Rational Practices to Manage Boll Weevils Colonization and Population Growth on Family Farms in the Semiárido Region of Brazil
Abstract
:1. Introduction
2. Experimental Section
2.1. Boll Weevil Preference towards Kaolin-Treated and -Untreated Plants: Choice Trial
2.2. Boll Weevil Establishment on Kaolin-Treated and -Untreated Plants: Non-Choice Trial
2.3. Boll Weevil Establishment under Kaolin Treatment in the Field
3. Results
3.1. Boll Weevil Preference towards Kaolin-Treated and Untreated Plants: Choice Trial

3.2. Boll Weevil Establishment on Kaolin-Treated and -Untreated Plants: Non-Choice Trial
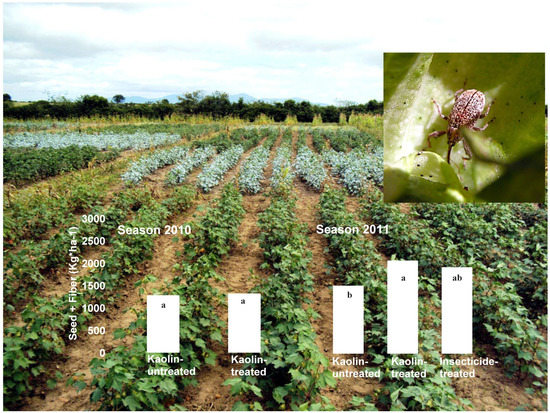
3.3. Boll Weevil Establishment under Kaolin Treatment in the Field
| Kaolin sprays | Season 2010 (May 27–August 14) | Season 2011 (June 16–October 2) | ||||||
|---|---|---|---|---|---|---|---|---|
| DAP 1 | Tm (°C) | RH (%) | Rainfall (mm) | DAP | Tm (°C) | RH (%) | Rainfall (mm) | |
| 1st | 47 | − | − | − | 47 | − | − | − |
| 2nd | 54 | 26.3 | 73.6 | 0 | 53 | 21.8 | 74.5 | 117 |
| 3rd | 62 | 24.9 | 77.8 | 48 | 62 | 21.5 | 68.5 | 101 |
| 4th | 73 | 22.8 | 62.2 | 280 | 70 | 22.8 | 64.0 | 27 |
| 5th | 81 | 22.6 | 60.1 | 28 | 77 | 22.2 | 66.2 | 0 |
| 6th | 89 | 22.4 | 54.6 | 5 | 84 | 21.9 | 67.6 | 2 |
| 7th | 97 | 23.1 | 78.2 | 10 | 90 | 22.0 | 64.4 | 60 |
| 8th | 104 | 22.0 | 86.0 | 4 | 98 | 22.8 | 64.7 | 3 |
| 9th | 111 | 21.9 | 83.7 | 5 | 105 | 22.4 | 78.2 | 0 |
| 10th | 118 | 21.4 | 85.2 | 10 | 111 | 22.6 | 79.0 | 2 |
| 11th | − | − | − | 6 | 117 | 23.3 | 73.9 | 3 |
| − | − | − | − | − | − | 24.8 | 70.0 | 0 |

| Collection | Season 2010 | Season 2011 | |||||
|---|---|---|---|---|---|---|---|
| DAP 1 | Untreated | Kaolin-treated | DAP | Untreated | Kaolin-treated | Insecticide-treated | |
| 1st | 62 | 0 (0) 2 | 0 (0) | 53 | 0 (0) | 0 (0) | 0 (0) |
| 2nd | 73 | 35 (10) | 37 (5) | 62 | 07 (3) | 01 (00) | 01 (0) |
| 3rd | 81 | 85 (20) | 154 (40) | 70 | 04 (2) | 01 (00) | 02 (2) |
| 4th | 89 | 258 (96) | 241 (87) | 77 | 15 (6) | 02 (01) | 08 (4) |
| 5th | 97 | 191 (112) | 202 (135) | 84 | 09 (5) | 02 (01) | 02 (1) |
| 6th | 104 | 671 (341) | 624 (247) | 90 | 30 (10) | 06 (03) | 08 (2) |
| 7th | 111 | 1350(830)a 3 | 483 (319) b | 98 | 70 (36) a | 12 (09) b | 19 (10) b |
| 8th | 118 | 744 (322) a | 199 (45) b | 105 | 82 (37) | 20 (13) | 54 (30) |
| 9th | − | − | − | 111 | 55 (25) a | 18 (08) b | 38 (23) ab |
| 10th | − | − | − | 117 | 114 (38) a | 79 (15) ab | 55 (22) b |
| Totals | − | 3,334 (1,731) | 1,930 (878) | − | 386 (162) | 141 (50) | 187 (94) |

4. Discussion
5. Conclusion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Almeida, R.P.; Silva, C.A.D.; Ramalho, F.S. Manejo integrado de pragas do algodoeiro no Brasil. In o Agronegócio do Algodão no Brasil; Beltrão, N.E.M., Azevedo, D.M.P., Eds.; Embrapa: Brasília, Brasil, 2008; pp. 1035–1098. [Google Scholar]
