Pheromone Autodetection: Evidence and Implications
Abstract
:1. Introduction
1.1. Background
1.2. History of Anosmia vs. Autodetection
2. Types of Evidence
2.1. Criterion for Selecting Literature
2.2. Sexual Dimorphism of Antennae
2.3. Behavioral Observations
2.4. Electroantennogram (EAG) and Single-Sensillum Recording (SSR)
2.5. Pheromone-Binding- and Pheromone-Receptor- Proteins in Female Antennae
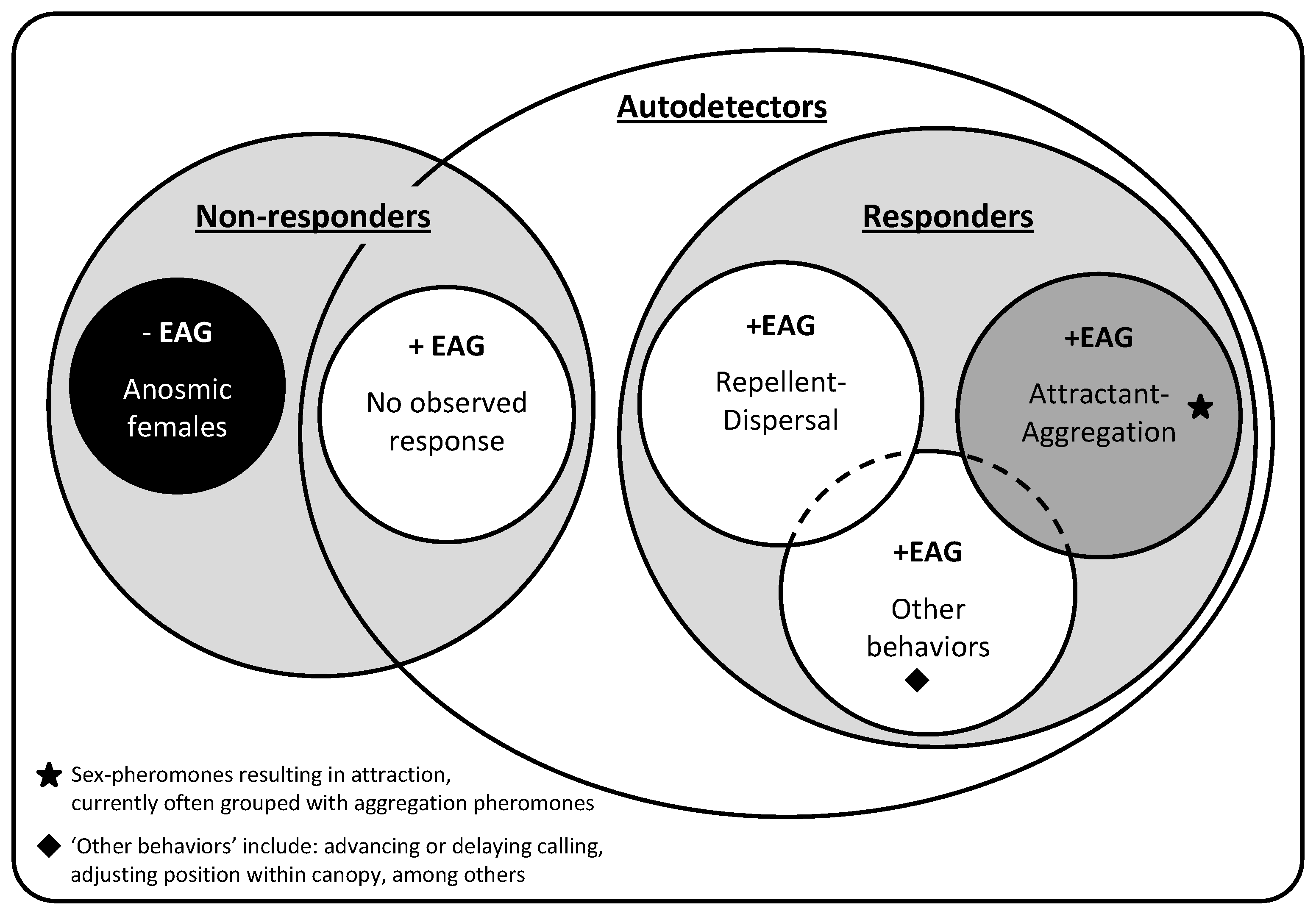
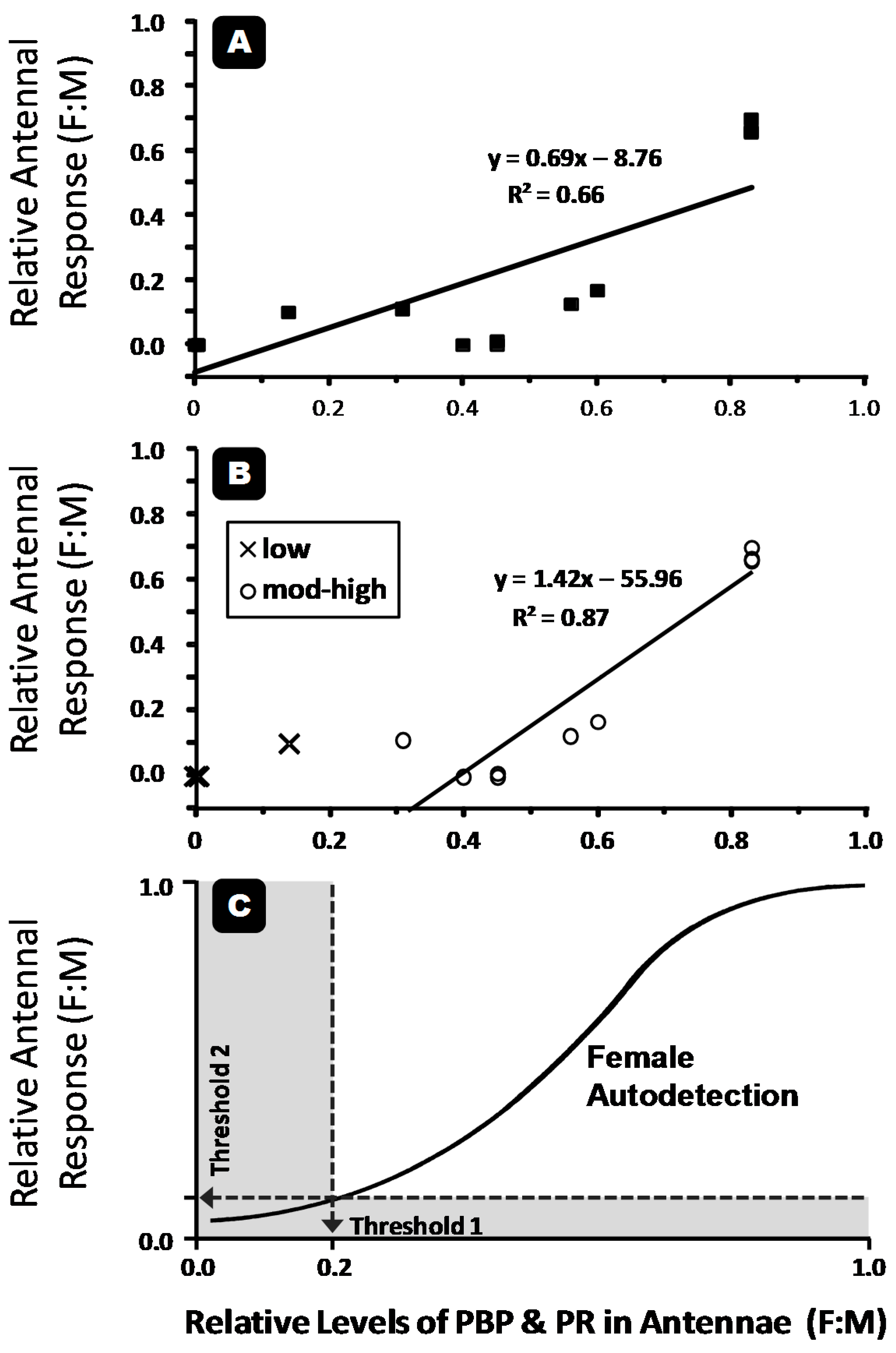
3. Relationships and Patterns
4. Selection Pressures Favoring Autodetection
4.1. Resource Limitation and Plume Competition
4.2. Cooperative and Dishonest Strategies in Aggregations
4.3. Effect of Movement on Female Fecundity and Progeny Survival
5. Implications of Autodetection for Pheromone Use in Agriculture
5.1. Pheromone-Baited Monitoring Traps
5.2. Pheromone Mating Disruption
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| EAG | Electroantennogram |
| SSR | Single-sensillum recording |
| CR | Cardiac response |
| GR | Glomerular response |
| PBP | Pheromone-binding protein |
| PR | Pheromone receptor protein |
References
- Cardé, R.; Baker, T. Sexual communication with pheromones. In Chemical Ecology of Insects; Bell, W., Cardé, R., Eds.; Springer: New York, NY, USA, 1984; pp. 355–383. [Google Scholar]
- Butenandt, A.; Beckmann, R.; Stamm, D.; Hecker, E. über den sexuallockstoff des seidenspinners Bombyx mori, Reindarstellung and konstitution. Z. Naturforsc. 1959, 14b, 283–284. (In German) [Google Scholar]
- Schneider, D. Electrophysiological investigation on the antennal receptors of the silk moth during chemical and mechanical stimulation. Experientia 1957, 13, 89–91. [Google Scholar] [CrossRef]
- Schneider, D. Electrophysiological investigation on the olfactory specificity of sexual attracting substances in different species of moths. J. Insect Physiol. 1962, 8, 15–30. [Google Scholar] [CrossRef]
- Roelofs, W. Electroantennogram assays: Rapid and convenient screening procedures for pheromones. In Techniques in Pheromone Research; Hummel, H., Miller, T., Eds.; Springer: New York, NY, USA, 1984; pp. 131–159. [Google Scholar]
- Schneider, D.; Schulz, S.; Priesner, E.; Ziesmann, J.; Francke, W. Autodetection and chemistry of female and male pheromone in both sexes of the tiger moth Panaxia. quadripunctaria. J. Comp. Physiol. A 1998, 182, 153–161. [Google Scholar] [CrossRef]
- Mitchell, E.R.; Webb, J.C.; Hines, R.W. Capture of male and female cabbage loopers in field traps baited with synthetic sex pheromone. Environ. Entomol. 1972, 4, 525–526. [Google Scholar] [CrossRef]
- Landolt, P.J.; Heath, R.R. Sexual role reversal in mate-finding strategies of the cabbage looper moth. Science 1990, 249, 1026–1028. [Google Scholar] [CrossRef] [PubMed]
- Heath, R.R.; Landolt, P.J.; Dueben, B.D.; Murphy, R.E.; Schneider, R.E. Identification of male cabbage looper sex pheromone attractive to females. J. Chem. Ecol. 1992, 18, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Birch, M.C. Response of both sexes of Trichoplusia ni (Lepidoptera: Noctuidae) to virgin females and to synthetic pheromone. Ecol. Entomol. 1977, 2, 99–104. [Google Scholar] [CrossRef]
- Larsdotter-Mellström, H.; Eriksson, K.; Janz, N.; Nylin, S.; Carlsson, M.A. Male butterflies use an anti-aphrodisiac pheromone to tailor ejaculates. Funct. Ecol. 2015. [Google Scholar] [CrossRef]
- Cuperus, P.L.; Thomas, G.; Den Otter, C.J. Interspecific variation and sexual dimorphism of antennal receptor morphology, in European Yponomeuta (Latreille) (Lepidoptera: Yponomeutidae). Int. J. Insect Morphol. Embryol. 1983, 12, 67–78. [Google Scholar] [CrossRef]
- Symonds, M.R.E.; Johnson, T.L.; Elgar, M.A. Pheromone production, male abundance, body size, and the evolution of elaborate antennae in moths. Ecol. Evol. 2012, 2, 227–246. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, R.N.; Rubin, R.E.; Mcfarland, S.U.; Shorey, H.H. Sex pheromones of noctuid moths. XXII. The external morphology of the antennae of Trichoplusia ni, Heliothis zea, Prodenia ornithogalli, and Spodoptera exigua. Ann. Entomol. Soc. Am. 1970, 63, 1227–1238. [Google Scholar]
- Hillier, N.K.; Kleineidam, C.; Vickers, N.J. Physiology and glomerular projections of olfactory receptor neurons on the antenna of female Heliothis virescens (Lepidoptera: Noctuidae) responsive to behaviorally relevant odors. J. Comp. Physiol. A 2006, 192, 199–219. [Google Scholar] [CrossRef] [PubMed]
- Hillier, N.K.; Kavanagh, R.M.B. Differential octopaminergic modulation of olfactory receptor neuron responses to sex pheromones in Heliothis virescens. PLoS ONE 2015, 10, e0143179. [Google Scholar] [CrossRef] [PubMed]
- Palaniswamy, P.; Seabrook, W.D. Behavioral responses of the female eastern spruce budworm Choristoneura fumiferana (Lepidoptera, Tortricidae) to the sex pheromone of her own species. J. Chem. Ecol. 1978, 4, 649–655. [Google Scholar] [CrossRef]
- Sanders, C.J. Flight and copulation of female spruce budworm in pheromone-permeated air. J. Chem. Ecol. 1987, 13, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.D.; Scott, D.R. Repellency of pheromones released by females of Heliothis armigera and H. zea to females of both species. Entomol. Exp. Appl. 1981, 30, 123–127. [Google Scholar] [CrossRef]
