1. Introduction
Cotton crops in Australia are attacked by a wide range of pests, the major ones being the two lepidopteran pests,
Helicoverpa armigera (Hübner) and
Helicoverpa punctigera (Wallengren) [
1]. These two pests are responsible for the use of synthetic insecticides in the Australian cotton industry from the 1960s until 1996–1997 [
2]. In 1996, transgenic cotton containing genes from
Bacillus thuringiensis (Bt) were introduced and then taken up by 90% of Australian cotton farmers. This cotton expresses the toxins Cry1Ac (Ingard
®) (in transgenic cotton from 1996) and Cry 1Ac + Cry2Ab (Bollgard II
®) (in transgenic cotton from 2004). The use of transgenic cotton has reduced the impact of the major pest (
Helicoverpa spp.) on cotton crops in Australia [
3,
4,
5,
6]. These transgenic cotton crops are toxic to
Helicoverpa spp. and other lepidopteran pests when ingested [
7]. Thus, the introduction of transgenic cotton crops has decreased the use of synthetic insecticides against
Helicoverpa spp. by 75%–80% [
6]. In contrast, 10% of cotton farmers in Australia still grow non-transgenic cotton and rely exclusively on the use of synthetic insecticides to manage
Helicoverpa spp. For these non-transgenic crops, there is a need to control
Helicoverpa spp. with synthetic insecticides early in the season, which inadvertently suppresses populations of beneficial insects and potentially causes outbreaks of secondary pests, such as green mirids (
Creontiades dilutus), cotton aphids (
Aphis gossypii) and green vegetable bugs (
Nezara viridula). The negative impact of this practice was of great concern to the cotton industry [
6,
7,
8] until the adoption of transgenic (Bt) cotton crops.
Many beneficial insects, particularly predatory insects and specialist parasitoids, have been recorded in cotton crops worldwide. The role of beneficial insects in regulating pests in cotton agro-ecosystems has become increasingly important [
9,
10,
11,
12,
13]. Although these beneficial insects do not show tightly coupled dynamics with their prey, because their population dynamics are not solely dependent on target pests, they often provide effective control, particularly in agro-ecosystems with non-chemical insecticide regimes [
10,
11,
14,
15,
16].
In Australia, cotton crops are often grown in remote areas that have very sparse natural vegetation [
11,
14] and that lay fallow for most of the year. With no natural refuges and food sources for the adult natural enemies of pests, beneficial insect populations decline quickly, thereby limiting natural biological control [
11,
14,
17]. Presently, cotton farmers in Australia are inadvertently discriminating against the natural enemies of
Helicoverpa spp. and other pests by exclusively using synthetic insecticides on monocultured crops to control
Helicoverpa spp. on conventional cotton and to control sucking pests on transgenic (Bt) cotton.
Helicoverpa spp. are difficult to manage on conventional cotton crops: first, because of the lack of diversity and stability in the cotton agro-ecosystem; second, because
Helicoverpa spp. are highly mobile and highly migratory and can rapidly infest cotton crops; and third, because their eggs can escape natural enemy attack, as the natural enemies may not be present or established in sufficiently high numbers [
2,
11,
14,
18,
19]. These factors have encouraged the use of synthetic broad-spectrum insecticides.
As a result, cotton farmers require new pest control methods to manage
Helicoverpa spp. and to complement integrated pest management (IPM) on conventional cotton crops. Entomopathogenic fungi are known to be important natural enemies of many pests of agricultural crops [
20,
21]. Over 750 different species of fungi have been identified to date; these are cosmopolitan organisms that have been isolated from soils and have infected insects around the world [
22,
23,
24]. All stages of insects, from the egg, larva or nymph to adult, can be killed by fungal pathogens. Fungi, unlike other pathogens, such as viruses and bacteria, do not need to be ingested to infect and kill the host. The advantage of using fungi for biological control is that the fungi are self-generating within or on the surface of the plant and can potentially provide ongoing protection as the plant grows, with little effect on non-herbivorous predators [
5]. Several species of entomopathogenic fungi have been developed as alternatives to synthetic insecticides because of the low risk to humans, low risk to the environment and low pest resistance [
22]. However, in Australia, only two isolates of
Metarhizium anisopliae are commercially available, for the control of plague locusts and sugar cane grubs.
The present study evaluated the efficacy of a naturally occurring entomopathogenic fungus, BC 639 (Aspergillus sp.), against Helicoverpa spp. populations and on retaining populations of beneficial insects (e.g., predatory beetles, predatory bugs, predatory lacewings and spiders) on commercial conventional cotton crops. The study objectives were: (1) to assess the dose-response of the BC 639 fungus on Helicoverpa spp. larvae; (2) to assess the efficacy of different application rates of the BC 639 formulated product; (3) to identify the optimum rate of the BC 639 formulated product needed to control Helicoverpa spp. populations; and (4) to assess the impact of the BC 639 formulated product on the natural enemies of the target pests.
