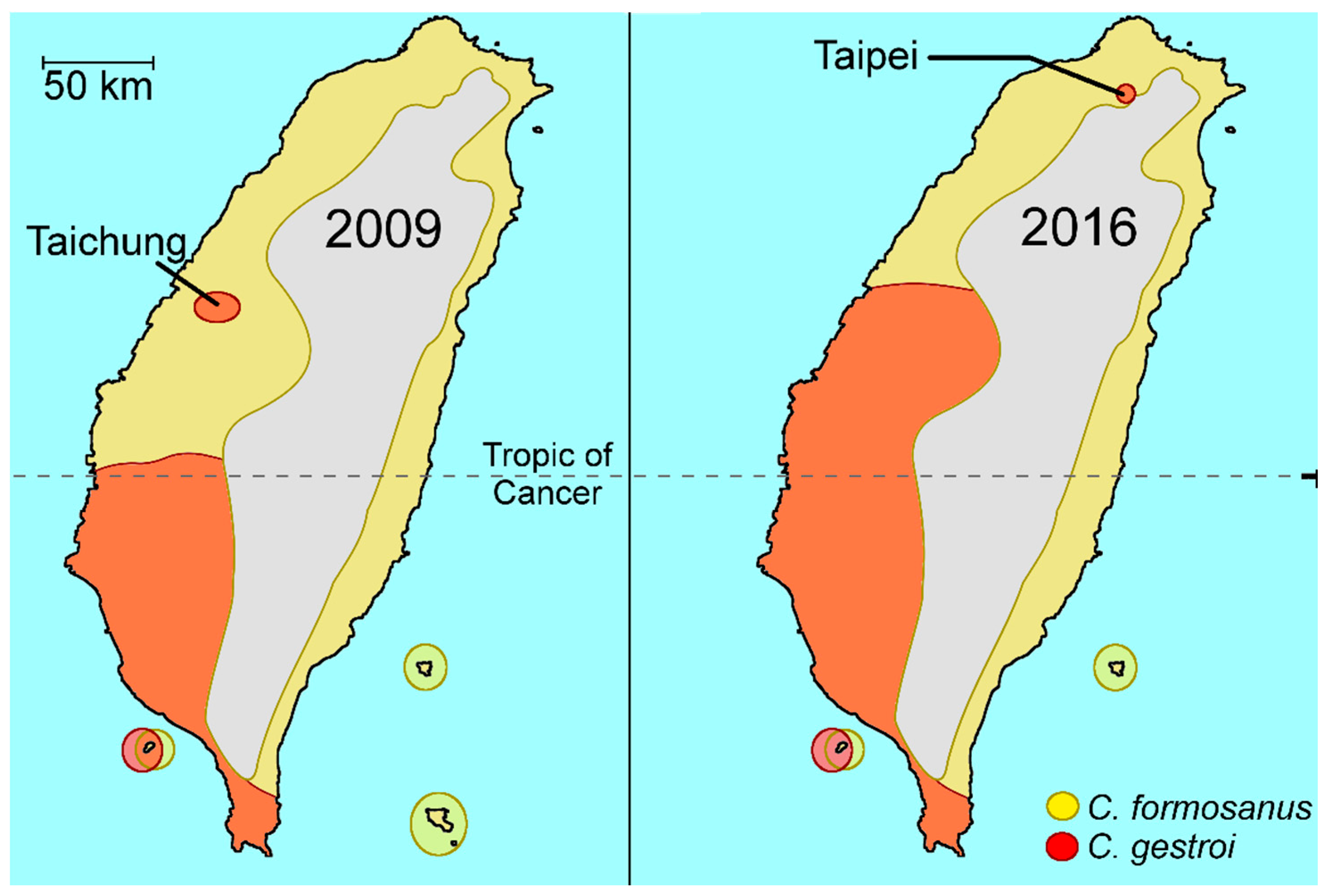
2.1. Coptotermes in Taiwan
2.2. Coptotermes in Hawaii
2.3. Coptotermes in Florida
3. Hybridization
3.1. Observations in Coptotermes
3.2. Hybridization in Social Hymenoptera
3.3. Hybridization in Other Termites
4. Future Prospects on Hybridization in Coptotermes
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Krishna, K.; Grimaldi, D.A.; Krishna, V.; Engel, M.S. Treatise on the Isoptera of the World: Volume 1. Bull. Am. Mus. Nat. History 2013, 377, 1–200. [Google Scholar] [CrossRef]
- Rust, M.; Su, N.-Y. Managing social insects of urban importance. Annu. Rev. Entomol. 2012, 57, 355–375. [Google Scholar] [CrossRef] [PubMed]
- Chouvenc, T.; Li, H.-F.; Austin, J.; Bordereau, C.; Bourguignon, T.; Cameron, S.L.; Cancello, E.M.; Constantino, R.; Costa-Leonardo, A.M.; Eggleton, P.; et al. Revisiting Coptotermes (Isoptera: Rhinotermitidae): A global taxonomic roadmap for species validity and distribution of an economically important subterranean termite genus. Syst. Entomol. 2016, 41, 299–306. [Google Scholar] [CrossRef]
- Evans, T.A.; Forschler, B.T.; Grace, J.K. Biology of invasive termites: A worldwide review. Annu. Rev. Entomol. 2013, 58, 455–474. [Google Scholar] [CrossRef] [PubMed]
- Crispo, E.; Moore, J.S.; Lee-Yaw, J.A.; Gray, S.M.; Haller, B.C. Broken barriers: Human-induced changes to gene flow and introgression in animals. BioEssays 2011, 33, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Scheffrahn, R.H.; Crowe, W. Ship-borne termite (Isoptera) border interceptions in Australia and onboard infestations in Florida, 1986–2009. Fla. Entomol. 2011, 94, 57–63. [Google Scholar] [CrossRef]
- Bourguignon, T.; Lo, N.; Šobotník, J.; Sillam-Dussès, D.; Roisin, Y.; Evans, T.A. Oceanic dispersal, vicariance and human introduction shaped the modern distribution of the termites Reticulitermes, Heterotermes and Coptotermes. Proc. R. Soc. B 2016. [Google Scholar] [CrossRef] [PubMed]