- Ellis, P.E.; Brimacombe, L.C.; Mcveigh, L.J.; Dignan, A. Laboratory experiments on the disruption of mating in the Egyptian cotton leafworm Spodoptera littoralis (Boisduval) (Lepidoptera: Noctuidae) by excesses of female pheromones. Bull. Entomol. Res. 1980, 70, 673–684. [Google Scholar] [CrossRef]
- Pearson, G.A. Pheromone Effects on Mating Success and Female Behavior in the Grape Root Borer. Ph.D. Thesis, North Carolina State University, Raleigh, NC, USA, 1992. [Google Scholar]
- Trematerra, P.; Battaini, F. Control of Ephestia kuehniella (Zeller) by mass-trapping. J. Appl. Entomol. 1987, 104, 336–340. [Google Scholar] [CrossRef]
- Stelinski, L.; Holdcraft, R.; Rodriguez-Saona, C. Female moth calling and flight behavior are altered hours following pheromone autodetection: Possible implications for practical management with mating disruption. Insects 2014, 5, 459–473. [Google Scholar] [CrossRef] [PubMed]
- Pearson, G.A.; Dillery, S.; Meyer, J.R. Modeling intra-sexual competition in a sex pheromone system: How much can female movement affect female mating success? J. Theor. Biol. 2004, 231, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, H.; Tamaki, Y. Conspecific female-sex pheromone delays calling behavior of Adoxophyes sp. and Homona magnanima (Ledipoptera: Tortricidae). Jpn. J. Appl. Entomol. Zool. 1985, 29, 113–118. [Google Scholar] [CrossRef]
- Gokce, A.; Stelinski, L.L.; Gut, L.J.; Whalon, M.E. Comparative behavioral and EAG responses of female obliquebanded and redbanded leafroller moths (Lepidoptera: Tortricidae) to their sex pheromone components. Eur. J. Entomol. 2007, 104, 187–194. [Google Scholar] [CrossRef]
- Palaniswamy, P.; Seabrook, W.D. The alteration of calling behaviour by female Choristoneura fumiferana when exposed to synthetic sex pheromone. Entomol. Exp. Appl. 1985, 37, 13–16. [Google Scholar] [CrossRef]
- Kuhns, E.; Pelz-Stelinski, K.; Stelinski, L. Reduced mating success of female tortricid moths following intense pheromone auto-exposure varies with sophistication of mating system. J. Chem. Ecol. 2012, 38, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Den Otter, C.J.; de Cristofaro, A.; Voskamp, K.E.; Rotundo, G. Electrophysiological and behavioural responses of chestnut moths, Cydia fagiglandana and C. splendana (Lep., Tortricidae), to sex attractants and odours of host plants. J. Appl. Entomol. 1996, 120, 413–421. [Google Scholar] [CrossRef]
- Weissling, T.J.; Knight, A.L. Oviposition and calling behavior of codling moth (Lepidoptera: Tortricidae) in the presence of codlemone. Ann. Entomol. Soc. Am. 1996, 89, 142–147. [Google Scholar] [CrossRef]
- El-Sayed, A.M.; Suckling, D.M. Behavioural observations of mating disruption in three lepidopteran pests. Behaviour 2005, 142, 717–729. [Google Scholar] [CrossRef]
- Stelinski, L.L.; Il’ichev, A.L.; Gut, L.J. Antennal and behavioral responses of virgin and mated oriental fruit moth (Lepidoptera: Tortricidae) females to their sex pheromone. Ann. Entomol. Soc. Am. 2006, 99, 898–904. [Google Scholar] [CrossRef]
- DeLury, N.C.; Judd, G.J.R.; Gardiner, M.G.T. Antennal detection of sex pheromone by female Pandemis limitata (Robinson) (Lepidoptera: Tortricidae) and its impact on their calling behaviour. J. Entmol. Soc. B. C. 2005, 102, 3–12. [Google Scholar]
- Groot, A.T.; Classen, A.; Staudacher, A.; Schal, C.; Heckel, D.G. Phenotypic plasticity in sexual communication signal of a noctuid moth. J. Evol. Biol. 2010, 23, 2731–2738. [Google Scholar] [CrossRef] [PubMed]
- Rehermann, G.; Altesor, P.; McNeil, J.N.; González, A. Conspecific females promote calling behaviour in the noctuid moth, Pseudaletia adultera. Entomol. Exp. Appl. 2016, in press. [Google Scholar]
- Cruz, D.; Eizaguirre, M. Response to conspecific and heterospecific semiochemicals by Sesamia nonagrioides (L.) (Lepidoptera: Noctuidae) gravid females. Bull. Entomol. Res. 2015, 105, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-W.; Dong, S.-L.; Chen, L. Electrophysiological and behavioral responses of female beet armyworm Spodoptera exigua (Hübner) to the conspecific female sex pheromone. J. Insect Behav. 2009, 22, 153–164. [Google Scholar] [CrossRef]
- Sadek, M.M.; Von Wowern, G.; Löfstedt, C.; Rosén, W.-Q.; Anderson, P. Modulation of the temporal pattern of calling behavior of female Spodoptera littoralis by exposure to sex pheromone. J. Insect Physiol. 2012, 58, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Greenfield, M.D. Female pheromonal chorusing in an arctiid moth, Utetheisa ornatrix. Behav. Ecol. 2007, 18, 165–173. [Google Scholar] [CrossRef]
- Lim, H.; Greenfield, M.D. Female arctiid moths, Utetheisa ornatrix, orient towards and join pheromonal choruses. Anim. Behav. 2008, 75, 673–680. [Google Scholar] [CrossRef]
- Lim, H.; Park, K.; Baker, T.; Greenfield, M. Perception of conspecific female pheromone stimulates female calling in an Arctiid moth, Utetheisa ornatrix. J. Chem. Ecol. 2007, 33, 1257–1271. [Google Scholar] [CrossRef] [PubMed]
- Pearson, G.A. Sesiid pheromone increases squash vine borer (Lepidoptera: Sesiidae) infestation. Environ. Entomol. 1995, 24, 1627–1632. [Google Scholar] [CrossRef]
- Leal, W.; Hasegawa, M.; Sawada, M.; Ono, M. Sex pheromone of oriental beetle, Exomala orientalis: Identification and field evaluation. J. Chem. Ecol. 1994, 20, 1705–1718. [Google Scholar] [CrossRef] [PubMed]
- Holdcraft, R.; Rodriguez-Saona, C.; Marucci Center for Blueberry and Cranberry Research and Extension, Rutgers University, Chatsworth, NJ 08019, USA. Unpublished data. 2011.
