Impact of an Invasive Insect and Plant Defense on a Native Forest Defoliator
Abstract
:1. Introduction
2. Materials and Methods
2.1. Performance Experiment
2.2. Preference Experiment
2.3. Statistical Analysis
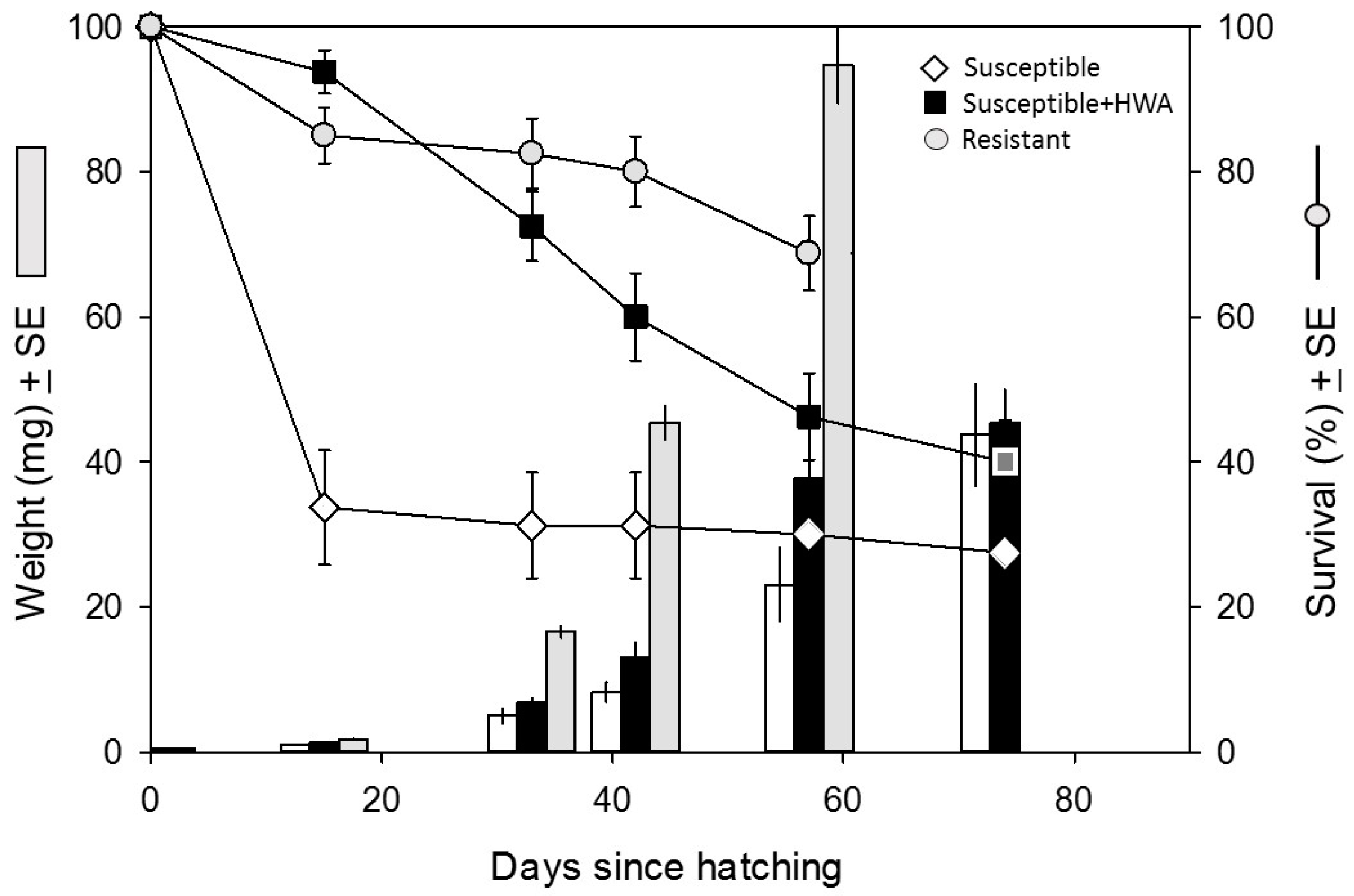
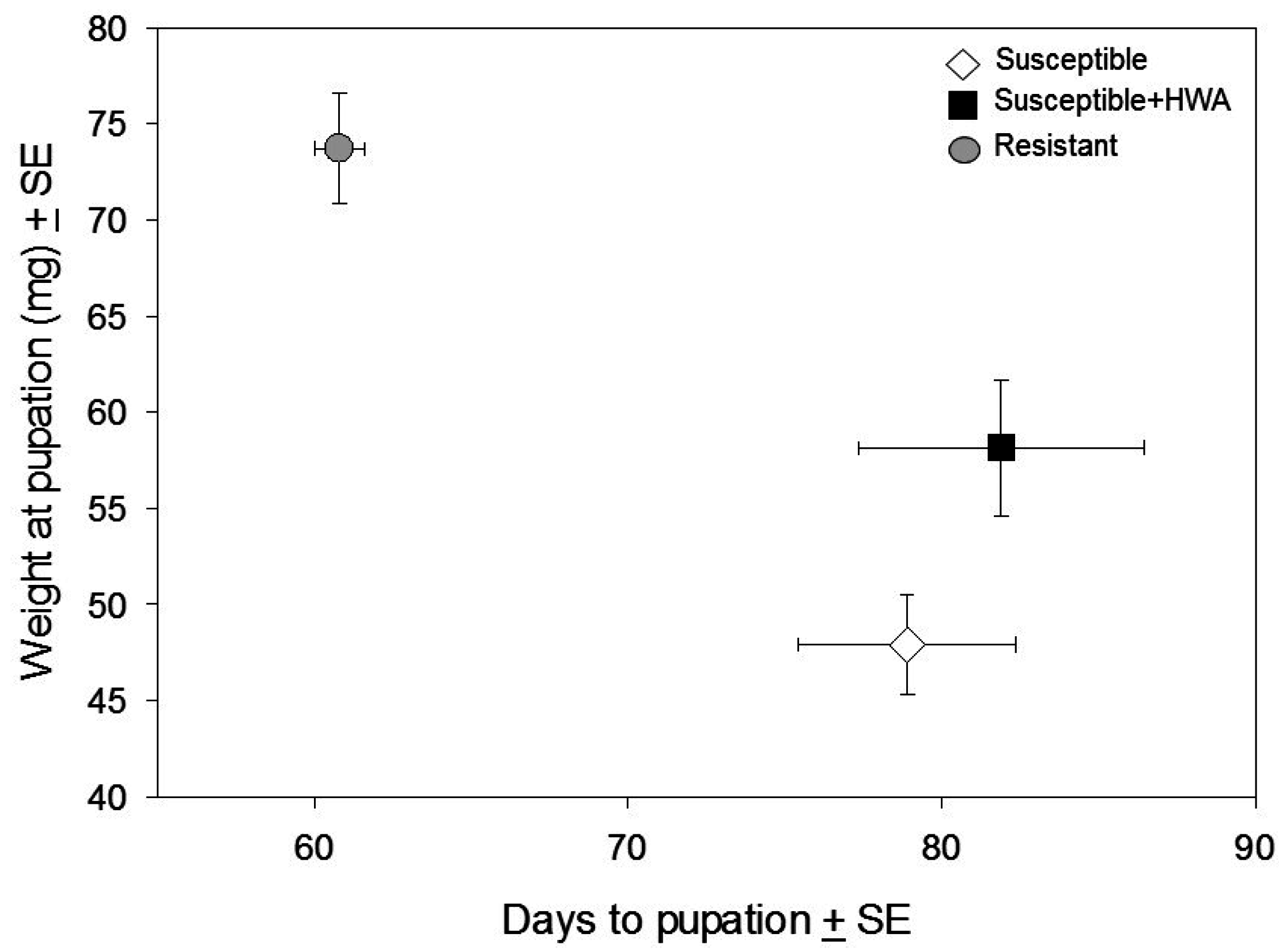
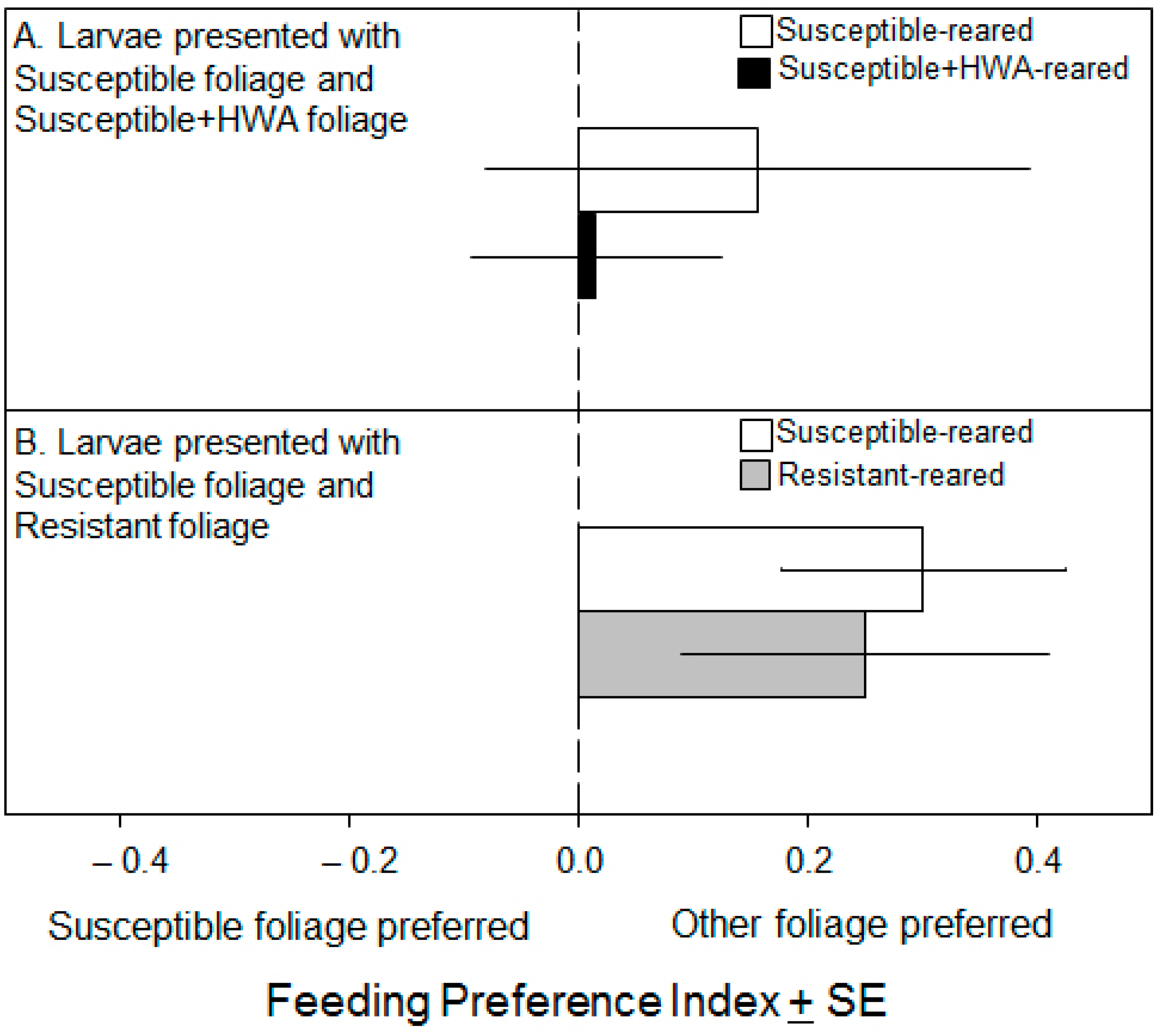
3. Results
3.1. Performance Experiment
3.2. Preference Experiments
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Karban, R.; Baldwin, I.T. Induced Responses to Herbivory; University of Chicago Press: Chicago, IL, USA, 1997. [Google Scholar]
- Green, T.R.; Ryan, C.A. Wound-induced proteinase inhibitor in plant leaves—possible defense mechanism against insects. Science 1972, 175, 776–777. [Google Scholar] [CrossRef] [PubMed]
- Heil, M.; Koch, T.; Hilpert, A.; Fiala, B.; Boland, W.; Linsenmair, K.E. Extrafloral nectar production of the ant-associated plant, Macaranga tanarius, is an induced, indirect, defensive response elicited by jasmonic acid. Proc. Natl. Acad. Sci. USA 2001, 98, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Karban, R.; Agrawal, A.A. Herbivore offense. Annu. Rev. Ecol. Syst. 2002, 33, 641–664. [Google Scholar] [CrossRef]
- Agrawal, A.A. Induced responses to herbivory in wild radish: Effects on several herbivores and plant fitness. Ecology 1999, 80, 1713–1723. [Google Scholar] [CrossRef]