- Chouvenc, T.; Helmick, E.E.; Su, N.-Y. Hybridization of two major termite invaders as a consequence of human activity. PLoS ONE 2015, 10, e0120745. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-C.; Chen, C.-S. First record of Coptotermes gestroi (Isoptera: Rhinotermitidae) from Taiwan. Formosan Entomol. 2003, 23, 157–161. [Google Scholar]
- Li, H.-F.; Su, N.-Y.; Wu, W.-J. Solving the hundred-year controversy of Coptotermes taxonomy in Taiwan. Am. Entomol. 2010, 56, 222–227. [Google Scholar] [CrossRef]
- Li, H.-F.; Su, N.-Y. Phylogeography of Coptotermes gestroi and Coptotermes formosanus (Isoptera: Rhinotermitidae) in Taiwan. Ann. Entomol. Soc. Am. 2009, 102, 684–693. [Google Scholar] [CrossRef]
- Li, H.-F. Taiwan Termite Identification Service Website. Available online: termite.nchu.edu.tw (accessed on 24 January 2017).
- Li, Z.Q.; Liu, B.R.; Li, Q.J.; Xiao, W.L.; Zhong, J.H. Two new synonyms of Coptotermes gestroi (Wasmann) (Isoptera: Rhinotermitidae) in China. Sociobiology 2011, 58, 448–455. [Google Scholar]
- Sweezy, O.H. Notes and exhibitions. Proc. HI. Entomol. Soc. 1914, 3, 27. [Google Scholar]
- Tamashiro, M.; Su, N.-Y. Biology and control of the Formosan subterranean termite. In Biology and Control of the Formosan Subterranean Termite; Tamashiro, M., Su, N.-Y., Eds.; College of Tropical Agriculture Human Resources, University of Hawaii: Honolulu, HI, USA, 1987; p. 1. [Google Scholar]
- Ehrhorn, E.M. The Termites of Hawaii, Their Economic Significance and Control and the Distribution of Termites by Commerce; Kofoid, C.A., Ed.; Termites and Termite Control University of California: Berkeley, CA, USA, 1934; pp. 321–333. [Google Scholar]
- Weesner, F.M. The Termites of the United States; The National Pest Control Association: Elizabeth, NJ, USA, 1965; p. 67. [Google Scholar]
- Woodrow, R.J.; Grace, J.K.; Higa, S.Y. Occurrence of Coptotermes vastator (Isoptera: Rhinotermitidae) on the island of Oahu, Hawaii. Sociobiology 2001, 38, 667–673. [Google Scholar]
- Uchima, S.Y.; Grace, J.K. Interspefic agonism and foraging competition between Coptotermes formosanus and Coptotermes gestroi (Blattodea: Rhinotermitidae). Sociobiology 2009, 54, 765–776. [Google Scholar]
- Grace, J.K.; University of Hawaii, Honolulu, HI, USA. Personal communication, 2016.