- Tamaki, Y.; Sugie, H.; Noguchi, H. Methyl (Z)-5-Tetradecenoate: Sex-Attractant Pheromone of the Soybean Beetle, Anomala rufocuprea (Motschulsky) (Coleoptera: Scarabaeidae). Appl. Entomol. Zool. 1985, 20, 359–361. [Google Scholar]
- Imai, T.; Tsuchiya, S.; Maekawa, M.; Fujimori, T.; Leal, S.W. Methyl anthranilate, a novel attractant for the soybean beetle, Anomala rufocuprea (Motschulsky) (Coleoptera: Scarabaeidae). Appl. Entomol. Zool. 1997, 32, 45–48. [Google Scholar]
- Domek, J.M.; Johnson, D.T. Evidence of a sex pheromone in the green June beetle, Cotinis nitida (Coleoptera: Scarabaeidae). J. Entomol. Sci. 1987, 22, 264–267. [Google Scholar]
- Domek, J.M.; Johnson, D.T. Demonstration of semiochemically induced aggregation in the green June beetle, Cotinis nitida (L.) (Coleoptera: Scarabaeidae). Environ. Entomol. 1988, 17, 147–149. [Google Scholar] [CrossRef]
- Leal, W.; Yadava, C.S.; Vijayvergia, J. Aggregation of the scarab beetle Holotrichia consanguinea in response to female-released pheromone suggests secondary function hypothesis for semiochemical. J. Chem. Ecol. 1996, 22, 1557–1566. [Google Scholar] [CrossRef] [PubMed]
- Arakaki, N.; Wakamura, S.; Yasui, H.; Sadoyama, Y.; Kishita, M. Sexually differentiated functions of female-produced pheromone of the black chafer Holotrichia loochooana loochooana (Sawada) (Coleoptera: Scarabaeidae). Chemoecology 2003, 13, 183–186. [Google Scholar] [CrossRef]
- Yasui, H.; Fukaya, M.; Wakamura, S.; Akino, T.; Yasuda, T.; Kobayashi, A.; Arakaki, N. Aggregation of the black chafer Holotrichia loochooana loochooana (Sawada) (Coleoptera: Scarabaeidae): Function of female pheromone and possible adaptive significance. Appl. Entomol. Zool. 2007, 42, 507–515. [Google Scholar] [CrossRef]
- Ganeshaiah, K.N.; Kumar, A.R.V. Self organization and chemically mediated aggregation of adults in the whitegrub, Holotichia serrata. In Chemical Ecology of Phytophagous Insects; Ananthakrishnan, T.N., Raman, A., Eds.; Oxford and IBH Publishing Co.: New Delhi, India, 1993; pp. 167–178. [Google Scholar]
- Yarden, G.; Shani, A. Evidence for volatile chemical attractants in the beetle Maladera matrida (argaman) (Coleoptera: Scarabaeidae). J. Chem. Ecol. 1994, 20, 2673–2685. [Google Scholar] [CrossRef] [PubMed]
- Falach, L.; Shani, A. Trapping efficiency and sex ratio of Maladera matrida beetles in yellow and black Traps. J. Chem. Ecol. 2000, 26, 2619–2624. [Google Scholar] [CrossRef]
- Baker, R.; Longhurst, C. Chemical control of insect behaviour. Philos. T. Roy. Soc. B 1981, 295, 73–82. [Google Scholar]
- Ross, M.H.; Tignor, K.R. Response of German cockroaches to a dispersant and other substances secreted by crowded adults and nymphs (Blattodea: Blattelidae). Proc. Entomol. Soc. Wash. 1986, 88, 25–29. [Google Scholar]
- Shields, V.D.C.; Hildebrand, J.G. Responses of a population of antennal olfactory receptor cells in the female moth Manduca sexta to plant-associated volatile organic compounds. J. Comp. Physiol. A 2001, 186, 1135–1151. [Google Scholar] [CrossRef]
- Kalinová, B.; Hoskovec, M.; Liblikas, I.; Unelius, C.R.; Hansson, B.S. Detection of sex pheromone components in Manduca sexta (L.). Chem. Senses 2001, 26, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Den Otter, C.J.; Schuil, H.A.; Oosten, A.S.-V. Reception of host-plant odours and female sex pheromone in Adoxophyes orana (Lepidoptera: Tortricidae): Electrophysiology and morphology. Entomol. Exp. Appl. 1978, 24, 570–578. [Google Scholar] [CrossRef]
- Roelofs, W.L.; Comeau, A. Sex pheromone perception: Electroantennogram responses of the red-banded leaf roller moth. J. Insect Physiol. 1971, 17, 1969–1982. [Google Scholar] [CrossRef]
- Palaniswamy, P.; Sivasubramanian, P.; Seabrook, W.D. Modulation of sex pheromone perception in female moths of the eastern spruce budworm, Choristoneura fumiferana by altosid. J. Insect Physiol. 1979, 25, 571–574. [Google Scholar] [CrossRef]
- Ross, R.J.; Palaniswamy, P.; Seabrook, W.D. Electroantennograms from spruce budworm moths (Choristoneura fumiferana) (Lepidoptera: Tortricidae) of different ages and for various pheromone concentrations. Can. Entomol. 1979, 111, 807–816. [Google Scholar] [CrossRef]
- Ansebo, L.; Coracini, M.D.A.; Bengtsson, M.; Liblikas, I.; Ramírez, M.; Borg-Karlson, A.K.; Tasin, M.; Witzgall, P. Antennal and behavioural response of codling moth Cydia pomonella to plant volatiles. J. Appl. Entomol. 2004, 128, 488–493. [Google Scholar] [CrossRef]
- Ansebo, L.; Ignell, R.; Löfqvist, J.; Hansson, B.S. Responses to sex pheromone and plant odours by olfactory receptor neurons housed in sensilla auricillica of the codling moth, Cydia pomonella (Lepidoptera: Tortricidae). J. Insect Physiol. 2005, 51, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Hansson, B.S.; Pers, J.N.C.V.D.; Löfqvist, J.N. Comparison of male and female olfactory cell response to pheromone compounds and plant volatiles in the turnip moth, Agrotis. segetum. Physiol. Entomol. 1989, 14, 147–155. [Google Scholar] [CrossRef]
- Nesbitt, B.F.; Beevor, P.S.; Cole, R.A.; Lestor, R.; Poppi, R.G. Sex pheromones of two noctuid moths. Nat. New Biol. 1973, 244, 208–209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-G.; Maida, R.; Steinbrecht, R.A. Immunolocalization of odorant-binding proteins in noctuid moths (Insecta, Lepidoptera). Chem. Senses 2001, 26, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Christensen, T.A.; Geoffrion, S.C.; Hildebrand, J.G. Physiology of interspecific chemical communication in Heliothis moths. Physiol. Entomol. 1990, 15, 275–283. [Google Scholar] [CrossRef]
- Groot, A.; Gemeno, C.; Brownie, C.; Gould, F.; Schal, C. Male and female antennal responses in Heliothis virescens and H. subflexa to conspecific and heterospecific sex pheromone compounds. Environ. Entomol. 2005, 34, 256–263. [Google Scholar] [CrossRef]
- Almaas, T.; Mustaparta, H. Heliothis virescens: Response characteristics of receptor neurons in sensilla trichodea type 1 and type 2. J. Chem. Ecol. 1991, 17, 953–972. [Google Scholar] [CrossRef] [PubMed]
- Angioy, A.M.L.; Desogus, A.; Hansson, B.S. Importance of sex pheromone autodetection in female moths. Soc. Neurosci. Abstr. 2001, 308, 9. [Google Scholar]
- Acín, P.; Carrascal, M.; Abián, J.; Guerrero, A.; Quero, C. Expression of differential antennal proteins in males and females of an important crop pest, Sesamia nonagrioides. Insect Biochem. Mol. Biol. 2009, 39, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Dickens, J.C.; Visser, J.H.; van der Pers, J.N.C. Detection and deactivation of pheromone and plant odor components by the beet armyworm, Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae). J. Insect Physiol. 1993, 39, 503–516. [Google Scholar] [CrossRef]
- Malo, E.A.; Castrej, N.G.; Mez, V.R.; Cruz, L.; Pez, L.; Rojas, J.C. Antennal sensilla and electrophysiological response of male and female Spodoptera frugiperda (Lepidoptera: Noctuidae) to conspecific sex pheromone and plant odors. Ann. Entomol. Soc. Am. 2004, 97, 1273–1284. [Google Scholar] [CrossRef]
- Ljungberg, H.; Anderson, P.; Hansson, B.S. Physiology and morphology of pheromone-specific sensilla on the antennae of male and female Spodoptera littoralis (Lepidoptera: Noctuidae). J. Insect Physiol. 1993, 39, 253–260. [Google Scholar] [CrossRef]
- Anderson, P.; Hansson, B.S.; Löfqvist, J. Plant-odour-specific receptor neurones on the antennae of female and male Spodoptera. littoralis. Physiol. Entomol. 1995, 20, 189–198. [Google Scholar] [CrossRef]
- Light, D.M.; Birch, M.C. Electrophysiological basis for the behavioural response of male and female Trichoplusia ni to synthetic female pheromone. J. Insect Physiol. 1979, 25, 161–167. [Google Scholar] [CrossRef]
- Seabrook, W.D.; Linn, C.E.; Dyer, L.J.; Shorey, H.H. Comparison of electroantennograms from female and male cabbage looper moths (Trichoplusia ni) of different ages and for various pheromone concentrations. J. Chem. Ecol. 1987, 13, 1443–1453. [Google Scholar] [CrossRef] [PubMed]
- Todd, J.L.; Baker, T.C. Response of single antennal neurons of female cabbage loopers to behaviorally active attractants. Naturwissenschaften 1993, 80, 183–186. [Google Scholar] [CrossRef]
