Insecticide Resistance in Eggs and First Instars of the Bed Bug, Cimex lectularius (Hemiptera: Cimicidae)
Abstract
:1. Introduction
2. Experimental Section
2.1. Experimental Insects
2.2. Epic Center Adult Resistance Assessment
2.3. Egg Resistance Assessment
2.4. First Instar Resistance Assessment
2.5. Statistical Analysis
3. Results
3.1. Epic Center Strain Resistance Assessment
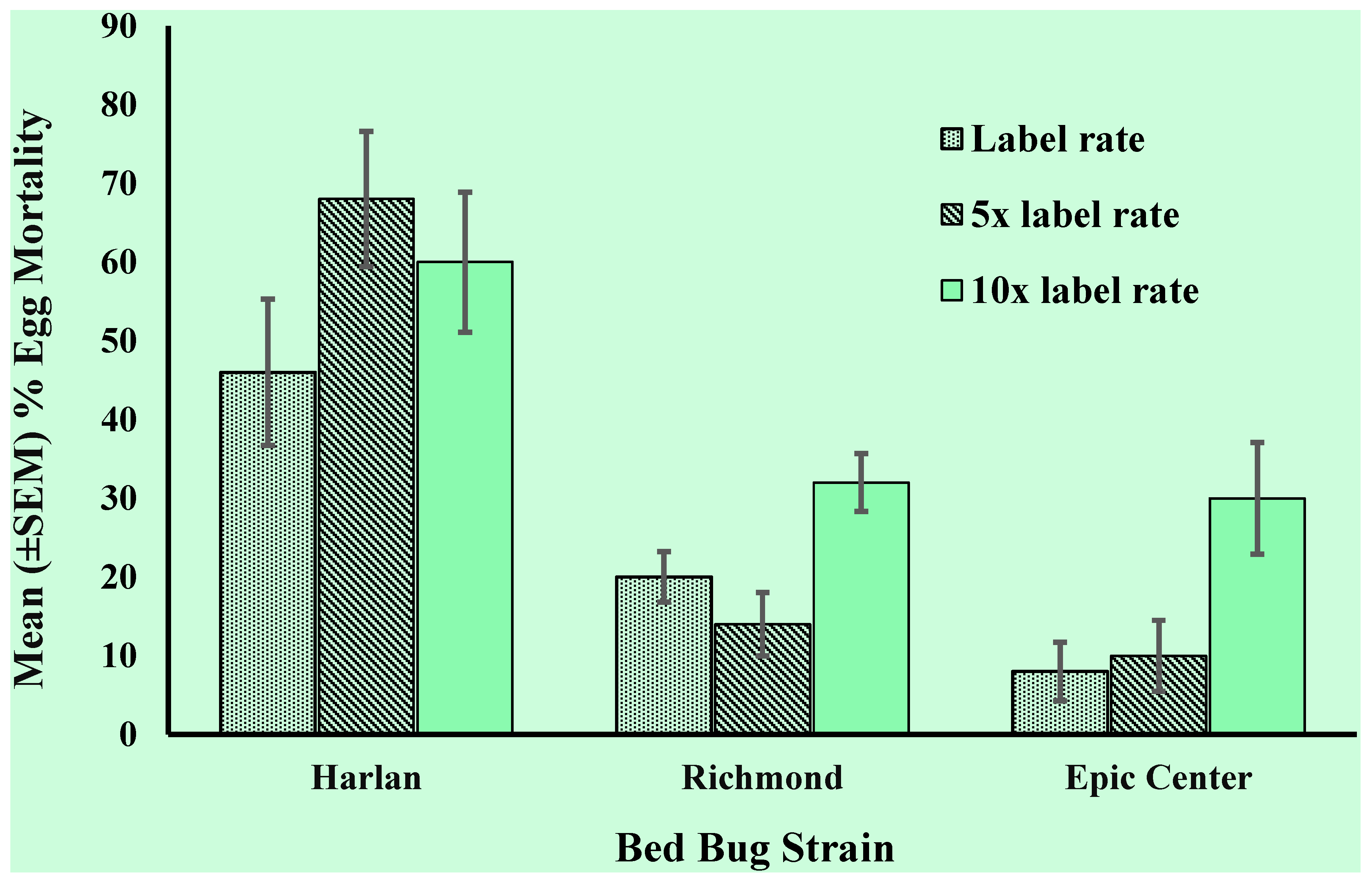
3.2. Egg Resistance Assessment
3.3. First Instar Resistance Assessment
| Strain | n | LC50 (95% CI) | Slope ± SE | X2 (df) | RR |
|---|---|---|---|---|---|
| Imidacloprid/β-cyfluthrin | |||||
| Harlan | 250 | 0.41 μL/mL (0.28–0.55) b | 1.86 ± 0.24 | 33.42 (23) | |
| Richmond | 320 | 1.23 μL/mL (0.59–2.10) a | 1.13 ± 0.14 | 82.57 (30) | 3.0 |
| Epic Center | 400 | 2.10 μL/mL (1.05–4.59) a | 0.95 ± 0.10 | 149.91 (38) | 5.1 |
| Acetamiprid/bifenthrin | |||||
| Harlan | 250 | 0.02 ng/mL (0.02–0.03) c | 2.33 ± 0.25 | 26.90 (23) | |
| Richmond | 310 | 0.78 ng/mL (0.37–1.44) b | 0.58 ± 0.10 | 29.17 (29) | 39 |
| Epic Center | 240 | 21.6 ng/mL (6.4–51.3) a | 0.48 ± 0.09 | 25.71 (22) | 1080 |
| Strain | n | LC50 (95% CI) | Slope ± SE | X2 (df) | RR |
|---|---|---|---|---|---|
| Imidacloprid/β-cyfluthrin | |||||
| Harlan | 150 | 0.04 µL/mL (0.03–0.06) b | 2.16 ± 0.34 | 38.17 (28) | |
| Richmond | 195 | 4.81 µL/mL (1.94–10.26) a | 0.66 ± 0.12 | 45.87 (37) | 121 |
| Epic Center | 190 | 19.72 µL/mL (8.18–184.48) a | 0.75 ± 0.17 | 45.39 (36) | 493 |
| Acetamiprid/bifenthrin | |||||
| Harlan | 155 | 0.007 ng/mL (0.005–0.008) c | 3.29 ± 0.48 | 33.95 (29) | |
| Richmond | 125 | 0.69 ng/mL (0.21–1.43) b | 0.94 ± 0.19 | 29.91 (23) | 99 |
| Epic Center | 115 | 13.6 ng/mL (3.9–1215.8) a | 0.50 ± 0.13 | 23.31 (21) | 1943 |
3.4. Stage Resistance Comparisons
| Strain | Stage with > LC50 | Stage RR |
|---|---|---|
| Imidacloprid/β-cyfluthrin | ||
| Harlan | egg | 9.98 |
| Richmond | 1st instar | 3.91 |
| Epic Center | 1st instar | 9.4 |
| Acetamiprid/bifenthrin | ||
| Harlan | egg | 3.28 |
| Richmond | egg | 1.13 |
| Epic Center | egg | 1.51 |
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Delaunay, P.; Blanc, V.; del Giudice, P.; Levy-Bencheton, A.; Chosidow, O.; Marty, P.; Brouqui, P. Bedbugs and infectious diseases. Clin. Infect. Dis. 2011, 52, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J. Bedbugs in relation to transmission of human diseases: Review of the literature. Public Health Rep. 1963, 78, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Sabou, M.; Imperiale, D.G.; Andrès, E.; Abou-Bacar, A.; Foeglé, J.; Lavigne, T.; Candolfi, E. Bed bugs reproductive life cycle in the clothes of a patient suffering from Alzheimer’s disease results in iron deficiency anemia. Parasite 2013, 20. [Google Scholar] [CrossRef]
