Hypoxia Treatment of Callosobruchus maculatus Females and Its Effects on Reproductive Output and Development of Progeny Following Exposure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insect Colonies
2.2. Baseline Bruchid Reproductive Output
2.3. Effect of Reduced Oxygen on Bruchid Egg Production
2.4. Statistical Analysis
3. Results
3.1. Baseline Reproductive Output of Female Bruchids
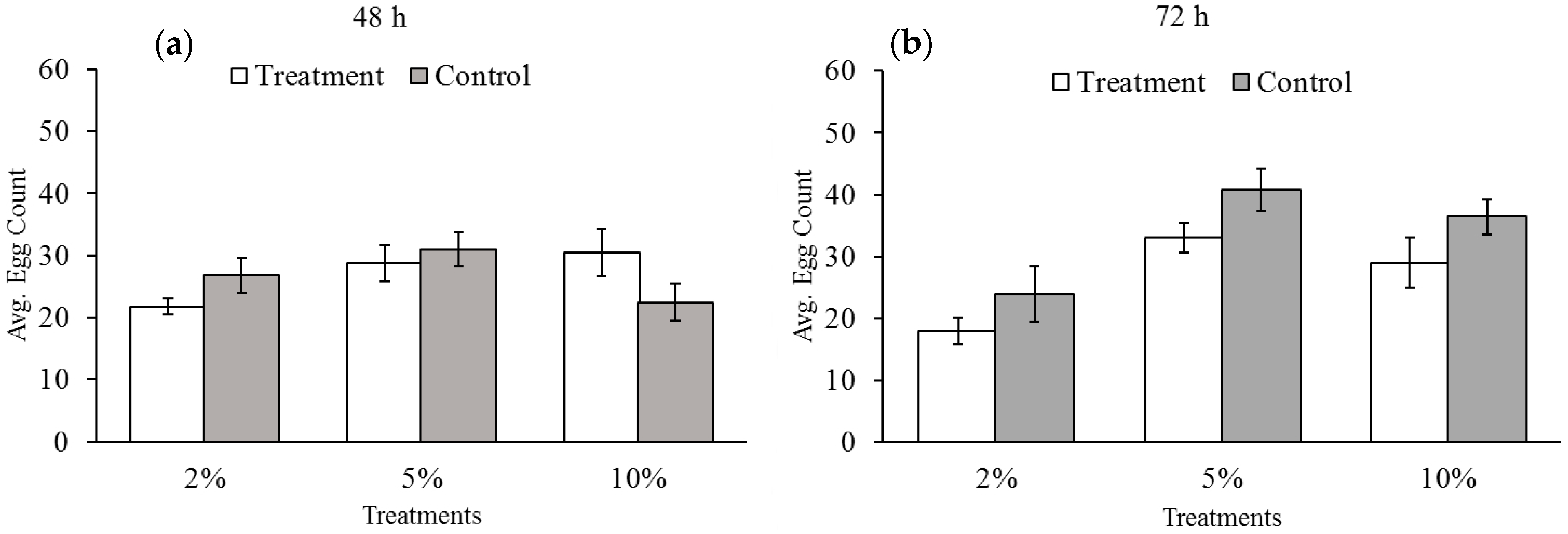
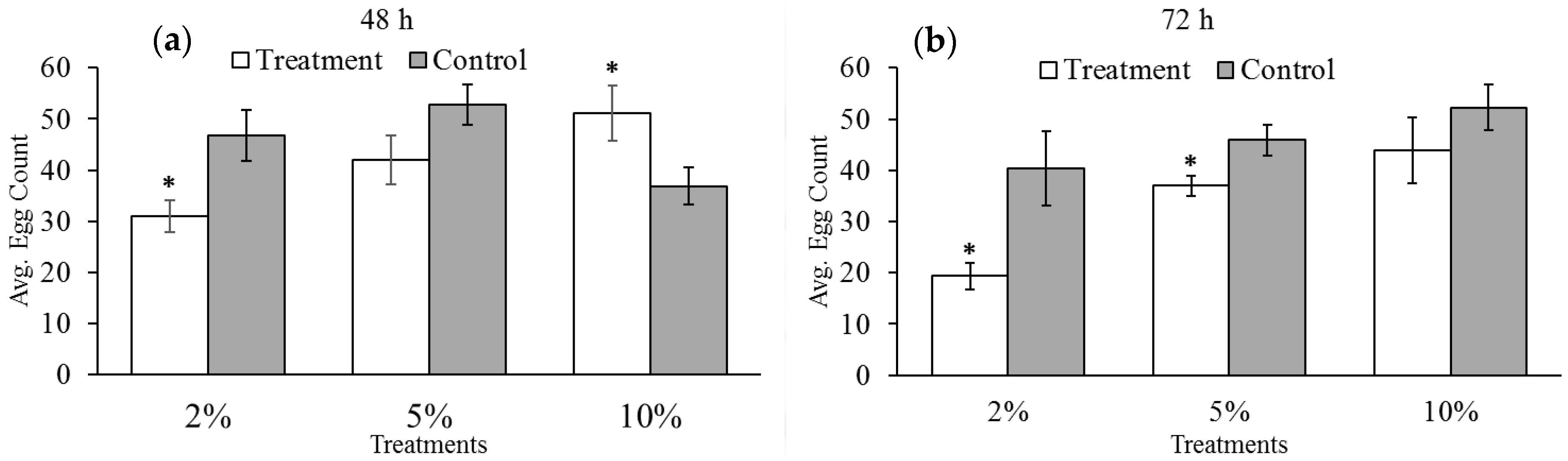
3.2. Effect of Reduced Ambient Oxygen on Bruchid Egg Production
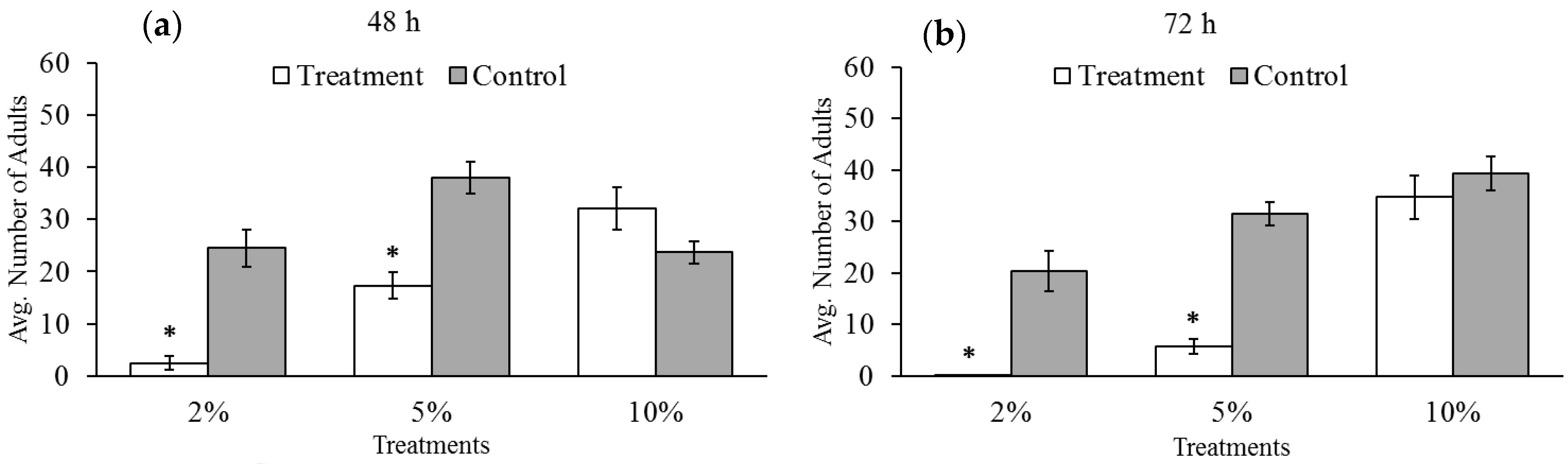
3.3. Adult-Emergence
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| MA | Modified Atmosphere |
| PICS | Purdue Improved Crop Storage |
| HDPE | High-density polyethylene |
| ANOVA | Analysis of Variance |
| HIF | Hypoxia Inducible Factor |
References
- Cheng, C.; Yang, R.; Cheng, I.; Horng, S. Egg dumping: A strategy for host range expansion in Callosobruchus maculatus (Coleoptera: Bruchidae). Ann. Entomol. Soc. Am. 2008, 101, 950–954. [Google Scholar] [CrossRef]
- Langyintuo, A.S.; Lowenberg-DeBoer, J.; Faye, M.; Lambert, D.; Ibro, G.; Moussa, B.; Kergna, A.; Kushwaha, S.; Musa, S.; Ntoukam, G. Cowpea supply and demand in West and Central Africa. Field Crops Res. 2003, 82, 215–231. [Google Scholar] [CrossRef]
- Cheng, W.N.; Lei, J.X.; Ahn, J.E.; Wang, Y.; Lei, C.; Zhu-Salzman, K. CO2 enhances effects of hypoxia on mortality, development, and gene expression in cowpea bruchid, Callosobruchus maculatus. J. Stored Prod. Res. 2013, 59, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- Zhu-Salzman, K.; Murdock, L.L. Cowpea: Insects, ecology and control. In The Encyclopedia of Pest Management; Taylor & Francis Group: New York, NY, USA, 2006; pp. 1–3. [Google Scholar]
- Boeke, S.J.; Baumgart, I.R.; van Loon, J.J.A.; van Huis, A.; Dicke, M.; Kossou, D.K. Toxicity and repellence of African plants traditionally used for the protection of stored cowpea against Callosobruchus maculatus. J. Stored Prod. Res. 2004, 40, 423–438. [Google Scholar] [CrossRef]