- Neff, D.L.; Vanderzant, E.S. Methods of evaluating the chemotropic response of boll weevils to extracts of the cotton plant and various other substances. J. Econ. Entomo. 1963, 56, 761–766. [Google Scholar]
- Smith, G.L.; Cleveland, T.C.; Clark, J.C. Boll weevil movement from hibernation sites to fruiting cotton. J. Econ. Entomol. 1965, 58, 357–358. [Google Scholar]
- Showler, A.T. Relationships of abscised cotton fruit to boll weevil (Coleoptera: Curculionidae) feeding, oviposition, and development. J. Econ. Entomol. 2008, 101, 68–73. [Google Scholar]
- White, J.R.; Rummel, D.R. Emergence profile of overwintered boll weevils and entry into cotton. Environ. Entomol. 1978, 7, 7–14. [Google Scholar]
- Neves, R.C.S.; Torres, J.B.; Silva, M.N.B. Época apropriada para a poda apical do algodoeiro para o controle de pragas. Pesqu. Agropec. Bras. 2010, 45, 1342–1350. [Google Scholar]
- Summy, K.R.; Cate, J.R.; Bar, D. Overwinter survival of boll weevil in South Texas: Entrapment in desiccated bolls. J. Econ. Entomol. 1993, 86, 421–426. [Google Scholar]
- Neves, R.C.S.; Showler, A.T.; Pinto, E.S.; Bastos, C.S.; Torres, J.B. Reducing boll weevil populations by clipping terminal buds and removing abscised fruiting bodies. Entomol. Exp. Appl. 2013, 146, 276–285. [Google Scholar]
- Ramalho, F.S.; Wanderley, P.A. Ecology and management of the boll weevil in South American cotton. Am. Entomol. 1995, 42, 41–47. [Google Scholar]
- Santos, R.L.; Neves, R.C.S.; Colares, F.; Torres, J.B. Parasitóides do bicudo Anthonomus grandis e predadores residentes em algodoeiro pulverizado com caulim. Semina Ciên. Agr. 2013, 34, 3463–3474. [Google Scholar]
- Lima Junior, I.S.; Degrande, P.E.; Miranda, J.E.; Santos, W.J. Evaluation of the boll weevil Anthonomus grandis Boheman (Coleoptera: Curculionidae) suppression program in the state of Goiás, Brazil. Neotrop. Entomol. 2013, 42, 82–88. [Google Scholar]
- Torres, J.B.; Ruberson, J.R.; Whitehouse, M.W. Transgenic cotton for sustainable pest management: A review. In Organic Farming, Pest Control and Remediation of Soil Pollutants; Lichtfouse, E., Ed.; Springer: Dordrecht, The Netherlands, 2009; pp. 15–53. [Google Scholar]
- Showler, A.T. Effects of kaolin-based particle film application on boll weevil (Coleoptera: Curculionidae) injury to cotton. J. Econ. Entomol. 2002, 95, 754–762. [Google Scholar]
- Silva, C.A.D.; Ramalho, F.S. Kaolin spraying protects cotton plants against damages by boll weevil Anthonomus grandis Boheman (Coleoptera: Curculionidae). J. Pest Sci. 2013, 86, 563–569. [Google Scholar]
