Host Plant Volatiles and the Sexual Reproduction of the Potato Aphid, Macrosiphum euphorbiae
Abstract
:1. Introduction
2. Experimental Section
2.1. Insects
2.2. Bioassays
2.3. Statistics
3. Results
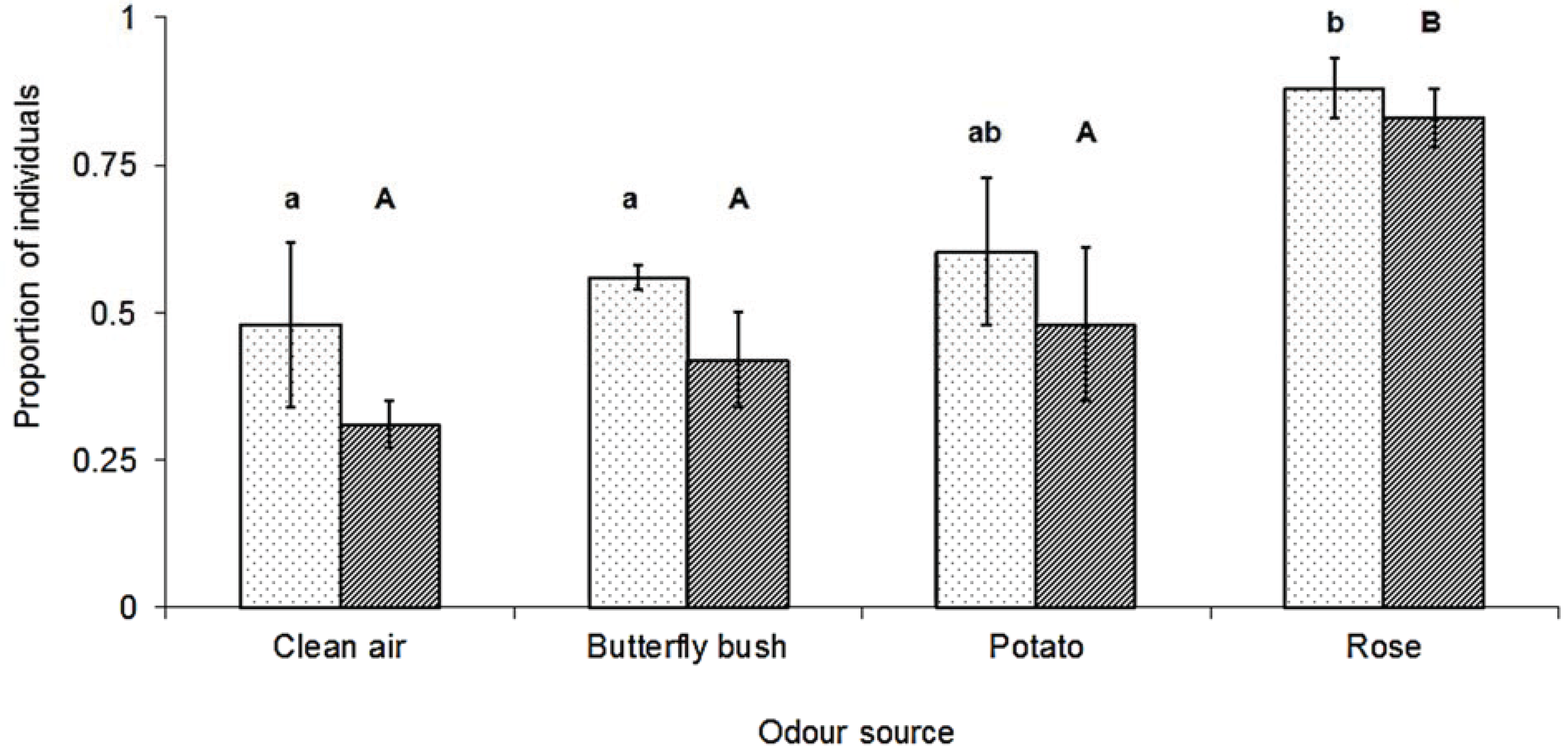
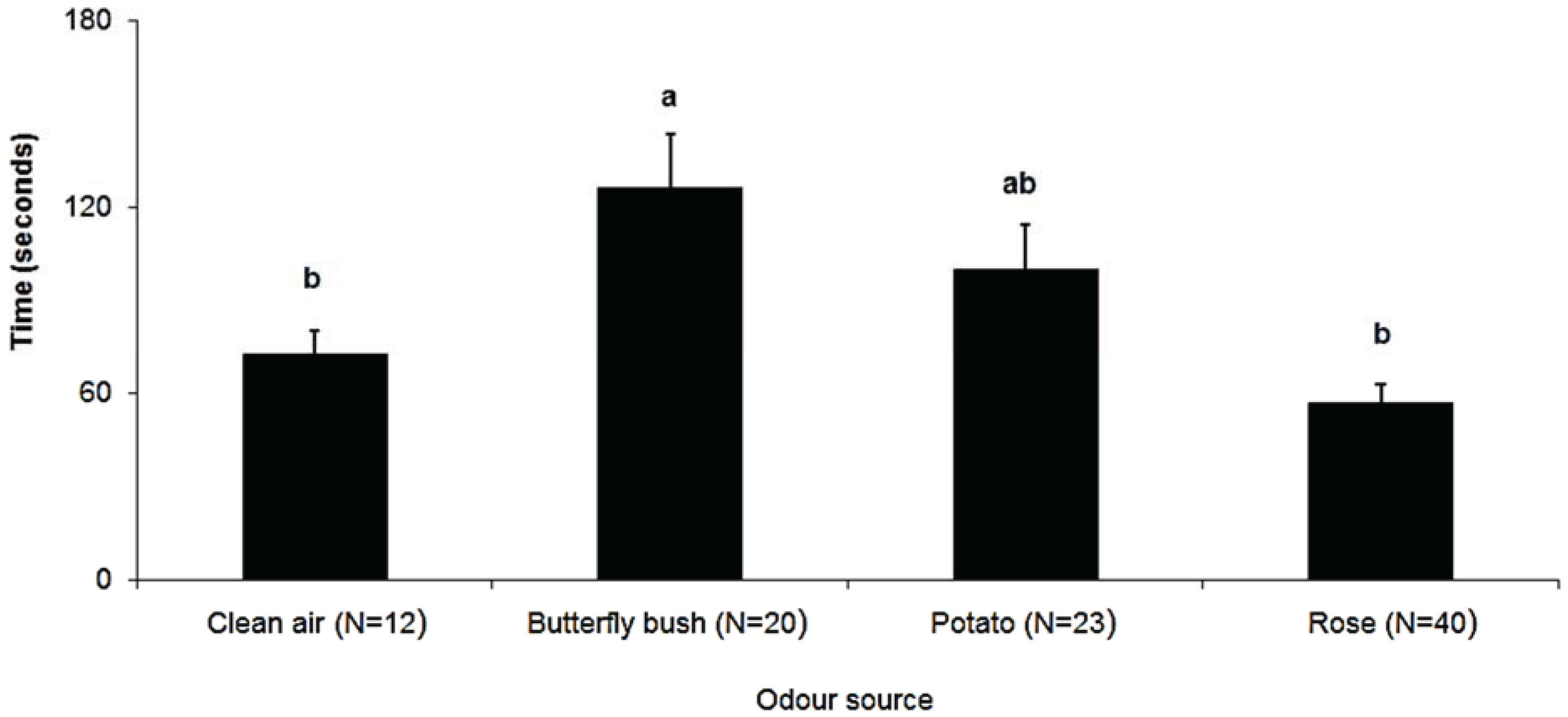

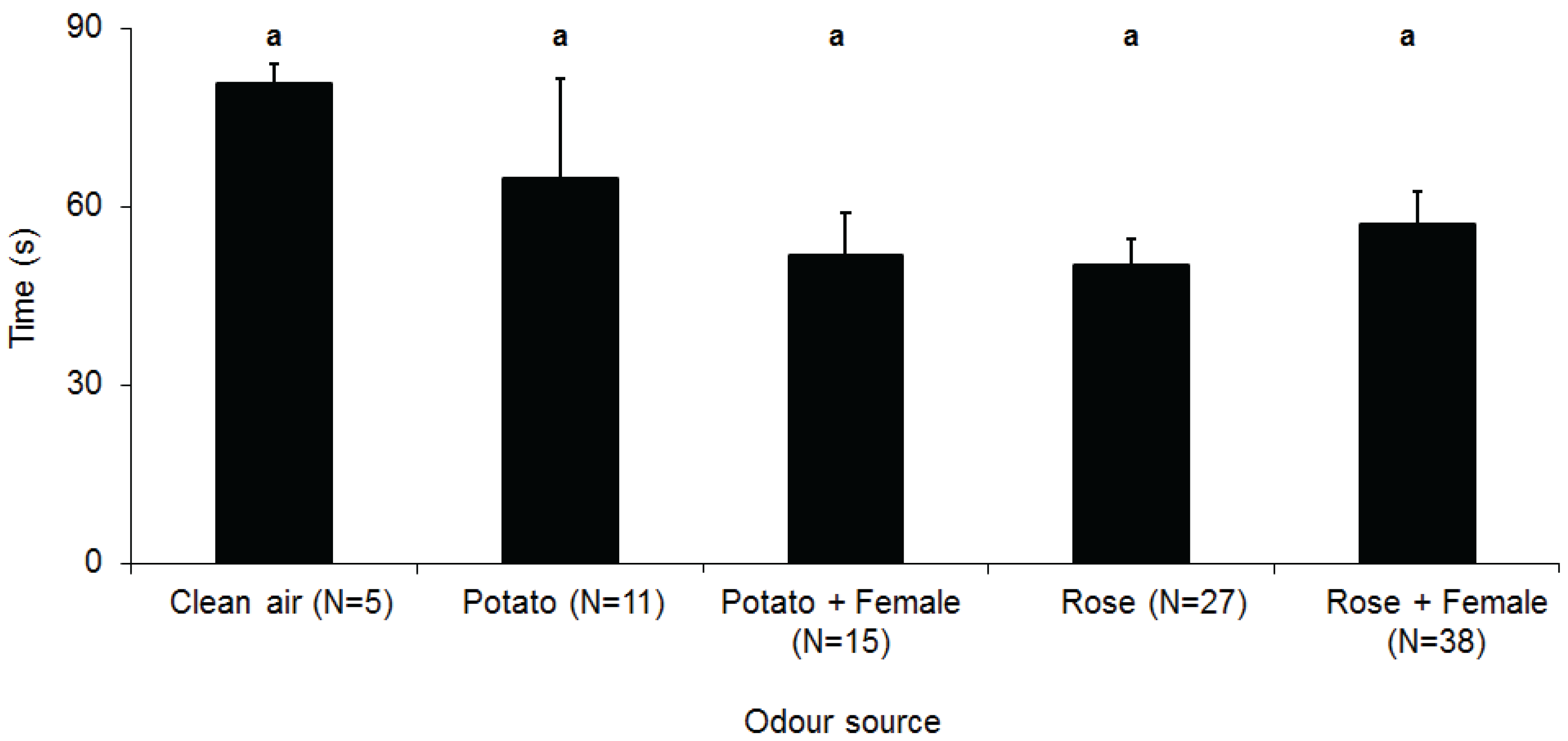
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Miller, J.R.; Stickler, K.L. Finding and accepting host plants. In Chemical Ecology of Insects; Bell, W.J., Carde, R.T., Eds.; Sinauer Associates: Sunderland, MA, USA, 1984; pp. 127–158. [Google Scholar]
- Stadler, E. Plant chemical cues important for egg deposition by herbivorous insects. In Chemoecology of Insect Eggs and Egg Deposition; Hilker, M., Meiners, T., Eds.; Blackwell Publishing: Oxford, UK, 2002; pp. 171–204. [Google Scholar]