- Fields, P.G.; White, N.D.G. Alternatives to methyl bromide treatments for stored-product and quarantine insects. Annu. Rev. Entomol. 2006, 47, 331–359. [Google Scholar] [CrossRef] [PubMed]
- Benhalima, H.; Chaudhry, M.Q.; Mills, K.A.; Price, N.R. Phosphine resistance in stored-product insects collected from various grain storage facilities in Morocco. J. Stored Prod. Res. 2006, 40, 241–249. [Google Scholar] [CrossRef]
- Pimentel, M.A.G.; Faroni, L.R.D.; Guedes, R.N.C.; Sousa, A.H.; Totola, M.R. Phosphine resistance in Brazilian populations of Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae). J. Stored Prod. Res. 2009, 45, 71–74. [Google Scholar] [CrossRef]
- Zettler, J.L.; Cuperus, G.W. Pesticidal resistance in Tribolium castaneum (Coleoptera: Tenebrionidae) and Rhysopertha dominica (Coleoptera: Bostrichidae) in wheat. J. Econ. Entomol. 1990, 83, 1677–1681. [Google Scholar] [CrossRef]
- Silver, P. Alternatives to methyl bromide sought. Pestic. News 1994, 24, 12–27. [Google Scholar]
- Banks, H.J.; Annis, P.C. Comparative advantages of high CO2 and low O2 types of controlled atmospheres for grain storage. In Food Preservation by Modified Atmospheres; Calderon, M., Barkai-Golan, R., Eds.; CRC Press: Boca Raton, FL, USA, 1990; pp. 93–122. [Google Scholar]
- Fleurat-Lessard, F. Effect of modified atmospheres on insects and mites infesting stored products. In Food Preservation by Modified Atmospheres; Calderon, M., Barkai-Golan, R., Eds.; CRC Press: Boca Raton, FL, USA, 1990; pp. 21–38. [Google Scholar]
- Navarro, S. Modified atmospheres for the control of stored-product insects and mites. In Insect Management for Food Storage and Processing; Heaps, J.W., Ed.; AACC International: St. Paul, MN, USA, 2006; pp. 105–146. [Google Scholar]
- Navarro, S.; Timlick, B.; Demianyk, C.J.; White, N.D.G. Controlled and modified atmospheres. In Stored Product Protection; Hagstrum, D., Phillips, T., Cupperus, G., Eds.; Kansas State Research and Extension: Manhattan, KS, USA, 2012; pp. 1–25. [Google Scholar]