- Gregoire, D.M.; Quiring, D.T.; Royer, L.; Heard, S.B.; Bauce, E. Indirect host-mediated effects of an exotic phloem-sap feeder on a native defoliator of balsam fir. For. Ecol. Manag. 2015, 341, 1–7. [Google Scholar] [CrossRef]
- Zhang, P.J.; Zheng, S.J.; van Loon, J.J.A.; Boland, W.; David, A.; Mumm, R.; Dicke, M. Whiteflies interfere with indirect plant defense against spider mites in lima bean. Proc. Natl. Acad. Sci. USA 2009, 106, 21202–21207. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Dicke, M.; Harvey, J.A.; Gols, R. Intra-specific variation in wild Brassica oleracea for aphid-induced plant responses and consequences for caterpillar-parasitoid interactions. Oecologia 2014, 174, 853–862. [Google Scholar] [CrossRef] [PubMed]
- De Vos, M.; Van Oosten, V.R.; Van Poecke, R.M.P.; Van Pelt, J.A.; Pozo, M.J.; Mueller, M.J.; Buchala, A.J.; Metraux, J.P.; Van Loon, L.C.; Dicke, M.; et al. Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Mol. Plant Microbe Interact. 2005, 18, 923–937. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Saona, C.R.; Musser, R.O.; Vogel, H.; Hum-Musser, S.M.; Thaler, J.S. Molecular, biochemical, and organismal analyses of tomato plants simultaneously attacked by herbivores from two feeding guilds. J. Chem. Ecol. 2010, 36, 1043–1057. [Google Scholar] [CrossRef] [PubMed]