2. Materials and Methods
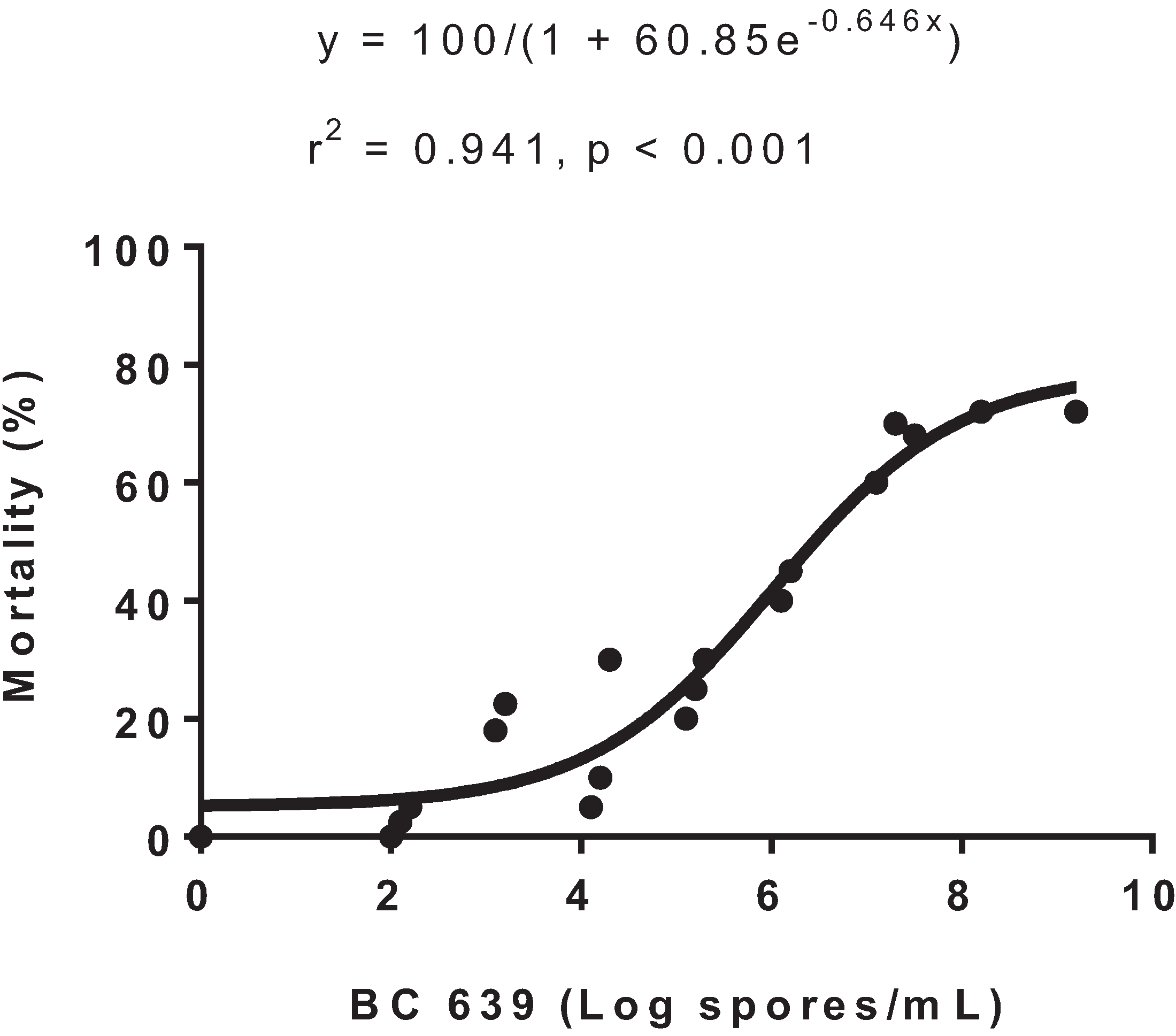
2.2. Experiment 1: Dose-Response of Helicoverpa spp. Second Instar Larvae in the Laboratory to BC 639
The experiment was conducted in the laboratory at the Australian Cotton Research Institute in Narrabri, NSW, Australia, from 10 to 28 February 2006. Helicoverpa armigera larvae were cultured in the laboratory and used in the study at the second instar stage.