- Abdolmaleki, A.; Safavi, S.A.; Safaralizadeh, M.H.; Allahvaisi, S.; Sadeghi, G.R. Lethal effects of low atmosphere pressures on various developmental stages of Tribolium castaneum (Herbst) and Ephestia kuehniella (Zeller) under Laboratory Conditions. Egypt. J. Biol. Pest Control 2011, 21, 159–163. [Google Scholar]
- Murdock, L.L.; Margam, V.; Baoua, I.; Balfe, S.; Shade, R.E. Death by desiccation: Effects of hermetic storage on cowpea bruchids. J. Stored Prod. Res. 2012, 49, 166–170. [Google Scholar] [CrossRef]
- Baoua, I.B.; Amadou, L.; Ousmane, B.; Baributsa, D.; Murdock, L.L. PICS bags for post-harvest storage of maize grain in West Africa. J. Stored Prod. Res. 2014, 58, 20–28. [Google Scholar] [CrossRef]
- Sudini, H.; Ranga Rao, G.V.; Gowda, C.L.L.; Chandrika, R.; Margam, V.; Rathore, A.; Murdock, L.L. Purdue Improved Crop Storage (PICS) bags for safe storage of groundnuts. J. Stored Prod. Res. 2015, 64, 133–138. [Google Scholar] [CrossRef]
- Williams, S.B.; Baributsa, D.; Woloshuk, C. Assessing Purdue Improved Crop Storage (PICS) bags to mitigate fungal growth and aflatoxin contamination. J. Stored Prod. Res. 2014, 59, 190–196. [Google Scholar] [CrossRef]
- Baributsa, D.; Lowenberg-DeBoer, J.; Murdock, L.; Moussa, B. Profitable chemical-free cowpea storage technology for smallholder farmers in Africa: Opportunities and challenges. In Proceedings of the 10th International Working Conference on Stored Product Protection, Estoril, Portugal, 27 June–2 July 2010; pp. 1046–1052.
- Baributsa, D.; Tahirou, A.; Lowenberg-DeBoer, J.; Dabiré, C.; Moussa, B.; Coulibaly, O.; Baoua, I. Market building for post-harvest technology through large-scale extension efforts. J. Stored Prod. Res. 2014, 58, 59–66. [Google Scholar] [CrossRef]
- Murdock, L.L.; Shade, R.E.; Kitch, L.W.; Ntoukam, G.; Lowenberg-Deboer, J.; Huesing, J.E.; Moar, W.; Chambliss, O.L.; Endondo, C.; Wolfson, J.L. Postharvest storage of cowpea in sub-Saharan Africa. In Advances in Cowpea Reseach; Singh, B.B., Ed.; Co-publication of IITA and JIRCAS: Ibadan, Nigeria, 1997; pp. 302–312. [Google Scholar]