- Schwartzberg, E.G.; Boroczky, K.; Tumlinson, J.H. Pea aphids, Acyrthosiphon pisum, suppress induced plant volatiles in broad bean, Vicia faba. J. Chem. Ecol. 2011, 37, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Stam, J.M.; Kroes, A.; Li, Y.H.; Gols, R.; van Loon, J.J.A.; Poelman, E.H.; Dicke, M. Plant interactions with multiple insect herbivores: From community to genes. Annu. Rev. Plant Biol. 2014, 65, 689–713. [Google Scholar] [CrossRef] [PubMed]
- Ellison, A.M.; Bank, M.S.; Clinton, B.D.; Colburn, E.A.; Elliott, K.; Ford, C.R.; Foster, D.R.; Kloeppel, B.D.; Knoepp, J.D.; Lovett, G.M.; et al. Loss of foundation species: Consequences for the structure and dynamics of forested ecosystems. Front. Ecol. Environ. 2005, 3, 479–486. [Google Scholar] [CrossRef]
- Snyder, C.; Young, J.; Lemarié, D.; Smith, D. Influence of eastern hemlock (Tsuga canadensis) forests on aquatic invertebrate assemblages in headwater streams. Can. J. Fish. Aquat. Sci. 2002, 59, 262–275. [Google Scholar] [CrossRef]
- Orwig, D.; Cobb, R.; D’Amato, A.; Kizlinski, M.; Foster, D. Multi-year ecosystem response to hemlock woolly adelgid infestation in southern New England forests. Can. J. For. Res. 2008, 38, 834–843. [Google Scholar] [CrossRef]
- Allen, M.C.; Sheehan, J.; Master, T.L.; Mulvihill, R. Responses of acadian flycatchers (Empidonax virescens) to hemlock woolly adelgid (Adelges tsugae) infestation in Appalachian riparian forests. Auk 2009, 126, 543–553. [Google Scholar] [CrossRef]