- Reinhardt, K.; Kempke, D.; Naylor, R.A.; Siva-Jothy, M.T. Sensitivity to bites by the bedbug, Cimex lectularius. Med. Vet. Entomol. 2009, 23, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Leverkus, M.; Jochim, R.C.; Schäd, S.; Bröcker, E.B.; Andersen, J.F.; Valenzuela, J.G.; Trautmann, A. Bullous allergic hypersensitivity to bed bug bites mediated by IgE against salivary nitrophorin. J. Investig. Dermatol. 2006, 126, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, C.L.; Ardern-Jones, M.R.; Hay, R.J. Widespread bullous eruption due to multiple bed bug bites. Clin. Exp. Dermatol. 2002, 27, 74–75. [Google Scholar] [CrossRef] [PubMed]
- Goddard, J.; de Shazo, R. Multiple feeding by the common bed bug, Cimex lectularis, without sensitization. Midsouth Entomol. 2009, 2, 90–92. [Google Scholar]
- Churchill, T.P. Urticaria due to bedbug bites. J. Am. Med. Assoc. 1930, 95. [Google Scholar] [CrossRef]
- Goddard, J.; Edwards, K.T.; de Shazo, R.D. Observations on development of cutaneous lesions from bites by the common bed bug, Cimex lectularius L. Midsouth Entomol. 2011, 4, 49–52. [Google Scholar]
- Pritchard, M.J.; Hwang, S.W. Severe anemia from bedbugs. Can. Med. Assoc. J. 2009, 181, 287–288. [Google Scholar] [CrossRef]
- Goddard, J.; de Shazo, R. Psychological effects of bed bug attacks (Cimex lectularius L.). Am. J. Med. 2012, 125, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Susser, S.R.; Perron, S.; Fournier, M.; Jacques, L.; Denis, G.; Tessier, F.; Roberge, P. Mental health effects from urban bed bug infestation (Cimex lectularius L.): A cross-sectional study. Br. Med. J. Open 2012. [Google Scholar] [CrossRef]
- Pinto, L.J.; Cooper, R.; Kraft, S.K. Bed Bug Handbook: The Complete Guide to Bed Bugs and Their Control; Pinto & Associates, Inc.: Mechanicsville, MD, USA, 2007. [Google Scholar]
- Goddard, J. Laboratory assays of various insecticides against bed bugs (Hemiptera: Cimicidae) and their eggs. J. Entomol. Sci. 2013, 48, 65–69. [Google Scholar]
- Callaway, S.; Musgrave, A.J. Laboratory tests with liquid insecticides on the eggs of the bed-bug, Cimex lectularius L. Ann. Appl. Biol. 1940, 27, 252–261. [Google Scholar] [CrossRef]
- Cueto, G.M.; Zerba, E.N.; Picollo, M.I. Evidence of pyrethroid resistance in eggs of Pediculus humanus capitis (Phthiraptera: Pediculidae) from Argentina. J. Med. Entomol. 2008, 45, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.H.; Goh, P.M. Deltamethrin as a potential ovicidal pyrethroid against Plutella xylostella L. Toxicol. Lett. 1984, 22, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Toloza, A.C.; Germano, M.; Cueto, G.M.; Vassena, C.; Zerba, E.; Picollo, M.I. Differential patterns of insecticide resistance in eggs and first instars of Triatoma infestans (Hemiptera: Reduviidae) from Argentina and Bolivia. J. Med. Entomol. 2008, 45, 421–426. [Google Scholar] [CrossRef]