- SAS Institute. SAS/STAT User’s Guide, Version 8.02, TS level 2MO; SAS Institute: Cary, NC, USA, 2001. [Google Scholar]
- Degrande, P.E. Guia Prático de Controle das Pragas do Algodoeiro; UFMS: Dourados, Brazil, 1998; p. 60. [Google Scholar]
- Weaver Junior, J.B.; Reddy, M.S. Boll weevil non-preference, antibiosis and hatchability studies utilizing cotton lines with multiple non-preferred characters. J. Econ. Entomol. 1977, 70, 283–285. [Google Scholar]
- Showler, A.T. Effects of kaolin particle film on beet armyworm, Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae): Oviposition, larval feeding and development on cotton, Gossypium hirsutum L. Agric. Ecos. Environ. 2003, 95, 265–271. [Google Scholar]
- Sisterson, M.S.; Liu, Y.B.; Kerns, D.L.; Tabashnik, B.E. Effects of kaolin particle film on oviposition, larval mining, and infestation of cotton by pink bollworm (Lepidoptera: Gelechiidae). J. Econ. Entomol. 2003, 96, 805–810. [Google Scholar]
- Alavo, T.B.C.; Yarou, B.B.; Atachi, P. Field effects of kaolin particle film formulation against major cotton lepidopteran pests in North Benin, West African. Int. J. Pest Manag. 2010, 56, 287–290. [Google Scholar]
- Showler, A.T.; Armstrong, J.S. Kaolin particle film associated with increased cotton aphid infestations in cotton. Entomol. Exp. Appl. 2007, 124, 55–60. [Google Scholar]
- Alavo, T.B.C.; Abagli, A.Z.; Tégbéssou, K.J.C.; Dunphy, G.B. Kaolin potential for the integrated management of Aphis gossypii Glov. (Homoptera: Aphididae) on cotton. Int. Arch. Phytop. Pl. Prot. 2011, 44, 764–770. [Google Scholar]
- Khan, M.; Quade, A. QDPI&F; Plant Science; Kingaroy. Kaolin cons cotton suckers. Aust. Cotton Grower 2006, 18–19. [Google Scholar]
- Burt, E.C.; Lloyd, E.; Smith, D.B. Control of the boll weevil by mechanically destroying fallen infested cotton squares. J. Econ. Entomo. 1969, 62, 862–865. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neves, R.C.S.; Colares, F.; Torres, J.B.; Santos, R.L.; Bastos, C.S. Rational Practices to Manage Boll Weevils Colonization and Population Growth on Family Farms in the Semiárido Region of Brazil. Insects 2014, 5, 818-831. https://doi.org/10.3390/insects5040818
Neves RCS, Colares F, Torres JB, Santos RL, Bastos CS. Rational Practices to Manage Boll Weevils Colonization and Population Growth on Family Farms in the Semiárido Region of Brazil. Insects. 2014; 5(4):818-831. https://doi.org/10.3390/insects5040818
Chicago/Turabian StyleNeves, Robério C. S., Felipe Colares, Jorge B. Torres, Roberta L. Santos, and Cristina S. Bastos. 2014. "Rational Practices to Manage Boll Weevils Colonization and Population Growth on Family Farms in the Semiárido Region of Brazil" Insects 5, no. 4: 818-831. https://doi.org/10.3390/insects5040818