- Havill, N.; Montgomery, M.; Yu, G.; Shiyake, S.; Caccone, A. Mitochondrial DNA from hemlock woolly adelgid (Hemiptera: Adelgidae) suggests cryptic speciation and pinpoints the source of the introduction to eastern North America. Ann. Entomol. Soc. Am. 2006, 99, 195–203. [Google Scholar] [CrossRef]
- Orwig, D.; Foster, D.; Mausel, D. Landscape patterns of hemlock decline in New England due to the introduced hemlock woolly adelgid. J. Biogeogr. 2002, 29, 1475–1487. [Google Scholar] [CrossRef]
- McClure, M. Density-dependent feedback and population cycles in Adelges tsugae (Homoptera: Adelgidae) on Tsuga canadensis. Environ. Entomol. 1991, 20, 258–264. [Google Scholar] [CrossRef]
- Young, R.; Shields, K.; Berlyn, G. Hemlock woolly adelgid (Homoptera: Adelgidae): Stylet bundle insertion and feeding sites. Ann. Entomol. Soc. Am. 1995, 88, 827–835. [Google Scholar] [CrossRef]
- Oten, K.L.F.; Cohen, A.C.; Hain, F.P. Stylet bundle morphology and trophically-related enzymes of the hemlock woolly adelgid (Hemiptera: Adelgidae). Ann. Entomol. Soc. Am. 2014, 107, 680–690. [Google Scholar] [CrossRef]
- Radville, L.; Chaves, A.; Preisser, E. Variation in plant defense against invasive herbivores: Evidence for a hypersensitive response in eastern hemlocks (Tsuga canadensis). J. Chem. Ecol. 2011, 37, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Domec, J.