BC 639 was tested at 101, 102, 103, 104, 105, 106, 108 and 109 spores/mL. For each treatment dose, 10 H. armigera second instar larvae were placed in a 90-mm Petri dish lined with filter paper and sprayed separately. The larvae from each treatment were then each transferred into separate 45-mm Petri dishes containing a fresh 19-mm conventional cotton leaf disc on filter paper. The Petri dishes were sealed and placed in an incubator set at 26–28 °C. Therefore, each treatment had 10 larvae placed individually in 10 Petri dishes.
The larvae were checked on 1, 2 and 3 days after treatment (DAT), and the number of dead and alive larvae were recorded. The mortality for each treatment was calculated, and a dose-response curve was developed for the concentration of BC 639 (log spores/mL) per mortality (%) of Helicoverpa spp. using GraphPad Prism 6 (GraphPad Software, Inc. San Diego, CA, USA). The number of spores/mL required to cause 50% mortality was extrapolated from the dose-response curve.
2.3. Experiment 2: Efficacy of BC 639 against Helicoverpa spp. First and Second Instar Larvae in the Laboratory
The experiment was conducted in the laboratory at the Australian Cotton Research Institute in Narrabri, NSW, Australia. Helicoverpa armigera first and second instar larvae were used for the experiment. The spore concentration used for this study was 1.0 × 107. The conidial germination was 100%.
BC 639 was formulated into an oil-based product by Becker Underwood Pty. Ltd., Australia. The product was evaluated at 1% v/v (1.0 L/ha), and water was used as the control treatment. First instar larvae were used in the first experiment, and second instar larvae were used in the second experiment. For each experiment and each treatment, one larva per plant was placed on a cotton leaf