- Moore, D.J.; Miller, D.M. Laboratory evaluations of insecticide product efficacy for control of Cimex lectularius. J. Econ. Entomol. 2006, 99, 2080–2086. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.; Potter, M.F.; Potter, D.A.; Haynes, K.F. Insecticide resistance in the bed bug: A factor in the pest’s sudden resurgence? J. Med. Entomol. 2007, 44, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Adelman, Z.N.; Kilcullen, K.A.; Koganemaru, R.; Anderson, M.A.; Anderson, T.D.; Miller, D.M. Deep sequencing of pyrethroid-resistant bed bugs reveals multiple mechanisms of resistance within a single population. PLOS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Seong, K.M.; Lee, D.Y.; Yoon, K.S.; Kwon, D.H.; Kim, H.C.; Klein, T.A.; Lee, S.H. Establishment of quantitative sequencing and filter contact vial bioassay for monitoring pyrethroid resistance in the common bed bug, Cimex lectularius. J. Med. Entomol. 2010, 47, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Kilpinen, O.; Kristensen, M.; Jensen, K.M.V. Resistance differences between chlorpyrifos and synthetic pyrethroids in Cimex lectularius population from Denmark. Parasitol. Res. 2011, 109, 1461–1464. [Google Scholar] [CrossRef] [PubMed]
- Potter, M.F.; Haynes, K.F.; Henriksen, M.; Rosenberg, B. The 2011 Bed Bugs without Borders Survey 2011. Available online: http://www.edrants.com/_img/pestworldsurvey2011.pdf (accessed on 30 October 2014).
- Robertson, J.L.; Preisler, H.K.; Russel, R.M. PoloPlus: Probit and Logit Analysis User’s Guide; LeOra Software: Petaluna, CA, USA, 2003. [Google Scholar]
- Smith, E.H.; Salkeld, E.H. The Use and Action of Ovicides. Annu. Rev. Entomol. 1966, 11, 331–368. [Google Scholar] [CrossRef] [PubMed]
- Potter, M.F.; Haynes, K.F.; Gordon, J.R.; Hardebeck, E.; Wickemeyer, W. Dual Action Bed Bug Killers. Pest Control Technol. Available online: http://www.pctonline.com/pct0312-temprid-transport-insecticides-testing.aspx (accessed on 30 October 2014).
- Gordon, J.R.; Goodman, M.H.; Potter, M.F.; Haynes, K.F. Population variation in and selection for resistance to pyrethroid-neonicotinoid insecticides in the bed bug. Sci. Rep. 2014. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campbell, B.E.; Miller, D.M. Insecticide Resistance in Eggs and First Instars of the Bed Bug, Cimex lectularius (Hemiptera: Cimicidae). Insects 2015, 6, 122-132. https://doi.org/10.3390/insects6010122
Campbell BE, Miller DM. Insecticide Resistance in Eggs and First Instars of the Bed Bug, Cimex lectularius (Hemiptera: Cimicidae). Insects. 2015; 6(1):122-132. https://doi.org/10.3390/insects6010122
Chicago/Turabian StyleCampbell, Brittany E., and Dini M. Miller. 2015. "Insecticide Resistance in Eggs and First Instars of the Bed Bug, Cimex lectularius (Hemiptera: Cimicidae)" Insects 6, no. 1: 122-132. https://doi.org/10.3390/insects6010122