-C.; Rivera, L.N.; King, J.S.; Peszlen, I.; Hain, F.; Smith, B.; Frampton, J. Hemlock woolly adelgid (Adelges tsugae) infestation affects water and carbon relations of eastern hemlock (Tsuga canadensis) and carolina hemlock (Tsuga caroliniana). New Phytol. 2013, 199, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Pezet, J.; Elkinton, J.; Gomez, S.; McKenzie, E.A.; Lavine, M.; Preisser, E. Hemlock woolly adelgid and elongate hemlock scale induce changes in foliar and twig volatiles of eastern hemlock. J. Chem. Ecol. 2013, 39, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Pezet, J.; Elkinton, J.S. Hemlock woolly adelgid (Hemiptera: Adelgidae) induces twig volatiles of eastern hemlock in a forest setting. Environ. Entomol. 2014, 43, 1275–1285. [Google Scholar] [CrossRef] [PubMed]
- Bhiry, N.; Filion, L. Mid-holocene hemlock decline in eastern North America linked with phytophagous insect activity. Quat. Res. 1995, 45, 312–320. [Google Scholar] [CrossRef]
- Foster, D.; Oswald, W.; Faison, E.; Doughty, E.; Hansen, B. A climatic driver for abrupt mid-holocene vegetation dynamics and the hemlock decline in New England. Ecology 2006, 87, 2959–2966. [Google Scholar] [CrossRef]
- Trial, J.H.; Devine, M.E. The Impact of the Current Hemlock Looper, Lambdina Fiscellaria (Guen.), Outbreak in Selected Severely Damaged Stands of Eastern Hemlock; Insect and Disease Management Division, Maine Forest Service: Augusta, ME, USA, 1994.