and sprayed until run-off. Each treatment used 40 cotton plants infested with 40 larvae. After spraying, each larvae was transferred into a 35-mL clear plastic container (P10M; Solo, Urbana, IL, USA) containing a soybean-based artificial diet. The number of dead larvae were counted and recorded daily until all of the larvae had pupated. The mortality (%) was calculated for each treatment.
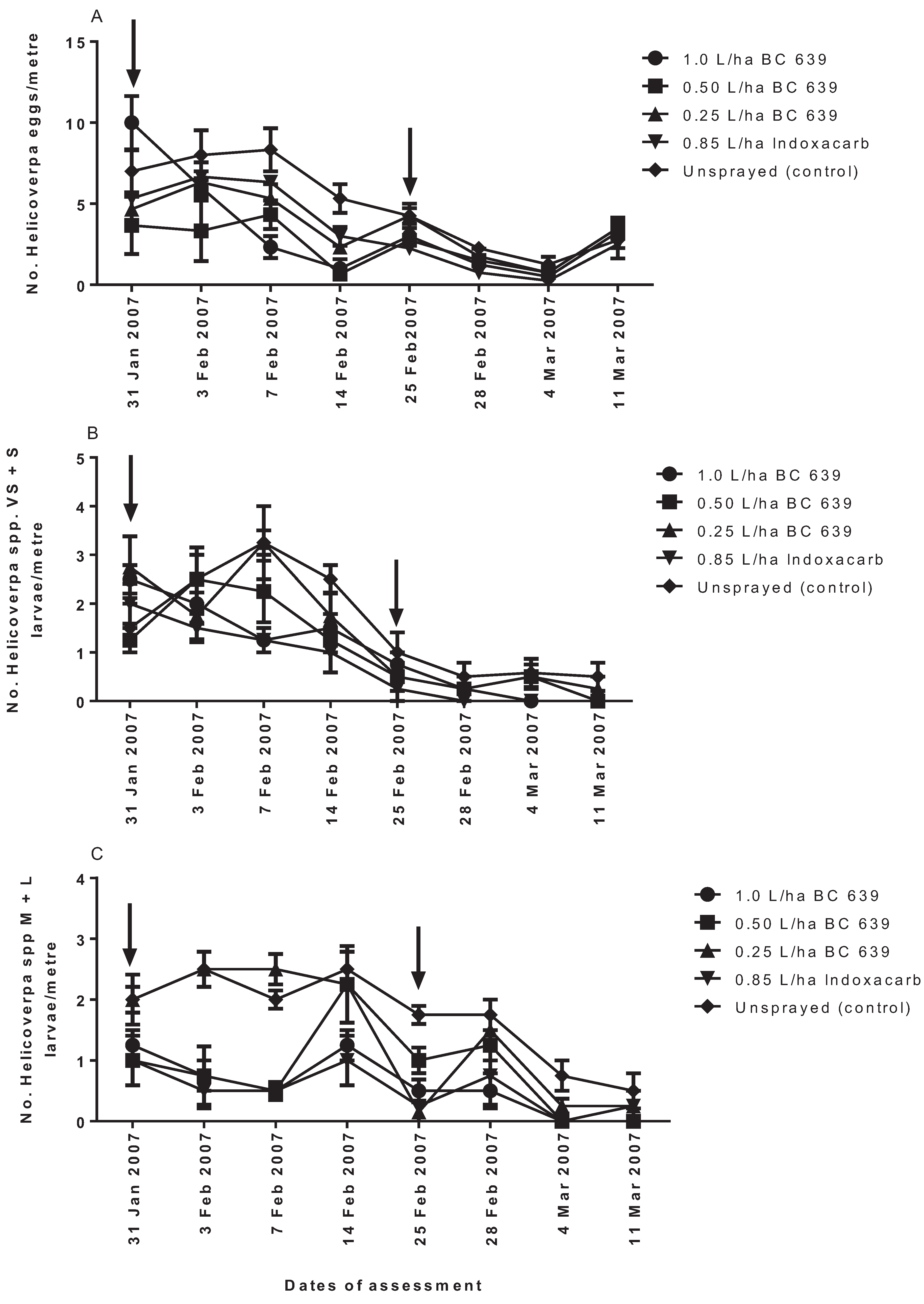
2.4. Experiment 3: Efficacy of Different Rates of BC 639 on the Survival of Helicoverpa spp. and Beneficial Insects on Commercial Cotton Crops
The trial was conducted on a dryland, commercial, conventional cotton farm at Getta Getta near Goondiwindi in the Macintyre Valley in Queensland in the 2006–2007 growing season.
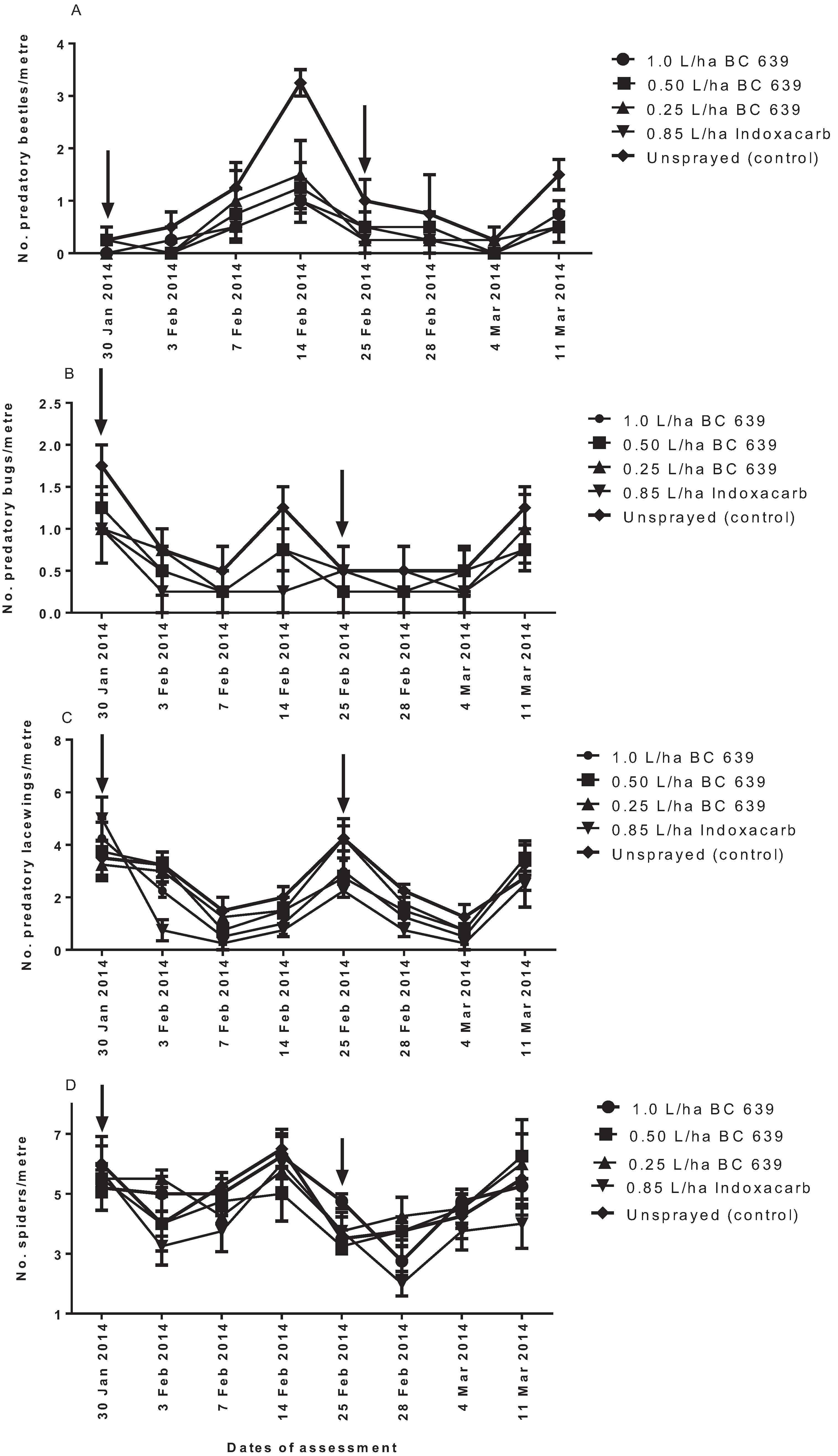
The following treatments were evaluated for their effect on Helicoverpa spp., predatory beetles, predatory bugs, predatory lacewings and spiders: (1) 1.0 L/ha BC 639; (2) 0.50 L/ha BC 639; (3) 0.25 L/ha BC 639; (4) 0.85 L/ha Indoxacarb; and (5) unsprayed (untreated) control. The treatment plots were arranged in a randomized complete block design with six replicates per treatment. Each replicated plot measured 8-m wide and 220-m long.
The first foliar treatment was applied on 31 January 2007 and the second on 25 February 2007. Foliar treatments were applied using a ground rig sprayer fitted with flat fan nozzles (3 nozzles/row of cotton) to achieve a droplet size of 200 µm and an application volume of 100 L/ha. The treatment was applied in the morning when the temperature was between 20 °C and 28 °C. The decision for when to apply the treatment was based on the IPM Guidelines and the economic threshold of 2.0 larvae per metre as recommended by CottonLogic [
1].