- Grant, A.J.; Connell, R.J. Responses of olfactory receptor neurons in Utetheisa ornatrix to gender-specific odors. J. Comp. Physiol. A 2000, 186, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Bouwer, M.C.; Slippers, B.; Degefu, D.; Wingfield, M.J.; Lawson, S.; Rohwer, E.R. Identification of the sex pheromone of the tree infesting Cossid moth Coryphodema tristis (Lepidoptera: Cossidae). PLoS ONE 2015, 10, e0118575. [Google Scholar] [CrossRef] [PubMed]
- Pearson, G.A.; Schal, C. Electroantennogram responses of both sexes of grape root borer (Lepidoptera: Sesiidae) to synthetic female sex pheromone. Environ. Entomol. 1999, 28, 943–946. [Google Scholar] [CrossRef]
- Van der Pers, J.N.C.; den Otter, C.J. Single cell responses from olfactory receptors of small ermine moths to sex-attractants. J. Insect Physiol. 1978, 24, 337–343. [Google Scholar] [CrossRef]
- Pophof, B.; Stange, G.; Abrell, L. Volatile organic compounds as signals in a plant–herbivore System: Electrophysiological responses in olfactory sensilla of the moth Cactoblastis cactorum. Chem. Senses 2005, 30, 51. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, E.S.; Sanes, J.R.; Hildebrand, J.G. Ontogeny of electroantennogram responses in the moth, Manduca sexta. J. Insect Physiol. 1976, 22, 955–960. [Google Scholar] [CrossRef]
- De Silva, E.C.A.; Silk, P.J.; Mayo, P.; Hillier, N.K.; Magee, D.; Cutler, G.C. Identification of sex pheromone components of blueberry spanworm Itame argillacearia (Lepidoptera: Geometridae). J. Chem. Ecol. 2013, 39, 1169–1181. [Google Scholar] [CrossRef] [PubMed]
- Daimon, T.; Fujii, T.; Fujii, T.; Yokoyama, T.; Katsuma, S.; Shinoda, T.; Shimada, T.; Ishikawa, Y. Reinvestigation of the sex pheromone of the wild silkmoth Bombyx mandarina: The effects of bombykal and bombykyl acetate. J. Chem. Ecol. 2012, 38, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Boeckh, J.; Kaissling, K.E.; Schneider, D. Insect olfactory receptors. Cold Spring Harbor Symp. Quant. Biol. 1965, 30, 263–280. [Google Scholar] [CrossRef] [PubMed]
- Maida, R.; Ziesmann, J. Female Attacus atlas respond to pheromones of Antheraea polyphemus: A comparative electrophysiological and biochemical study. Chem. Senses 2001, 26, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Leal, W.; Mochizuki, F.; Wakamura, S.; Yasuda, T. Electroantennographic detection of Anomala cuprea (Hope) (Coleoptera: Scarabaeidae) sex pheromone. Appl. Entomol. Zool. 1992, 27, 289–291. [Google Scholar]
- Larsson, M.C.; Leal, W.S.; Hansson, B.S. Olfactory receptor neurons specific to chiral sex pheromone components in male and female Anomala cuprea beetles (Coleoptera: Scarabaeidae). J. Comp. Physiol. A 1999, 184, 353–359. [Google Scholar] [CrossRef]
- Larsson, M.C.; Leal, W.S.; Hansson, B.S. Olfactory receptor neurons detecting plant odours and male volatiles in Anomala cuprea beetles (Coleoptera: Scarabaeidae). J. Insect Physiol. 2001, 47, 1065–1076. [Google Scholar] [CrossRef]
- Leal, W.; Hasegawa, M.; Sawada, M.; Ono, M.; Ueda, Y. Identification and field evaluation of Anomala octiescostata (Coleoptera: Scarabaeidae) sex pheromone. J. Chem. Ecol. 1994, 20, 1643–1655. [Google Scholar] [CrossRef] [PubMed]
- Nikonov, A.A.; Peng, G.; Tsurupa, G.; Leal, W.S. Unisex pheromone detectors and pheromone-binding proteins in scarab beetles. Chem. Senses 2002, 27, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Subaharan, K.; Kumar, A.R.V.; Ganiger, P. Electrophysiological responses of chafer beetle, Holotrichia serrata (F.) (Coleoptera: Scarabaeidae). J. Saudi Soc. Agric. Sci. 2013, 12, 155–159. [Google Scholar] [CrossRef]
- Washio, H.; Nishino, C.; Bowers, W.S. Antennal receptor response to sex pheromone mimics in the American cockroach. Nature 1976, 262, 487–489. [Google Scholar] [CrossRef] [PubMed]
- Nishino, H.; Iwasaki, M.; Mizunami, M. Pheromone detection by a pheromone emitter: A small sex pheromone-specific processing system in the female American cockroach. Chem. Senses 2011, 36, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Kanaujia, S.; Kaissling, K.E. Interactions of pheromone with moth antennae: Adsorption, desorption and transport. J. Insect Physiol. 1985, 31, 71–81. [Google Scholar] [CrossRef]
- Breer, H. Molecular Mechanisms of Pheromone Reception in Insect Antennae. In Insect Pheromone Research; Cardé, R., Minks, A., Eds.; Springer: New York, NY, USA, 1997; pp. 115–130. [Google Scholar]
- Vogt, R.G.; Riddiford, L.M. Pheromone binding and inactivation by moth antennae. Nature 1981, 293, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Györgyi, T.K.; Roby-Shemkovitz, A.J.; Lerner, M.R. Characterization and cDNA cloning of the pheromone-binding protein from the tobacco hornworm, Manduca sexta: A tissue-specific developmentally regulated protein. Proc. Nat. Acad. Sci. USA 1988, 85, 9851–9855. [Google Scholar] [CrossRef] [PubMed]
- Steinbrecht, R.A.; Laue, M.; Ziegelberger, G. Immunolocalization of pheromone-binding protein and general odorant-binding protein in olfactory sensilla of the silk moths Antheraea and Bombyx. Cell. Tissue Res. 1995, 282, 203–217. [Google Scholar] [CrossRef]
- Krieger, J.; Große-Wilde, E.; Gohl, T.; Breer, H. Candidate pheromone receptors of the silkmoth Bombyx mori. Eur. J. Neurosci. 2005, 21, 2167–2176. [Google Scholar] [CrossRef] [PubMed]
- Forstner, M.; Breer, H.; Krieger, J. A receptor and binding protein interplay in the detection of a distinct pheromone component in the silkmoth Antheraea polyphemus. Int. J. Biol. Sci. 2009, 5, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, J.M.; Trona, F.; Montagne, N.; Anfora, G.; Ignell, R.; Witzgall, P.; Jacquin-Joly, E. Putative chemosensory receptors of the codling moth, Cydia pomonella, identified by antennal transcriptome analysis. PLoS ONE 2012, 7, e31620. [Google Scholar] [CrossRef] [PubMed]
- Picimbon, J.F.; Gadenne, C. Evolution of noctuid pheromone binding proteins: Identification of PBP in the black cutworm moth, Agrotis ipsilon. Insect Biochem. Mol. Biol. 2002, 32, 839–846. [Google Scholar] [CrossRef]
- Gu, S.-H.; Sun, L.; Yang, R.-N.; Wu, K.-M.; Guo, Y.-Y.; Li, X.-C.; Zhou, J.-J.; Zhang, Y.-J. Molecular characterization and differential expression of olfactory genes in the antennae of the black cutworm moth Agrotis ipsilon. PLoS ONE 2014, 9, e103420. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.-H.; Zhou, J.-J.; Wang, G.-R.; Zhang, Y.-J.; Guo, Y.-Y. Sex pheromone recognition and immunolocalization of three pheromone binding proteins in the black cutworm moth Agrotis ipsilon. Insect Biochem. Mol. Biol. 2013, 43, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Steinbrecht, R.A.; Ozaki, M.; Ziegelberger, G. Immunocytochemical localization of pheromone-binding protein in moth antennae. Cell. Tissue Res. 1992, 270, 287–302. [Google Scholar] [CrossRef]
- Zhang, T.; Gu, S.; Wu, K.; Zhang, Y.; Guo, Y. Construction and analysis of cDNA libraries from the antennae of male and female cotton bollworms Helicoverpa. armigera (Hübner) and expression analysis of putative odorant-binding protein genes. Biochem Biophys. Res. Commun. 2011, 407, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gu, S.; Zhang, Y.; Guo, Y.; Wang, G. Candidate olfaction genes identified within the Helicoverpa. armigera antennal transcriptome. PLoS ONE 2012, 7, e48260. [Google Scholar] [CrossRef] [PubMed]
- Callahan, F.E.; Vogt, R.G.; Tucker, M.L.; Dickens, J.C.; Mattoo, A.K. High level expression of “male specific” pheromone binding proteins (PBPs) in the antennae of female noctuiid moths. Insect Biochem. Mol. Biol. 2000, 30, 507–514. [Google Scholar] [CrossRef]
- Krieger, J.; Gänβle, H.; Raming, K.; Breer, H. Odorant binding proteins of Heliothis virescens. Insect Biochem. Mol. Biol. 1993, 23, 449–456. [Google Scholar] [CrossRef]
- Krieger, J.; Grosse-Wilde, E.; Gohl, T.; Dewer, Y.M.E.; Raming, K.; Breer, H. Genes encoding candidate pheromone receptors in a moth (Heliothis virescens). Proc. Nat. Acad. Sci. USA 2004, 101, 11845–11850. [Google Scholar] [CrossRef] [PubMed]