- Su, N.-Y.; Scheffrahn, R.H. Formosan Subterranean Termite, Coptotermes formosanus Shiraki. “Featured Creatures”, University Florida Department Entomology/Nematology Website. EENY-121. 2016. Available online: http://entomology.ifas.ufl.edu/creatures/urban/termites/formosan_termite.htm (accessed on 24 January 2017).
- Su, N.-Y.; Scheffrahn, R.H.; Weissling, T. A new introduction of a subterranean termite, Coptotermes havilandi Holmgren (Isoptera: Rhinotermitidae) in Miami, Florida. Fla. Entomol. 1997, 80, 408–411. [Google Scholar] [CrossRef]
- Scheffrahn, R.H.; Su, N.-Y. Asian subterranean termite, Coptotermes gestroi (=havilandi) (Wasmann) (Insecta: Isoptera: Rhinotermitidae). “Featured Creatures”, University Florida Department Entomology/Nematology Website. EENY-128. 2014. Available online: http://entomology.ifas.ufl.edu/creatures/urban/termites/havilandi.htm (accessed on 24 January 2017).
- Chouvenc, T.; Scheffrahn, R.H.; Su, N.-Y. Establishment and spread of two invasive subterranean termite species (C. formosanus and C. gestroi; Isoptera; Rhinotermitidae) in metropolitan southeastern Florida (1990–2015). Fla. Entomol. 2016, 99, 187–191. [Google Scholar] [CrossRef]
- Scheffrahn, R.H. Overview and current status of non-native termites (Isoptera: Rhinotermitidae) in Florida. Fla. Entomol. 2013, 96, 781–788. [Google Scholar] [CrossRef]
- Chouvenc, T. Flight Phenology of Coptotermes formosanus and C. gestroi in Southeastern Florida. J. Econ. Entomol. 2017. in preparation. [Google Scholar]
- Feldhaar, H.; Foitzik, S.; Heinze, J. Lifelong commitment to the wrong partner: Hybridization in ants. Phil. Trans. R. Soc. B 2008, 363, 2891–2899. [Google Scholar] [CrossRef] [PubMed]
- Seifert, B. Interspecific hybridizations in natural populations of ants by example of a reginal fauna (Hymenoptera: Formicidae). Insect Soc. 1999, 46, 45–52. [Google Scholar] [CrossRef]
- Pearson, B. Hybridization between the ant species Lasius niger and Lasius alienus—The genetic evidence. Insect Soc. 1983, 30, 402–411. [Google Scholar] [CrossRef]
- Umphrey, G.J.; Danzman, R.G. Electrophoretic evidence for hybridization in the ant genus Acanthomyops (Hymenoptera: Formicidae). Biochem. Syst. Ecol. 1998, 26, 431–440. [Google Scholar] [CrossRef]
- Douwes, P.; Stille, B. Hybridization and variation in the Leptothorax tuberum group (Hymenoptera, Formicidae). Z. Zool. Syst. Evolutionsforsch. 1991, 29, 165–175. [Google Scholar] [CrossRef]
- Julian, G.E.; Fewell, J.H.; Gadau, J.; Johnson, R.A.; Larrabee, D. Genetic determination of the queen caste in an ant hybrid zone. Proc. Nat. Acad. Sci. USA 2002, 99, 8157–8160. [Google Scholar] [CrossRef] [PubMed]
- Plateaux, L. Ovarian polymorphism of queens in Leptothorax ants and interspecific variations: Inferiority of interspecific hybrids. Arch. Zool. Exp. Gen. 1979, 120, 381–398. [Google Scholar]
- Helms Cahan, S.H.; Vinson, S.B. Reproductive division of labor between hybrid and nonhybrid offspring in a fire ant hybrid zone. Evolution 2003, 57, 1562–1570. [Google Scholar] [CrossRef] [PubMed]
- Axen, H.J.; Wildermuth, A.; Helms Cahan, S. Environmental filtering of foraging strategies meditates patterns of coexistence in the fire ants Solenopsis geminate and Solenopsis xyloni, and their interspecific hybrids. Ecol. Entomol. 2014, 39, 290–299. [Google Scholar] [CrossRef]