Visual counts of Helicoverpa spp. eggs and larvae, predatory beetles, bugs, lacewings and spiders on cotton plants for each treatment were made at approximately weekly intervals. For each of the six treatment replicates, a randomly selected 1-m length of planted row was examined. The number of Helicoverpa spp., predatory beetles, predatory bugs, predatory lacewings and spiders per metre for each treatment was determined.
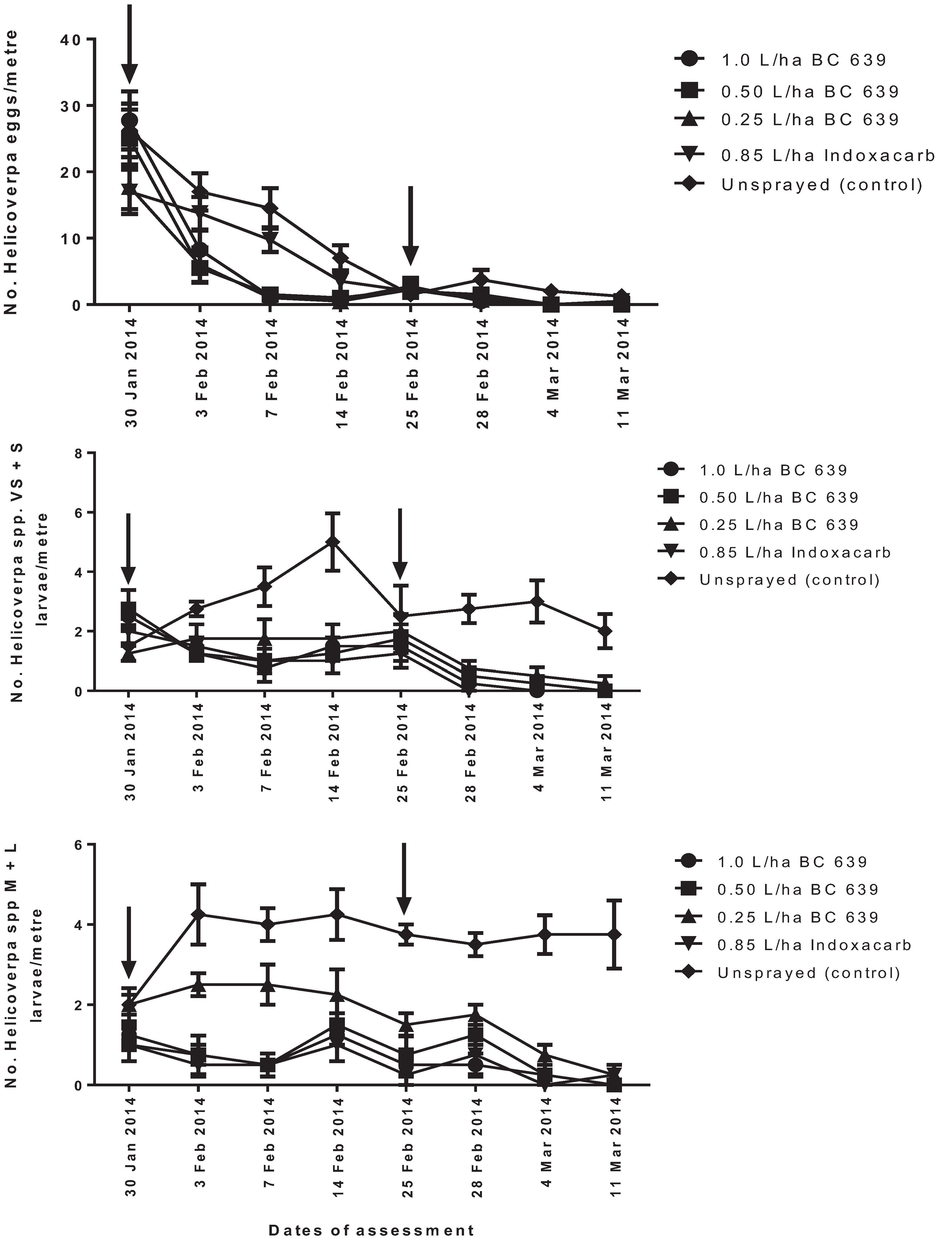
2.5. Experiment 4: Efficacy of Different Rates of BC 639 against Helicoverpa spp. and Beneficial Insects on Conventional Cotton Crops at Norwood in 2007–2008
The trial was conducted on a commercial conventional cotton farm at Norwood, near Moree. The trial was conducted from 16 November 2007 to 11 March 2008.
The following treatments were evaluated for their effect on Helicoverpa spp. eggs, very small and small, medium and large larvae and beneficial insects: (1) 1.0 L/ha BC 639; (2) 0.50 L/ha BC 639; (3) 0.25 L/ha BC 639 (4) 0.85 L/ha Indoxacarb; and (5) unsprayed (untreated) control. The treatment plots were arranged in a randomized complete block design with four replicates per treatment. Each replicated plot measured 40-m wide (equivalent to the number of rows) and 90-m long. A 40-m wide buffer separated each of the Indoxacarb-treated plots, fungus plots and unsprayed plots.
The number of insects were counted 24 h before treatment and then 3, 7 and 14 DAT for each spray application. Foliar treatments were applied on 30 January 2008 (first spray) and 25 February 2008 (second spray) using a ground rig sprayer fitted with flat fan nozzles (3 nozzles/row of cotton plants) to achieve a droplet size of 200 µm and an application volume of 100 L/ha. The treatments were applied in the morning when the temperature was between 20 °C and 28 °C. On each occasion, the treatments were applied using 100 L of water per hectare. The control plot was unsprayed.