- Krieger, J.; Gondesen, I.; Forstner, M.; Gohl, T.; Dewer, Y.; Breer, H. Hr11 and hr13 receptor-expressing neurons are housed together in pheromone-responsive sensilla trichodea of male Heliothis virescens. Chem. Senses 2009, 34, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Nagnan-Le Meillour, P.; Huet, J.-C.; Maibeche, M.; Pernollet, J.-C.; Descoins, C. Purification and characterization of multiple forms of odorant/pheromone binding proteins in the antennae of Mamestra brassicae (Noctuidae). Insect Biochem. Mol. Biol. 1996, 26, 59–67. [Google Scholar] [CrossRef]
- Maïbèche-Coisne, M.; Sobrio, F.; Delaunay, T.; Lettere, M.; Dubroca, J.; Jacquin-Joly, E.; Meillour, P.N.-L. Pheromone binding proteins of the moth Mamestra brassicae: Specificity of ligand binding. Insect Biochem. Mol. Biol. 1997, 27, 213–221. [Google Scholar]
- Mitsuno, H.; Sakurai, T.; Murai, M.; Yasuda, T.; Kugimiya, S.; Ozawa, R.; Toyohara, H.; Takabayashi, J.; Miyoshi, H.; Nishioka, T. Identification of receptors of main sex-pheromone components of three Lepidopteran species. Eur. J. Neurosci. 2008, 28, 893–902. [Google Scholar] [CrossRef] [PubMed]
- De Santis, F.; François, M.-C.; Merlin, C.; Pelletier, J.; Maïbèche-Coisné, M.; Conti, E.; Jacquin-Joly, E. Molecular cloning and in situ expression patterns of two new pheromone-binding proteins from the corn stemborer Sesamia nonagrioides. J. Chem. Ecol. 2006, 32, 1703–1717. [Google Scholar] [CrossRef] [PubMed]
- Xiu, W.-M.; Dong, S.-L. Molecular characterization of two pheromone binding proteins and quantitative analysis of their expression in the beet armyworm, Spodoptera exigua (Hübner). J. Chem. Ecol. 2007, 33, 947–961. [Google Scholar] [CrossRef] [PubMed]
- Legeai, F.; Malpel, S.; Montagne, N.; Monsempes, C.; Cousserans, F.; Merlin, C.; Francois, M.C.; Maibeche-Coisne, M.; Gavory, F.; Poulain, J.; et al. An expressed sequence tag collection from the male antennae of the noctuid moth Spodoptera littoralis: A resource for olfactory and pheromone detection research. BMC Genom. 2011, 12, 1471–2164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiu, W.-M.; Zhou, Y.-Z.; Dong, S.-L. Molecular characterization and expression pattern of two pheromone-binding proteins from Spodoptera litura (Fabricius). J. Chem. Ecol. 2008, 34, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Liu, Y.; Walker, W.B.; Liu, C.; Lin, K.; Gu, S.; Zhang, Y.; Zhou, J.; Wang, G. Identification and characterization of pheromone receptors and interplay between receptors and pheromone binding proteins in the diamondback moth, Plutella xyllostella. PLoS ONE 2013, 8, e62098. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.J.; Zhou, W.W.; Yu, H.Z.; Mao, C.G.; Zhang, C.X.; Cheng, J.A.; Zhu, Z.R. Cloning, expression and functional analysis of a general odorant-binding protein 2 gene of the rice striped stem borer, Chilo suppressalis (Walker) (Lepidoptera: Pyralidae). Insect Mol. Biol. 2009, 18, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Liu, Y.; Wei, J.; Liao, X.; Walker, W.B.; Li, J.; Wang, G. Identification of candidate olfactory genes in Chilo suppressalis by antennal transcriptome analysis. Int. J. Biol. Sci. 2014, 10, 846–860. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.-F.; Sun, X.; Dong, H.-B.; Wang, M.-Q. Analysis of a cDNA library from the antenna of Cnaphalocrocis medinalis and the expression pattern of olfactory genes. Biochem. Biophys. Res. Commun. 2013, 433, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Vogt, R.G.; Rybczynski, R.; Lerner, M.R. Molecular cloning and sequencing of general odorant-binding proteins GOBP1 and GOBP2 from the tobacco hawk moth Manduca sexta: Comparisons with other insect OBPs and their signal peptides. J. Neurosci. 1991, 11, 2972–2984. [Google Scholar] [PubMed]
- Vogt, R.G.; Rogers, M.E.; Franco, M.-D.; Sun, M. A comparative study of odorant binding protein genes: Differential expression of the PBP1-GOBP2 gene cluster in Manduca sexta (Lepidoptera) and the organization of OBP genes in Drosophila melanogaster (Diptera). J. Exper. Biol. 2002, 205, 719–744. [Google Scholar]
- Nardi, J.B.; Miller, L.A.; Walden, K.K.; Rovelstad, S.; Wang, L.; Frye, J.C.; Ramsdell, K.; Deem, L.S.; Robertson, H.M. Expression patterns of odorant-binding proteins in antennae of the moth Manduca sexta. Cell Tissue Res. 2003, 313, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Große-Wilde, E.; Stieber, R.; Forstner, M.; Krieger, J.; Wicher, D.; Hansson, B.S. Sex-specific odorant receptors of the tobacco hornworm Manduca sexta. Front. Cell. Neurosci. 2010. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Nakagawa, T.; Mitsuno, H.; Mori, H.; Endo, Y.; Tanoue, S.; Yasukochi, Y.; Touhara, K.; Nishioka, T. Identification and functional characterization of a sex pheromone receptor in the silkmoth Bombyx mori. Proc. Nat. Acad. Sci. USA 2004, 101, 16653–16658. [Google Scholar] [CrossRef] [PubMed]
- Maida, R.; Mameli, M.; Müller, B.; Krieger, J.; Steinbrecht, R.A. The expression pattern of four odorant-binding proteins in male and female silk moths, Bombyx. mori. J. Neurocytol. 2005, 34, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Forstner, M.; Gohl, T.; Breer, H.; Krieger, J. Candidate pheromone binding proteins of the silkmoth Bombyx mori. Invertebr. Neurosci. 2006, 6, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Raming, K.; Krieger, J.; Breer, H. Molecular cloning of an insect pheromone-binding protein. FEBS Lett. 1989, 256, 215–218. [Google Scholar] [CrossRef]
- Laue, M.; Steinbrecht, R.A.; Ziegelberger, G. Immunocytochemical localization of general odorant-binding protein in olfactory sensilla of the silkmoth Antheraea polyphemus. Naturwissenschaften 1994, 81, 178–180. [Google Scholar] [CrossRef]
- Greenfield, M.D. Moth Sex Pheromones: An evolutionary perspective. Fla. Entomol. 1981, 64, 4–17. [Google Scholar] [CrossRef]
- Harari, A.R.; Steinitz, H. The evolution of female sex pheromones. Curr. Zool. 2013, 59, 569–578. [Google Scholar] [CrossRef]
- Lundberg, S.; Löfstedt, C. Intra-specific competition in the sex communication channel: A selective force in the evolution of moth pheromones? J. Theor. Biol. 1987, 125, 15–24. [Google Scholar] [CrossRef]
- Harari, A.R.; Zahavi, T.; Thiéry, D. Fitness cost of pheromone production in signaling female moths. Evolution 2011, 65, 1572–1582. [Google Scholar] [CrossRef] [PubMed]
- Ochieng, S.A.; Anderson, P.; Hansson, B.S. Antennal lobe projection patterns of olfactory receptor neurons involved in sex pheromone detection in Spodoptera littoralis (Lepidoptera: Noctuidae). Tissue Cell. 1995, 27, 221–232. [Google Scholar] [CrossRef]
- Foster, S.P.; Anderson, K.G. Sex pheromones in mate assessment: Analysis of nutrient cost of sex pheromone production by females of the moth Heliothis virescens. J. Exp. Biol. 2015, 218, 1252–1258. [Google Scholar] [CrossRef] [PubMed]
- Régnière, J.; Delisle, J.; Pureswaran, D.S.; Trudel, R. Mate-finding allee effect in spruce budworm population dynamics. Entomol. Exp. Appl. 2013, 146, 112–122. [Google Scholar] [CrossRef]
- Evenden, M.L.; Mori, B.A.; Sjostrom, D.; Roland, J. Forest tent caterpillar, Malacosoma disstria (Lepidoptera: Lasiocampidae) mate-finding behavior is greatest at intermediate population densities: Implications for interpretation of moth capture in pheromone-baited traps. Front. Ecol. Evol. 2015. [Google Scholar] [CrossRef]
- Wall, C.; Perry, J.N. Interactions between pheromone traps for the pea moth, Cydia nigricana (F.). Entomol. Exp. Appl. 1978, 24, 155–162. [Google Scholar] [CrossRef]
- Wall, C.; Perry, J.N. Effects of spacing and trap number on interactions between pea moth pheromone traps. Entomol. Exp. Appl. 1980, 28, 313–321. [Google Scholar] [CrossRef]
- Miller, J.R.; Gut, L.J.; Lame, F.M.; Stelinski, L.L. Differentiation of competitive vs. non-competitive mechanisms mediating disruption of moth sexual communication by point sources of sex pheromone (Part 1): Theory. J. Chem. Ecol. 2006, 32, 2089–2114. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.R.; Gut, L.J.; Lame, F.M.; Stelinski, L.L. Differentiation of competitive vs. non-competitive mechanisms mediating disruption of moth sexual communication by point sources of sex pheromone (Part 2): Case studies. J. Chem. Ecol. 2006, 32, 2115–2143. [Google Scholar] [CrossRef] [PubMed]