- Gibboson, L.; Simberloff, D. Interaction of hybrid imported fire ants (Solenopsis invicta x S. richteri) with native ants at baits in southeastern Tennessee. Southeast Nat. 2005, 4, 303–320. [Google Scholar]
- James, S.S.; Pereira, R.M.; Vail, K.M.; Ownley, B.H. Survival of imported fire ant (Hymenoptera: Formicidae) species subjected to freezing and near freezing temperatures. Environ. Entomol. 2002, 31, 127–133. [Google Scholar] [CrossRef]
- Umphrey, G.J. Sperm parasitism in ants: Selection for interspecific mating and hybridization. Ecology 2006, 87, 2148–2159. [Google Scholar] [CrossRef]
- Anderson, K.E.; Novak, S.J.; Smith, J.F. Populations composed entirely of hybrid colonies: Bidirectional hybridization and polyandry in harvester ants. Biol. J. Linn. Soc. 2008, 95, 320–336. [Google Scholar] [CrossRef]
- Scott Schneider, S.; De Grandi-Hoffman, G.; Smith, D.R. The African honey bee: Factors contributing to a successful biological invasion. Annu. Rev. Entomol. 2004, 49, 351–376. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, B.T.; Kambhampati, S. Preliminary analysis of a hybrid zone between two subspecies of Zootermopsis nevadensis. Insect Soc. 2009, 56, 439–450. [Google Scholar] [CrossRef]
- Lefebvre, T.; Châline, N.; Limousin, D.; Dupont, S.; Bagnères, A.G. From speciation to introgressive hybridization: The phylogeographic structure of an island subspecies of termite, Reticulitermes lucifugus corsicus. BMC Evol. Biol. 2008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connétable, S.; Robert, A.; Bordereau, C. Dispersal flight and colony development in the fungus-growing termites Pseudacanthotermes spiniger and P. militaris. Insect Soc. 2012, 59, 269–277. [Google Scholar] [CrossRef]
- Hartke, T.R.; Rosengaus, R.B. Heterospecific pairing and hybridization between Nasutitermes corniger and N. ephratae. Naturwissenschaften 2011, 98, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Chunco, A.J. Hybridization in a warmer world. Ecol. Evol. 2015, 4, 2019–2031. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Su, N.Y. Temperature Preferences of Four Subterranean Termite Species (Isoptera: Rhinotermitidae) and Temperature-Dependent Survivorship and Wood-Consumption Rate. Ann. Entomol. Soc. Am. 2016, 109, 64–71. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, N.-Y.; Chouvenc, T.; Li, H.-F. Potential Hybridization between Two Invasive Termite Species, Coptotermes formosanus and C. gestroi (Isoptera: Rhinotermitidae), and Its Biological and Economic Implications. Insects 2017, 8, 14. https://doi.org/10.3390/insects8010014
Su N-Y, Chouvenc T, Li H-F. Potential Hybridization between Two Invasive Termite Species, Coptotermes formosanus and C. gestroi (Isoptera: Rhinotermitidae), and Its Biological and Economic Implications. Insects. 2017; 8(1):14. https://doi.org/10.3390/insects8010014
Chicago/Turabian StyleSu, Nan-Yao, Thomas Chouvenc, and Hou-Feng Li. 2017. "Potential Hybridization between Two Invasive Termite Species, Coptotermes formosanus and C. gestroi (Isoptera: Rhinotermitidae), and Its Biological and Economic Implications" Insects 8, no. 1: 14. https://doi.org/10.3390/insects8010014