Visual counts of Helicoverpa spp. eggs and larvae, predatory beetles, bugs, lacewings and spiders for each treatment were made at approximately weekly intervals. For each of the four treatment replicates, a randomly selected 1-m length of planted row was examined. The number of Helicoverpa spp., predatory beetles, predatory bugs, predatory lacewings and spiders per metre for each treatment was determined.
2.6. Data Analysis
All experimental data were analysed using repeated measures analysis of variance (v. 2.03; Graphpad Instat and Prism Software Inc., San Diego, CA, USA). Treatments and sample dates were the independent variables. Tukey-Kramer multiple comparison tests were used to compare the treatment means.
4. Discussion
The study has shown that the application of a formulated product of BC 639 entomopathogenic fungus effectively controlled populations of
Helicoverpa spp. on commercial cotton crops with minimal or no effect against predators of
Helicoverpa spp. One exception occurred when the product was applied at 1.0 L/ha (the highest rate in this study), and some negative effect was evident on predatory insects at the Getta Getta study site. In our study, the fungus killed
Helicoverpa spp. larvae within 3–7 DAT. Application of BC 639 at 1.0 and 0.50 L/ha reduced the number of
Helicoverpa spp. per metre, and these rates were consistently more effective than the lower 0.25 L/ha rate. Thus, the optimum effective rate of application of BC 639 against
Helicoverpa spp. on cotton crops to reduce pest damage was 0.50 L/ha. Similar results were obtained when BC 639 was applied to
Creontiades dilutus on commercial conventional cotton crops [
15].