- Harari, A.; Ben-Yakir, D.; Rosen, D. Mechanism of aggregation behavior in Maladera matrida (Argaman)(Coleoptera: Scarabaeidae). J. Chem. Ecol. 1994, 20, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Hasegawa, M.; Leal, W.S. Individual variation in pheromone emission and termination patterns in female Anomala cuprea. Chemoecology 2002, 12, 121–124. [Google Scholar] [CrossRef]
- Shorey, H.H.; Mcfarland, S.U.; Gaston, L.K. Sex pheromones of noctuid moths. XIII. Changes in pheromone quantity, as related to reproductive age and mating history, in females of seven species of Noctuidae (Lepidoptera). Ann. Entomol. Soc. Am. 1968, 61, 372–376. [Google Scholar] [CrossRef]
- Schal, C.; Charlton, R.; Cardé, R. Temporal patterns of sex pheromone titers and release rates in Holomelina lamae (Lepidoptera: Arctiidae). J. Chem. Ecol. 1987, 13, 1115–1129. [Google Scholar] [CrossRef] [PubMed]
- Delisle, J. Age related changes in the calling behaviour and the attractiveness of obliquebanded leafroller virgin females, Choristoneura rosaceana, under different constant and fluctuating temperature conditions. Entomol. Exp. Appl. 1992, 63, 55–62. [Google Scholar] [CrossRef]
- Knight, A.L.; Turner, J.E. Sexual biology of Pandemis pyrusana (Lepidoptera: Tortricidae) under laboratory conditions. J. Entomol. Soc. B. C. 1998, 95, 89–94. [Google Scholar]
- Gemeno, C.; Haynes, K. Periodical and age-related variation in chemical communication system of black cutworm moth, Agrotis ipsilon. J. Chem. Ecol. 2000, 26, 329–342. [Google Scholar]
- Bailey, W.J.; Haythornthwaite, S. Risks of calling by the field cricket Teleogryllus oceanicus; potential predation by Australian long-eared bats. J. Zool. 1998, 244, 505–513. [Google Scholar] [CrossRef]
- Cade, W. Acoustically orienting parasitoids: Fly phonotaxis to cricket song. Science 1975, 190, 1312–1313. [Google Scholar] [CrossRef]
- Branco, M.; Lettere, M.; Franco, J.; Binazzi, A.; Jactel, H. Kairomonal response of predators to three pine bast scale sex pheromones. J. Chem. Ecol. 2006, 32, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- Branco, M.; Franco, J.C.; Dunkelblum, E.; Assael, F.; Protasov, A.; Ofer, D.; Mendel, Z. A common mode of attraction of larvae and adults of insect predators to the sex pheromone of their prey (Hemiptera: Matsucoccidae). Bull. Entomol. Res. 2006, 96, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Dweck, H.K.M.; Svensson, G.P.; Gündüz, E.A.; Anderbrant, O. Kairomonal response of the parasitoid, Bracon hebetor (Say), to the male-produced sex pheromone of its host, the greater waxmoth, Galleria mellonella (L.). J. Chem. Ecol. 2010, 36, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Arakaki, N.; Wakamura, S.; Yasuda, T.; Yamagishi, K. Two regional strains of a phoretic egg parasitoid, Telenomus euproctidis; (Hymenoptera: Scelionidae), that use different sex pheromones of two allopatric tussock moth species as kairomones. J. Chem. Ecol. 1997, 23, 153–161. [Google Scholar] [CrossRef]
- Milonas, P.; Mazomenos, B.E.; Konstantopoulou, M.A. Kairomonal effect of sex pheromone components of two lepidopteran olive pests on Trichogramma wasps. Insect Sci. 2009, 16, 131–136. [Google Scholar] [CrossRef]
- Hissmann, K. Strategies of mate finding in the European field cricket (Gryllus campestris) at different population densities: A field study. Ecol. Entomol. 1990, 15, 281–291. [Google Scholar] [CrossRef]
- Walker, S.E.; Cade, W.H. A simulation model of the effects of frequency dependence, density dependence and parasitoid flies on the fitness of male field crickets. Ecol. Model. 2003, 169, 119–130. [Google Scholar] [CrossRef]
- Outram, I. Aspects of mating in the Spruce budworm, Choristoneura fumiferana (Lepidoptera: Tortricidae). Can. Entomol. 1971, 103, 1121–1128. [Google Scholar] [CrossRef]
- Fraser, H.W.; Trimble, R.M. Effect of delayed mating on reproductive biology of the Oriental fruit moth (Lepidoptera: Tortricidae). Can. Entomol. 2001, 133, 219–227. [Google Scholar] [CrossRef]
- Torres-Vila, L.M.; Rodríguez-Molina, M.C.; Stockel, J. Delayed mating reduces reproductive output of female European grapevine moth, Lobesia botrana (Lepidoptera: Tortricidae). Bull. Entomol. Res. 2002, 92, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Pérez, A.; Wang, Q. Effect of mating delay on the reproductive performance of Cnephasia jactatana (Lepidoptera: Tortricidae). J. Econ. Entomol. 2003, 96, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Jones, V.P.; Wiman, N.G.; Brunner, J.F. Comparison of delayed female mating on reproductive biology of codling moth and obliquebanded leafroller. Environ. Entomol. 2008, 37, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Wenninger, E.J.; Averill, A.L. Effects of delayed mating on reproductive output of female oriental beetle Anomala orientalis (Coleoptera: Scarabaeidae). Agric. For. Entomol. 2006, 8, 221–231. [Google Scholar] [CrossRef]
- Mori, B.A.; Evenden, M.L. When mating disruption does not disrupt mating: Fitness consequences of delayed mating in moths. Entomol. Exp. Appl. 2013, 146, 50–65. [Google Scholar] [CrossRef]
- Stelinski, L.L.; Rodriguez-Saona, C.; Meyer, W.L. Recognition of foreign oviposition-marking pheromone in a multi-trophic context. Naturwissenschaften 2009, 96, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Noldus, L.P.J.J.; Potting, R.P.J.; Barendregt, H.E. Moth sex pheromone adsorption to leaf surface: Bridge in time for chemical spies. Physiol. Entomol. 1991, 16, 329–344. [Google Scholar] [CrossRef]
- McNeil, J.N. Behavioral ecology of pheromone-mediated communication in moths and its importance in the use of pheromone traps. Annu. Rev. Entomol. 1991, 36, 407–430. [Google Scholar] [CrossRef]
- Cardé, R.T. Utilization of pheromones in the population management of moth pests. Environ. Health Perspec. 1976, 14, 133–144. [Google Scholar] [CrossRef]
- Blight, M.M.; Wadhams, L.J. Male-produced aggregation pheromone in pea and bean weevil, Sitona lineatus (L.). J. Chem. Ecol. 1987, 13, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Merkl, M.E.; Cross, W.H.; Johnson, W.L. Boll weevil: Detection and monitoring of small populations with in-field traps. J. Econ. Entomol. 1978, 71, 29–30. [Google Scholar] [CrossRef]
- Rodriguez-Saona, C.; Stelinski, L. Behavior-modifying strategies in IPM: Theory and practice. In Integrated Pest Management: Innovation-Development Process; Peshin, R., Dhawan, A.K., Eds.; Springer: New York, NY, USA, 2009; Volume 1, pp. 263–315. [Google Scholar]
- Ding, B.-J.; Hofvander, P.; Wang, H.-L.; Durrett, T.P.; Stymne, S.; Löfstedt, C. A plant factory for moth pheromone production. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Shorey, H.H.; Mckelvey, J.J. Chemical Control of Insect Behavior: Theory and Application; Wiley: Hoboken, NJ, USA, 1977. [Google Scholar]
- Cardé, R.T. Using pheromones to disrupt mating of moth pests. In Perspectives in Ecological Theory and Integrated Pest Management; Kogan, M., Jepson, P., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 122–169. [Google Scholar]
- Rodriguez-Saona, C.; Polk, D.; Holdcraft, R.; Chinnasamy, D.; Mafra-Neto, A. SPLAT-OB reveals competitive attraction as a mechanism of mating disruption in oriental beetle. Environ. Entomol. 2010, 39, 1980–1989. [Google Scholar] [CrossRef] [PubMed]
- Schneider, D.; Seibt, U. Sex pheromone of the queen butterfly: Electroantennogram responses. Science 1969, 164, 1173–1174. [Google Scholar] [CrossRef] [PubMed]
- Willis, M.A.; Birch, M.C. Male lek formation and female calling in a population of the arctiid moth Estigmene acrea. Science 1982, 218, 168–170. [Google Scholar] [CrossRef] [PubMed]
- Grant, A.; O’Connell, R.; Eisner, T. Pheromone-mediated sexual selection in the moth Utetheisa ornatrix: Olfactory receptor neurons responsive to a male-produced pheromone. J. Insect Behav. 1989, 2, 371–385. [Google Scholar] [CrossRef]
- Andersson, J.; Borg-Karlson, A.K.; Vongvanich, N.; Wiklund, C. Male sex pheromone release and female mate choice in a butterfly. J. Exp. Biol. 2007, 210, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Byers, J. Sex-specific responses to aggregation pheromone: Regulation of colonization density in the bark beetle Ips paraconfusus. J. Chem. Ecol. 1983, 9, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Birch, M.C. Aggregation in bark beetles. In Chemical Ecology of Insects; Bell, W., Cardé, R., Eds.; Springer: New York, NY, USA, 1984; pp. 331–353. [Google Scholar]