- Hébert, C.; Jobin, L. The Hemlock Looper; Natural Resources Canada, Canadian Forest Service, Laurentian Forestry Centre: Sainte-Foy, QC, Canada, 2001. [Google Scholar]
- Carroll, A.L. Physiological adaptation to temporal variation in conifer foliage by a caterpillar. Can. Entomol. 1999, 131, 659–669. [Google Scholar] [CrossRef]
- Alfaro, R.I.; Taylor, S.; Brown, G.; Wegwitz, E. Tree mortality caused by the western hemlock looper in landscapes of central British Columbia. For. Ecol. Manag. 1999, 124, 285–291. [Google Scholar] [CrossRef]
- Otvos, I.S.; Clark, R.C.; Clarke, L.J. The Hemlock Looper in Newfoundland: The Outbreak 1966 to 1971 and Aerial Spraying, 1968 and 1969; Newfoundland Forest Research Center: St. Johns, NL, Canada, 1971; pp. 4–5. [Google Scholar]
- Kaloshian, I.; Walling, L.L. Hemipterans as plant pathogens. Annu. Rev. Phytopathol. 2005, 43, 491–521. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, E.A.; Elkinton, J.S.; Casagrande, R.A.; Preisser, E.L.; Mayer, M. Terpene chemistry of eastern hemlocks resistant to hemlock woolly adelgid. J. Chem. Ecol. 2014, 40, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Ingwell, L.L.; Preisser, E.L. Using citizen science programs to identify host resistance in pest-invaded forests. Conserv. Biol. 2011, 25, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.; Phillip Alampi Beneficial Insect Laboratory, New Jersey Department of Agriculture, Division of Plant Industry, Trenton, NJ, USA. Personal communication, 2014.
- Casagrande, R.; Department of Plant Sciences and Entomology, College of the Environment and Life Science, The University of Rhode Island, Kingston, RI, USA. Personal communication, 2014.
- Preisser, E.; Department of Biological Sciences, College of the Environment and Life Science, The University of Rhode Island, Kingston, RI, USA. Unpublished data. 2013.