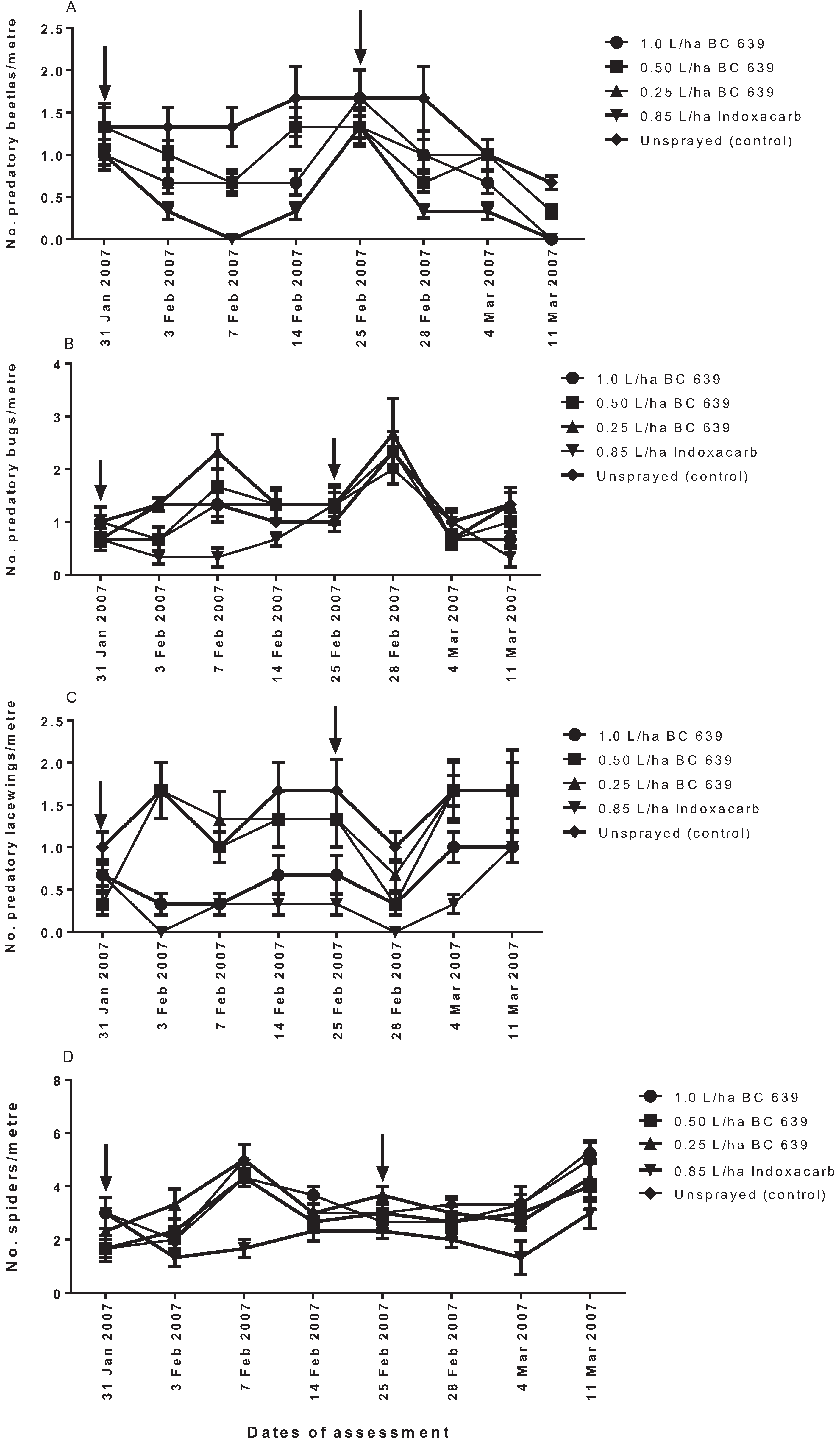
According to [
5,
25,
26], entomopathogenic fungi, such as BC 639, can negatively affect the survival of soft-bodied beneficial insects. In the present study, application of BC 639 at 0.5 and 0.25 L/ha did not significantly affect predatory beetle population, but when BC 639 was applied at 1.0 L/ha, it affected the number of predatory beetles per metre, with a similar effect to that of Indoxacarb (see
Figure 3A). Although the application of 1.0 L/ha BC 639 reduced the number of predatory beetles per metre, the negative effect of the 1.0 L/ha was significantly less than the negative effect of Indoxacarb. The present results also showed that following the first spraying, 1.0 and 0.50 L/ha BC 639 had a negative effect on the predatory bugs at three DAT, but the population recovered at seven and 14 DAT. In contrast, BC 639 applied at 0.25 L/ha had no effect on the predatory bugs. In the case of predatory lacewings, the 1.0 L/ha BC 639 had a similar negative effect on predatory lacewings as the Indoxacarb treatment. Furthermore, Indoxacarb was consistently disruptive to predatory bugs and spiders, whereas BC 639 had no effect on the spiders. These results suggest that the application of lower rates of BC 639 had a more selective effect on predatory beetles, bugs, lacewings and spiders than the Indoxacarb. Thus, the number of predatory insects recorded for the lower rates of BC 639-treated plots and the unsprayed plots was the same and significantly higher than the 1.0 L/ha BC 639 rate, which prevents us from speculating that BC 639 is essentially benign at all rates on soft-bodied insects. In the case of Indoxacarb, our study found that the selectivity of Indoxacarb only occurred in the treated plots that were adjacent to the unsprayed plots, indicating a possible influx of predators from the unsprayed plots to the Indoxacarb-treated plots.