- Dickens, J. Olfaction in the boll weevil, Anthonomus grandis Boh. (Coleoptera: Curculionidae): Electroantennogram studies. J. Chem. Ecol. 1984, 10, 1759–1785. [Google Scholar] [CrossRef] [PubMed]
- Iwabuchi, K.; Takahashi, J.; Nakagawa, Y.; Sakai, T. Electroantennogram responses of grape borer Xylotrechus pyrrhoderus (Bates) (Coleoptera: Cerambycidae) to its male sex pheromone components. J. Chem. Ecol. 1985, 11, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Gries, G.; Gries, R.; Perez, A.L.; Oehlschlager, A.C.; Gonzales, L.M.; Pierce, H.D., Jr. Aggregation pheromone of the African rhinoceros beetle, Oryctes monoceros (Olivier) (Coleoptera: Scarabaeidae). Z. Naturforsh. C 1994, 49, 363–366. [Google Scholar]
- Landolt, P.J. Sex attractant and aggregation pheromones of male phytophagous insects. Am. Entomol. 1997, 43, 12–22. [Google Scholar] [CrossRef]
- Lacey, E.; Ginzel, M.; Millar, J.; Hanks, L. Male-produced aggregation pheromone of the cerambycid beetle Neoclytus acuminatus acuminatus. J. Chem. Ecol. 2004, 30, 1493–1507. [Google Scholar] [CrossRef] [PubMed]
- Lacey, E.S.; Moreira, J.A.; Millar, J.G.; Ray, A.M.; Hanks, L.M. Male-produced aggregation pheromone of the cerambycid beetle Neoclytus mucronatus mucronatus. Entomol. Exp. Appl. 2007, 122, 171–179. [Google Scholar] [CrossRef]
- Blomquist, G.J.; Figueroa-Teran, R.; Aw, M.; Song, M.; Gorzalski, A.; Abbott, N.L.; Chang, E.; Tittiger, C. Pheromone production in bark beetles. Insect Biochem. Mol. Biol. 2010, 40, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Hardee, D.D.; Cross, W.H.; Mitchell, E.B. Male boll weevils are more attractive than cotton plants to boll weevils. J. Econ. Entomol. 1969, 62, 165–169. [Google Scholar] [CrossRef]
| Family | Species | Study Type | Response 1 | Dose Tested | Reference |
|---|---|---|---|---|---|
| Lepidoptera 2: Tortricidae | Adoxophyes orana (Fischer von Röslerstamm) | Observation chamber | Y | 0.01–100 µg | [25] |
| Adoxophyes honmai (Yasuda) | Observation chamber | Y | 0.01–100 µg | [25] | |
| Argyrotaenia velutinana (Walker) | Observation chamber | Y | [26] | ||
| Choristoneura fumiferana (Clemens) | Observation chamber | Y | 2 mg | [27] | |
| Observation chamber | Y | 1 pg–1 mg | [17] | ||
| Faraday cage | Y | (100–200 ng/h) | [18] | ||
| Choristoneura rosaceana (Harris) | Observation chamber | Y | [26] | ||
| Observation chamber | N | 255 mg | [28] | ||
| (0.04 mg/h) | |||||
| Observation chamber | Y | 0.002–0.05 mg | [23] | ||
| Flight mill | |||||
| Cydia fagiglandana (Zeller) | Observation chamber | Y | 10 µg blend | [29] | |
| Cydia pomonella (Linnaeus) | Observation chamber | Y | 100 µg | [30] | |
| (0.93 µg/h) | |||||
| Observation chamber | N | 109 mg | [28] | ||
| (0.02 mg/h) | |||||
| Cydia splendana (Hübner) | Observation chamber | Y | 10 µg blend | [29] | |
| Eupoecilia ambiguella (Hübner) | Observation chamber | N | [31] | ||
| Grapholita molesta (Busck) | Observation chamber | Y | [32] | ||
| Observation chamber | Y | 238 µg | [28] | ||
| (0.05 mg/h) | |||||
| Observation chamber Flight mill | Y | 0.002–0.05 mg | [23] | ||
| Homona magnanima (Diakonoff) | Observation chamber | Y | 0.01–100 µg | [25] | |
| Lobesia botrana (Denis and Schiffermüller) | Observation chamber | N | [31] | ||
| Pandemis limitata (Robinson) | Observation chamber | N | [33] | ||
| Pandemis pyrusana (Kearfott) | Observation chamber | Y | 255 mg (0.04 mg/h) | [28] | |
| Lepidoptera 2: Noctuidae | Helicoverpa armigera (Hübner) | Olfactometer | Y | [19] | |
| Helicoverpa zea (Boddie) | Olfactometer | Y | [19] | ||
| Heliothis subflexa (Guenée) | Olfactometer | N | [34] | ||
| Pseudaletia adultera (Schaus) | Observation chamber | Y | live ♀ | [35] | |
| Sesamia nonagrioides (Lefebvre) | Olfactometer | Y | [36] | ||
| Spodoptera exigua (Hübner) | Observation chamber | Y | 10 µg | [37] | |
| Spodoptera littoralis (Boisduval) | Observation chamber | Y | 0.1–5 mg | [20] | |
| Observation chamber | N | [31] | |||
| Observation chamber | Y | [38] | |||
| Trichoplusia ni (Hübner) | Field Trapping | Y | [7] | ||
| Field Trapping | Y | [10] | |||
| Lepidoptera 2: Arctiidae | Euplagia (Panaxia) quadripunctaria (Poda) | Field observation | ? | [6] | |
| Utetheisa ornatrix (Linnaeus) | Observation chamber | Y | [39,40] | ||
| Observation chamber | Y | [41] | |||
| Lepidoptera 2: Sesiidae | Melittia cucurbitae (Harris) | Field trapping | Y | [42] | |
| Vitacea polistiformis (Harris) | Field observation | Y | [21] | ||
| Lepidoptera 2: Pyralidae | Ephestia kuehniella (Zeller) | Field trapping | Y | [22] | |
| Coleoptera 3: Scarabaeidae | Anomala orientalis (Waterhouse) | Field trapping | Y | [43] | |
| Field observation | Y | [44] | |||
| Anomala rufocuprea (Motschulsky) | Field trapping | ? | [45] | ||
| Field trapping | Y | [46] | |||
| Cotinis nitida (Linnaeus) | Field trapping | Y | [47,48] | ||
| Holotrichia consanguinea (Blanchard) | Field trapping and observation | Y | [49] | ||
| Holotrichia loochooana loochooana (Sawada) | Field observation | Y | 1–10 mg | [50] | |
| Field observation and experiment | Y | (800 ng/h) | [51] | ||
| Holotrichia serrata (Fabricius) | Field observation | Y | [52] | ||
| Maladera (matrida) insanabilis (Brenske) | Field observation | Y | live ♀ | [53] | |
| Olfactometer | Y | live ♀ | |||
| Field trapping | Y | live ♀/extract | |||
| Field trapping | Y | live ♀ | [54] | ||
| Blattodea 4: Blattellidae | Blattella germanica (Linnaeus) | Observation and Olfactometer | Y | [55] | |
| Diptera 4: Tephritidae | Bactrocera (Dacus) oleae (Rossi) | Field trapping | Y | [56] |
| Order: Family | Species | Test/Result 1 | AD 2 | Threshold 3 | Reference | |
|---|---|---|---|---|---|---|
| Lepidoptera: Tortricidae | Adoxophyes orana (Fischer von Röslerstamm) | SSR | + | + | [59] | |
| Argyrotaenia velutinana (Walker) | EAG | − | + | [60] | ||
| EAG | + | ≤2 µg | [26] | |||
| Choristoneura fumiferana (Clemens) | EAG | + | + | <0.1 µg | [61] | |
| EAG | + | 0.9 µg | [62] | |||
| Choristoneura rosaceana (Harris) | EAG | + | + | ≤2 µg | [26] | |
| Cydia fagiglandana (Zeller) | EAG | + | + | ~0.1 µg | [29] | |
| Cydia pomonella (Linnaeus) | EAG | + | + | ≤100 ng | [63] | |
| SSR | + | [64] | ||||
| Cydia splendana (Hübner) | EAG | + | + | ~0.1 µg | [29] | |
| Grapholita molesta (Busck) | EAG | + | + | ≤2 µg | [32] | |
| Pandemis limitata (Robinson) | EAG | + | + | ≤10 µg | [33] | |
| Lepidoptera: Noctuidae | Agrotis segetum (Denis and Schiffermüller) | EAG | − | − | [65] | |
| SSR | − | |||||
| Diparopsis castanea (Hampson) | EAG | − | − | [66] | ||
| Helicoverpa armigera (Hübner) | SSR | − | − | [67] 4 | ||
| Helicoverpa zea (Boddie) | EAG | − | − | [68] | ||
| Heliothis subflexa (Guenée) | EAG | + | + | [69] | ||
| Heliothis virescens (Fabricius) | EAG | + | + | ≤10 µg | [70] | |
| SSR | + | 1 µg | ||||
| CR | + | ≤12 µg | [71] | |||
| EAG | + | [69] | ||||
| EAG | + | [15] | ||||
| SSR | + | |||||
| Pseudaletia adultera (Schaus) | EAG | + | + | 10 ng | [35] | |
| Sesamia nonagrioides (Lefebvre) | EAG | − | + | [72] | ||
| EAG | + | [36] | ||||
| Spodoptera exigua (Hübner) | EAG | − | + | [73] | ||
| EAG | + | 0.01 µg | [37] | |||
| Spodoptera frugiperda (Smith) | EAG | + | + | 10 µg | [74] | |
| Spodoptera littoralis (Boisduval) | EAG | + | + | [66] | ||
| EAG | + | [75] | ||||
| SSR | + | |||||
| SSR | + | 10–100 ng | [76] | |||
| Trichoplusia ni (Hübner) | EAG | + | + | 2–10 ng | [77] | |
| EAG | + | 0.1 µg | [78] | |||
| SSR | + | 5 ng | [79] | |||
| Lepidoptera: Arctiidae | Euplagia (Panaxia) quadripunctaria (Poda) | EAG | + | + | 0.01–1.0 µg | [6] |
| Utetheisa ornatrix (Linnaeus) | SSR | + | + | [80] | ||
| Lepidoptera: Cossidae | Coryphodema tristis (Drury) | EAG | + | + | [81] | |
| Lepidoptera: Sesiidae | Melittia cucurbitae (Harris) | EAG | + | + | 0.1 µg | [42] |
| Vitacea polistiformis (Harris) | EAG | + | + | 1 µg | [82] | |
| Lepidoptera: Yponomeuta | Yponomeuta rorellus (rorrella) (Hübner) | SSR | + | + | [83] | |
| Yponomeuta vigintipunctatus (Retzius) | SSR | + | + | [83] | ||
| Lepidoptera: Pyralidae | Cactoblastis cactorum (Berg) | EAG | + | + | [84] | |
| SSR | + | |||||