- Del Campo C, M.L.; Renwick, J.A.A. Dependence on host constituents controlling food acceptance by Manduca sexta larvae. Entomol. Exp. Appl. 1999, 93, 209–215. [Google Scholar] [CrossRef]
- Vlot, A.C.; Dempsey, D.A.; Klessig, D.F. Salicylic acid, a multifaceted hormone to combat disease. Annu. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef] [PubMed]
- Thaler, J.S.; Karban, R.; Ullman, D.E.; Boege, K.; Bostock, R.M. Cross-talk between jasmonate and salicylate plant defense pathways: Effects on several plant parasites. Oecologia 2002, 131, 227–235. [Google Scholar]
- Walling, L.L. Avoiding effective defenses: Strategies employed by phloem-feeding insects. Plant. Physiol. 2008, 146, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Scriber, J.M.; Slansky, F. The nutritional ecology of immature insects. Annu. Rev. Entomol. 1981, 26, 183–211. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C.M. Hormonal modulation of plant immunity. Annu. Rev. Cell. Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [PubMed]
- Walling, L.L. The myriad plant responses to herbivores. J. Plant Growth Regul. 2000, 19, 195–216. [Google Scholar] [PubMed]
- Soler, R.; Badenes-Perez, F.R.; Broekgaarden, C.; Zheng, S.J.; David, A.; Boland, W.; Dicke, M. Plant-mediated facilitation between a leaf-feeding and a phloem-feeding insect in a brassicaceous plant: From insect performance to gene transcription. Funct. Ecol. 2012, 26, 156–166. [Google Scholar] [CrossRef]
- Hebert, C.; Berthiaume, R.; Bauce, E.; Brodeur, J. Geographic biotype and host-associated local adaptation in a polyphagous species, Lambdina fiscellaria (Lepidoptera: Geometridae) feeding on balsam fir on Anticosti Island, Canada. Bull. Entomol. Res. 2006, 96, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Lerdau, M.; Matson, P.; Fall, R.; Monson, R. Ecological controls over monoterpene emissions from douglas-fir (Pseudotsuga menziesii). Ecology 1995, 76, 2640–2647. [Google Scholar] [CrossRef]
- Powell, J.S.; Raffa, K.F. Sources of variation in concentration and composition of foliar monoterpenes in tamarack (Larix laricina) seedlings: Roles of nutrient availability, time of season, and plant architecture. J. Chem. Ecol. 1999, 25, 1771–1797. [Google Scholar] [CrossRef]
- Franceschi, V.R.; Krokene, P.; Christiansen, E.; Krekling, T. Anatomical and chemical defenses of conifer bark against bark beetles and other pests. New Phytol. 2005, 167, 353–375. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.G.; Agrawal, A.A. Asymmetry of plant-mediated interactions between specialist aphids and caterpillars on two milkweeds. Funct. Ecol. 2014, 28, 1404–1412. [Google Scholar] [CrossRef]
- Foster, D. Hemlock’s Future in the Context of Its History: An Ecological Perspective. In Proceeding of Symposium on Sustainable Management of Hemlock Ecosystems in Eastern North America, Newtown Square, PA, USA, 22–24 June 1999; McManus, K., Shields, K., Souto, D., Eds.; US Forest Service: Newtown Square, PA, USA, 2000; pp. 1–4. [Google Scholar]
- Havill, N.; Campbell, C.; Vining, T.; LePage, B.; Bayer, R.; Donoghue, M. Phylogeny and biogeography of Tsuga (Pinaceae) inferred from nuclear ribosomal its and chloroplast DNA sequence data. Syst. Bot. 2008, 33, 478–489. [Google Scholar] [CrossRef]
- Lagalante, A.; Montgomery, M. Analysis of terpenoids from hemlock (Tsuga) species by solid-phase microextraction/gas chromatography/ion-trap mass spectrometry. J. Agric. Food Chem. 2003, 51, 2115–2120. [Google Scholar] [CrossRef] [PubMed]
- Lagalante, A.; Montgomery, M.; Calvosa, F.; Mirzabeigi, M. Characterization of terpenoid volatiles from cultivars of eastern hemlock (Tsuga canadensis). J. Agric. Food Chem. 2007, 55, 10850–10856. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilson, C.M.; Vendettuoli, J.F.; Orwig, D.A.; Preisser, E.L. Impact of an Invasive Insect and Plant Defense on a Native Forest Defoliator. Insects 2016, 7, 45. https://doi.org/10.3390/insects7030045
Wilson CM, Vendettuoli JF, Orwig DA, Preisser EL. Impact of an Invasive Insect and Plant Defense on a Native Forest Defoliator. Insects. 2016; 7(3):45. https://doi.org/10.3390/insects7030045
Chicago/Turabian StyleWilson, Claire M., Justin F. Vendettuoli, David A. Orwig, and Evan L. Preisser. 2016. "Impact of an Invasive Insect and Plant Defense on a Native Forest Defoliator" Insects 7, no. 3: 45. https://doi.org/10.3390/insects7030045