Entomopathogenic fungi in general are known to be important natural enemies of many pests of agricultural crops [
20] and have an advantage over synthetic insecticides in the conservation of beneficial insects [
20]. The BC 639 fungus used in this study is self-generating on the surface of the plant and can potentially provide ongoing protection as the plant grows, with little effect on non-herbivorous predators [
15,
5]. Cotton ecosystems contain many strains of fungi, such as in the soil and on plant debris, but long-term research is required to identify potential fungi and to increase its pathogenicity and adaptability to a particular environment, so as to enhance its ability to infect and control insects on agricultural crops.
The selectivity of BC 639 against predatory insects indicates that this fungus has the potential to enhance conservational biological control, to support IPM and to reduce synthetic insecticide use on conventional cotton crops. IPM involves using all means of managing pest populations with the aim of reducing insecticide use while maintaining profitability, yield and fibre quality [
15].
Similar to many other cotton production systems worldwide, the Australian cotton cropping system is strictly monoculture. This practice and the use of synthetic insecticides inadvertently discriminates against beneficial arthropods. Therefore, there is a need to use biological pesticides, such as lower rates of BC 639, to conserve beneficial insects and support IPM in cotton agro-ecosystems. By integrating BC 639 fungal biopesticide with beneficial insects, rather than relying on beneficial insects alone, we can effectively enhance the control of Helicoverpa spp. in monoculture cropping systems, such as cotton. This is because Helicoverpa spp. are highly migratory and can quickly infest cotton crops and lay their eggs. Such behaviour by Helicoverpa spp. means that by the time beneficial insects arrive and establish in the cotton crops, the pest might have developed into medium and large larvae (fourth to sixth instar), which are too large for the beneficial insects (mostly predators) to feed on and, thus, to control. Hence, beneficial insects (particularly predatory insects) need to be well established in the cotton crops prior to the arrival of the pest (Helicoverpa spp.) to enable a sufficiently rapid response for population control. Thus, an IPM strategy developed against Helicoverpa spp. should involve tools and strategies that enhance the control of these pests; these could include fungal biopesticides, such as BC 639, which is selective against beneficial insects.
An IPM program for large-, medium- and small-scale monoculture crops, such as cotton, should develop in a stepwise progression that initially involves strategies to establish beneficial insects in the cotton field [
13], followed by integration with IPM-compatible tools, such as the BC 639 entomopathogenic fungus that controls the target pest, but is selective for beneficial insects. Intervention with broad-spectrum synthetic pesticides should be used as a last resort when pests exceed thresholds and when no other effective selective management options are available.
The success of IPM programs in many cropping systems, especially conventional cotton systems, will depend on the availability of IPM-compatible tools and strategies to farmers. To adopt a true IPM program, farmers will need alternative pest control tools that have a minimal impact on the beneficial insects, whatever crop is grown. For cotton farmers in Australia and other parts of the world, the increase in
Helicoverpa spp. populations on conventional cotton crops, yield loss, high cost of cotton production, insecticide resistance, disruption of beneficial species and environmental impacts are considered to be the major factors influencing the implementation of a sustainable IPM program for cotton crops. Thus, it is crucial that research continues to identify and develop new IPM-compatible tools and alternative strategies for managing and controlling
Helicoverpa spp. and other pests [
6,
12,
13]. Hence, the use of biological pesticides, including lower rates of BC 639 entomopathogenic fungus, is very important to support IPM in cotton agro-ecosystems.
The microbial biopesticide BC 639 is selective at lower application rates of 0.5 and 0.25 L/ha against predatory insects and has been used to effectively control Helicoverpa spp. on commercial cotton crops.