- Murdock, L.L.; Seck, D.; Ntoukam, G.; Kitch, L.; Shade, R.E. Preservation of cowpea grain in Sub-Saharan Africa-bean/cowpea CRSP contributions. Field Crops Res. 2003, 82, 169–178. [Google Scholar] [CrossRef]
- Bailey, S.W. Air-tight storage of grain: Its effect on insect pests-IV Rhyzopertha dominica (F.) and some other Coleoptera that infest stored grain. J. Stored Prod. Res. 1965, 1, 25–33. [Google Scholar] [CrossRef]
- Dawson, C. The effect of carbon dioxide induced anaesthesia on fecundity of Callosobruchus maculatus (Coleoptera: Bruchidae). J. Stored Prod. Res. 1995, 31, 49–54. [Google Scholar] [CrossRef]
- Van der Meer, J.M. The specification of metameric order in the insect Callosobruchus maculatus Fabr. (Coleoptera). J. Embryol. Exp. Morphol. 1979, 51, 1–26. [Google Scholar] [PubMed]
- Dobie, P.; Haines, C.P.; Hodges, R.J.; Prevett, P.F.; Rees, D.P. Insects and Arachnids of Tropical Stored Products: Their Biology and Identification; Natural Resources Institute: Chatham Maritime, UK, 1991. [Google Scholar]
- Arnold, S.E.; Stevenson, P.C.; Belmain, S.R. Odour-mediated orientation of beetles is influenced by age, sex and morph. PLoS ONE 2012, 7, e49071. [Google Scholar] [CrossRef] [PubMed]
- Messina, F.J.; Bloxham, A.J.; Seargent, A.J. Mating compatibility between geographic populations of the seed beetle Callosobruchus maculatus. J. Insect Behav. 2007, 20, 489–501. [Google Scholar] [CrossRef]
- Hoback, W.W.; Stanley, D.W. Insects in hypoxia. J. Insect Physiol. 2001, 47, 533–542. [Google Scholar] [CrossRef]
- Greenlee, K.J.; Harrison, J.F. Respiratory changes throughout ontogeny in the tobacco hornworm caterpillar, Manduca sexta. J. Exp. Biol. 2005, 208, 1385–1392. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.; Frazier, M.R.; Henry, J.R.; Kaiser, A.; Klok, C.J.; Rascón, B. Responses of terrestrial insects to hypoxia or hyperoxia. Respir. Physiol. Neurobiol. 2006, 154, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Frazier, M.R. Alpine Insects: Physiology and Evolution in Cold, Thin Air. Ph.D. Thesis, University of Washington, Seattle, WA, USA, 2007. [Google Scholar]