| Lepidoptera: Sphingidae | Manduca sexta (Linnaeus) | EAG | − | ? | [85] | |
| EAG | − | [57] | ||||
| SSR | + | [58] | ||||
| Lepidoptera: Geometridae | Itame argillacearia (Packard) | EAG | − | − | [86] | |
| Lepidoptera: Bombycidae | Bombyx mandarina (Moore) | EAG | − | − | [87] | |
| Bombyx mori (Moore) | EAG | − | − | [4] | ||
| SSR | − | [88] | ||||
| Lepidoptera: Saturniidae | Antheraea pernyi (Guerin Meneville) | EAG | − | − | [4] | |
| Antheraea polyphemus (Cramer) | EAG | − | − | [89] | ||
| Attacus atlas (Linnaeus) | EAG | − | − | [89] | ||
| Callosamia promethea (Drury) | EAG | − | − | [4] | ||
| Eupackardia calleta (Westwood) | EAG | − | − | [4] | ||
| Hyalophora cecropia (Linnaeus) | EAG | − | − | [4] | ||
| Coleoptera: Scarabaeidae 4 | Anomala cuprea (Hope) | EAG | − | + | [90] | |
| SSR | + | [91] | ||||
| SSR | + | 10−100 ng | [92] | |||
| Anomala octiescostata (Burmeister) | EAG | + | + | [93] | ||
| EAG | + | 0.1−10 µg | [94] | |||
| Anomala orientalis (Waterhouse) | EAG | + | + | 30 µg | [44] | |
| Holotrichia serrata (Fabricius) | EAG | + | + | [95] | ||
| Blattodea: Blattidae 5 | Periplaneta americana (Linnaeus) | EAG | + | + | [96] | |
| GR | + | [97] | ||||
| Order: Family | Species | PBP 1 Present Female | PBP 1 Expression in Female 2 | PR 3 Present Female | PR 3,4 Expression in Female 2 | Reference |
|---|---|---|---|---|---|---|
| Lepidoptera: Tortricidae | Cydia pomonella (Linnaeus) | Y | moderate, similar to male | [105] | ||
| Lepidoptera: Noctuidae | Agrotis ipsilon (Hufnagel) | Y | moderate, similar to male | [106] | ||
| moderate, ~33% of male | [107] | |||||
| Agrotis segetum (Denis + Schiffermüller) | Y | moderate | Y | [67] | ||
| moderate, 30%–50% of male | [108] | |||||
| moderate, similar to male | low, less than male | [107] | ||||
| Autographa gamma (Linnaeus) | Y | moderate, similar to male | [109] | |||
| moderate | [67] | |||||
| Helicoverpa armigera (Hübner) | Y | high | Y | [110] | ||
| moderate-high, 37%–79% of male | [67] | |||||
| moderate, less than male | [111] | |||||
| Helicoverpa zea (Boddie) | Y | moderate, 40%–50% of male | [112] | |||
| Heliothis virescens (Fabricius) | Y | low, weakly expressed | Y | [113] | ||
| moderate, 40%–50% of male | [112] | |||||
| moderate | [67] | |||||
| not found | [114] | |||||
| found | [115] | |||||
| Mamestra brassicae (Linnaeus) | Y | unclear | [116] | |||
| present | [117] | |||||
| Mythimna separata (Walker) | Y | high | ? | not found (0:1) | [118] | |
| Sesamia nonagrioides (Lefebvre) | Y | abundant | [119] | |||
| moderate, 15%–47% of male | [72] | |||||
| Spodoptera exigua (Hübner) | Y | moderate-high, 39%–73% of male | [120] | |||
| Spodoptera frugiperda (Smith) | Y | moderate, 40%–50% of male | [112] | |||
| Spodoptera littoralis (Boisduval) | Y | high | Y | [102] 5 | ||
| high | [67] | |||||
| moderate, 30%–90% of male | [121] | |||||
| Spodoptera litura (Fabricius) | Y | low, 2%–7% of male | [122] | |||
| Lepidoptera: Plutellidae | Plutella xylostella (Linnaeus) | Y | high | Y | not found (0:1) | [117] |
| low, 1% of male | [123] | |||||
| Lepidoptera: Pyralidae | Chilo suppressalis (Walker) | Y | moderate, similar to male | Y | [124] | |
| moderate, similar to male | [125] | |||||
| Orthaga achatina (Butler) | Y | moderate, ~25%–33% | [111] | |||
| Lepidoptera: Crambidae | Cnaphalocrocis medinalis (Guenée) | Y | low, 1%–32% | [126] | ||
| Diaphania indica (Saunders) | Y | moderate | ? | not found (0:1) | [118] | |
| Lepidoptera: Sphingidae | Manduca sexta (Linnaeus) | Y | not detected | N | [100] | |
| low, 14% of male | [101] | |||||
| low | [127] | |||||
| low | [128] | |||||
| present | [129] | |||||
| not found (0:2) | [130] | |||||
| Lepidoptera: Bombycidae | Bombyx mori (Moore) | Y | low | Y | [109] | |
| rare | [102] | |||||
| not found (0:1) | [131] | |||||
| found (4:6) | [103] | |||||
| rare | [132] | |||||
| low | [133] | |||||
| Lepidoptera: Saturniidae | Antheraea pernyi (Guerin Meneville) | Y | not detected | [100] | ||
| not detected | [134] | |||||
| rare | [102] | |||||
| Antheraea polyphemus (Cramer) | Y | not detected | N | [100] | ||
| not detected | [109] | |||||
| very rare | [135] | |||||
| rare | [102] | |||||
| extremely low | [104] | |||||
| Hyalophora cecropia (Linnaeus) | N | not detected | [100] | |||
| Coleoptera: Scarabaeidae | Anomala cuprea (Hope) | Y | moderate, similar to male | [94] | ||
| Anomala octiescostata (Burmeister) | Y | moderate, similar to male | [94] |
| Order: Family | Species | Behavioral Response 2 | Electro-Physiological Response 1 | PBP/PR Presence 3,4 | Expression of Antennal Detection Proteins 5 | |
|---|---|---|---|---|---|---|
| PBP 3 | PR 3 | |||||
| Lepidoptera: Tortricidae | Adoxophyes orana | Y | + | + | ||
| Adoxophyes honmai | Y | |||||
| Argyrotaenia velutinana | Y | + | + | |||
| Choristoneura fumiferana | Y | + | + | |||
| Choristoneura rosaceana | Y | + | + | |||
| Cydia fagiglandana | Y | + | + | |||
| Cydia pomonella 6 | Y | + | + | • | • | |
| Cydia splendana | Y | + | + | |||
| Eupoecilia ambiguella | N | |||||
| Grapholita molesta | Y | + | + | |||
| Homona magnanima | Y | |||||
| Lobesia botrana | N | |||||
| Pandemis limitata | N | + | + | |||
| Pandemis pyrusana | Y | |||||
| Lepidoptera: Noctuidae | Agrotis ipsilon | + | 〇 | |||
| Agrotis segetum 6 | - | + | • | Ø | ||
| Autographa gamma | + | 〇 | ||||
| Diparopsis castanea | - | |||||
| Helicoverpa armigera | Y | - | + | • | 〇 | |
| Helicoverpa zea 6 | Y | - | + | 〇 | ||
| Heliothis subflexa | N | + | + | |||
| Heliothis virescens 6 | + | + | 〇 | 〇 | ||
| Mamestra brassicae | + | Ø | ||||
| Mythimna separate | + | • | ? | |||
| Pseudaletia adultera | Y | + | ||||
| Sesamia nonagrioides 6 | Y | + | + | 〇 | ||
| Spodoptera exigua 6 | Y | + | + | • | ||
| Spodoptera frugiperda | + | + | 〇 | |||
| Spodoptera littoralis 6 | Y | + | + | • | 〇 | |
| Spodoptera litura | + | Ø | ||||
| Trichoplusia ni | Y | + | + | |||
| Lepidoptera: Arctiidae | Euplagia quadripunctaria | ? | + | + | ||
| Utetheisa ornatrix | Y | + | + | |||
| Lepidoptera: Cossidae | Coryphodema tristis | + | ||||
| Lepidoptera: Sesiidae | Melittia cucurbitae | Y | + | + | ||
| Vitacea polistiformis | Y | + | + | |||
| Lepidoptera: Yponomeuta | Yponomeuta rorellus | + | + | |||
| Yponomeuta vigintipunctatus | + | + | ||||
| Lepidoptera: Plutellidae | Plutella xylostella | + | • | Ø | ||
| Lepidoptera: Pyralidae | Cactoblastis cactorum | + | + | |||
| Chilo suppressalis | + | 〇 | 〇 | |||
| Ephestia kuehniella | Y | |||||
| Orthaga achatina | + | 〇 | ||||
| Lepidoptera: Crambidae | Cnaphalocrocis medinalis | + | Ø | |||
| Diaphania indica | + | 〇 | ? | |||
| Lepidoptera: Sphingidae | Manduca sexta 6 | + * | + | Ø | X | |
| Lepidoptera: Geometridae | Itame argillacearia | - | ||||
| Lepidoptera: Bombycidae | Bombyx mandarina | - | ||||
| Bombyx mori 6 | - | + | Ø | Ø | ||
| Lepidoptera: Saturniidae | Antheraea pernyi 6 | - | + | Ø | ||
| Antheraea Polyphemus 6 | - | + | Ø | X | ||
| Attacus atlas | - | |||||
| Callosamia promethean | - | |||||
| Eupackardia calleta | - | |||||
| Hyalophora cecropia 6 | - | X | ||||
| Coleoptera: Scarabaeidae | Anomala cuprea 6 | + | + | 〇 | ||
| Anomala octiescostata 6 | + | + | 〇 | |||
| Anomala orientalis | Y | + | + | |||
| Anomala rufocuprea | Y | |||||
| Cotinis nitida | Y | |||||
| Holotrichia consanguinea | Y | |||||
| Holotrichia loochooana loochooana | Y | |||||
| Holotrichia serrata | Y | + | + | |||
| Maladera insanabilis | Y | |||||
| Blattodea: | Periplaneta Americana | + | + | |||
| Blattella germanica | Y | |||||
| Diptera: Tephritidae | Bactrocera oleae | Y | + | + | ||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holdcraft, R.; Rodriguez-Saona, C.; Stelinski, L.L. Pheromone Autodetection: Evidence and Implications. Insects 2016, 7, 17. https://doi.org/10.3390/insects7020017
Holdcraft R, Rodriguez-Saona C, Stelinski LL. Pheromone Autodetection: Evidence and Implications. Insects. 2016; 7(2):17. https://doi.org/10.3390/insects7020017
Chicago/Turabian StyleHoldcraft, Robert, Cesar Rodriguez-Saona, and Lukasz L. Stelinski. 2016. "Pheromone Autodetection: Evidence and Implications" Insects 7, no. 2: 17. https://doi.org/10.3390/insects7020017