- Reiling, J.H.; Hafen, E. The hypoxia-induced paralogs Scylla and Charybdis inhibit growth by down-regulating S6 K activity upstream of TSC in Drosophila. Genes Dev. 2004, 18, 2879–2892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, W.N.; Chi, Y.H.; Ahn, J.E.; Yun, D.J.; Lee, S.Y.; Liu, T.X.; Zhu-Salzman, K. Changes in oxygen and carbon dioxide environment alter gene expression of cowpea bruchids. J. Insect Physiol. 2005, 57, 220–230. [Google Scholar]
- Lavista-Llanos, S.; Centanin, L.; Irisarri, M.; Russo, D.M.; Gleadle, J.M.; Bocca, S.N.; Muzzopappa, M.; Ratcliffe, P.J.; Wappner, P. Control of the hypoxic response in Drosophila melanogaster by the basic helix-loop-helix PAS protein similar. Mol. Cell. Biol. 2002, 22, 6842–6853. [Google Scholar] [CrossRef] [PubMed]
- Gorr, T.A.; Gassmann, M.; Wappner, P. Sensing and responding to hypoxia via HIF in model invertebrates. J. Insect Physiol. 2006, 52, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.N.; Lei, J.X.; Ahn, J.E.; Liu, T.X.; Zhu-Salzman, K. Effects of decreased O2 and elevated CO2 on survival, development, and gene expression in cowpea bruchids. J. Insect Physiol. 2012, 58, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Lum, P.T.M. Oocyte degeneration in Plodia interpuntella Hubner and Cadra cautella (Walker) (Lepidoptera: Pyralidae): Influence of Temperature and Humidity! Environ. Entomol. 1983, 12, 1539–1541. [Google Scholar] [CrossRef]
- Lum, P.T.M.; Flaherty, B.R. Effect of carbon dioxide on production and hatchability of eggs of Plodia interpunctella (Lepidoptera: Phycitidae). Ann. Entomol. Soc. Am. 1972, 65, 976–977. [Google Scholar] [CrossRef]
- Janisch, E. Über die experimentelle Beeinflussung der Lebensdauer und des Alterns schadlicher Insekten. I. Mitt. Arb. Biol. Abt. 1924, 13, 173–195. (In German) [Google Scholar]
- Ofuya, T.I.; Reichmuth, C. Control of two bruchid pests of stored grain legumes in a nitrogen atmosphere. Crop Prot. 1993, 12, 394–396. [Google Scholar] [CrossRef]
- Woods, H.A.; Bonnecaze, R.T.; Zrubek, B. Oxygen and water flux across eggshells of Manduca sexta. J. Exp. Biol. 2005, 208, 1297–1308. [Google Scholar] [CrossRef] [PubMed]
- Murdock, L.L.; Baoua, I.B. On Purdue improved crop storage (PICS) technology: Background, mode of action, future prospects. J. Stored Prod. Res. 2014, 58, 3–11. [Google Scholar] [CrossRef]
| Day | Eggs Number | No. of Parent Adults Alive | First Adult Emerged (days) | Total Emerged Adults (60 days) | Percentage Emerged (%) |
|---|---|---|---|---|---|
| 1 | 14.8 ± 1.6 | 10 | 47 | 7.6 ± 1.2 | 51.4 ± 6.2 |
| 2 | 10.4 ± 1.2 | 10 | 47 | 6.9 ± 1.0 | 66.4 ± 5.9 |
| 3 | 8.5 ± 1.2 | 10 | 48 | 5.5 ± 0.9 | 64.7 ± 3.4 |
| 4 | 6.0 ± 1.8 | 10 | 49 | 2.9 ± 0.9 | 48.3 ± 9.2 |
| 5 | 3.0 ± 1.1 | 7 | 49 | 1.7 ± 0.6 | 56.7 ± 11.5 |
| 6 | 2.1 ± 1.2 | 5 | 54 | 0.6 ± 0.3 | 28.6 ± 7.2 |
| 7 | 0.4 ± 0.3 | 4 | NA | 0 ± 0 | 0.0 ± 0 |
| 8 | 0.2 ± 0.1 | 3 | NA | 0 ± 0 | 0.0 ± 0 |
| 9 | 0.0 ± 0.0 | 0 | NA | 0 ± 0 | 0.0 ± 0 |
| Oxygen (%) | Exposure (h) | t-Value | d.f. | p |
|---|---|---|---|---|
| 2 | 48 | −5.18 | 13 | <0.001 |
| 72 | −5.18 | 11 | <0.001 | |
| 5 | 48 | −5.25 | 20 | <0.001 |
| 72 | −9.73 | 18 | <0.001 | |
| 10 | 48 | 1.84 | 16 | 0.085 |
| 72 | 0.70 | 21 | 0.492 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.; Williams, S.B.; Baributsa, D.; Murdock, L.L. Hypoxia Treatment of Callosobruchus maculatus Females and Its Effects on Reproductive Output and Development of Progeny Following Exposure. Insects 2016, 7, 26. https://doi.org/10.3390/insects7020026
Yan Y, Williams SB, Baributsa D, Murdock LL. Hypoxia Treatment of Callosobruchus maculatus Females and Its Effects on Reproductive Output and Development of Progeny Following Exposure. Insects. 2016; 7(2):26. https://doi.org/10.3390/insects7020026
Chicago/Turabian StyleYan, Yan, Scott B. Williams, Dieudonne Baributsa, and Larry L. Murdock. 2016. "Hypoxia Treatment of Callosobruchus maculatus Females and Its Effects on Reproductive Output and Development of Progeny Following Exposure" Insects 7, no. 2: 26. https://doi.org/10.3390/insects7020026