- McNeil, J.N.; Delisle, J. Host plant pollen influences calling behaviour and ovarian development of the sunflower moth. Homeosoma Electellum Oecol. 1989, 80, 201–205. [Google Scholar]
- Raina, A.K.; Kingan, T.G.; Mattoo, A.K. Chemical signals from host plant and sexual behaviour in a moth. Science 1992, 255, 592–594. [Google Scholar] [CrossRef] [PubMed]
- Guldemond, J.A.; Dixon, A.F.G.; Pickett, J.A.; Wadhams, L.J.; Woodcock, C.M. Specificity of sex pheromones, the role of host plant odour in the olfactory attraction of males, and mate recognition in the aphid Cryptomyzus. Physiol. Entomol. 1993, 18, 137–143. [Google Scholar] [CrossRef]
- Campbell, C.A.M.; Dawson, G.W.; Griffiths, D.C.; Pettersson, J.; Pickett, J.A.; Wadhams, L.J.; Woodcock, C.M. Sex attractant pheromone of damson-hop aphid Phorodon humili (Homoptera, Aphididae). J. Chem. Ecol. 1990, 16, 3455–3465. [Google Scholar] [CrossRef] [PubMed]
- McNeil, J.N. Behavioural ecology of pheromone-mediated communication in moths and its importance in the use of pheromone traps. Ann. Rev. Entomol. 1991, 36, 407–430. [Google Scholar] [CrossRef]
- Landolt, P.J.; Phillips, T.W. Host plant influences on sex pheromone behaviour of phytophagous insects. Annu. Rev. Entomol. 1997, 42, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Xlangyu, J.; Geng, W.; Zhang, Z. Relevance of plant volatiles to sex pheromone in luring aphids in the field. Insect Sci. 2008, 7, 178–184. [Google Scholar]
- Pope, T.W.; Campbell, C.A.M.; Hardie, J.; Pickett, J.A.; Wadhams, L.J. Interactions between host-plant volatiles and the sex pheromones of the bird cherry-oat aphid, Rhopalosiphum padi and the damson-hop aphid, Phorodon humuli. J. Chem. Ecol. 2007, 33, 156–163. [Google Scholar]
- Dixon, A.F.G. Biology of Aphids; Edward Arnold: London, UK, 1973. [Google Scholar]
- Minks, A.K.; Harrewijn, P. World Crop Pests, Vol. 2. Aphids; Their Biology, Natural Enemies and Control, Part B; Elsevier: Amsterdam, The Netherlands, 1987. [Google Scholar]
- Pickett, J.A.; Wadhams, L.J.; Woodcock, C.M.; Hardie, J. The chemical ecology of aphids. Annu. Rev. Entomol. 1992, 37, 67–90. [Google Scholar] [CrossRef]
- Dixon, A.F.G. Aphid Ecology, 2nd ed.; Chapman and Hall: London, UK, 1998. [Google Scholar]
- Goldansaz, S.; Dewhirst, S.; Birkett, M.A.; Cooper, T.; Smiley, D.W.M.; Pickett, J.A.; Wadhams, L.; McNeil, J.N. Identification of two sex pheromone components of the potato aphid, Macrosiphum euphorbiae. (Thomas). J. Chem. Ecol. 2004, 30, 819–834. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, J. An aphid sex attractant I. Biological studies. Entomol. Scand. 1970, 1, 63–73. [Google Scholar] [CrossRef]
- Pettersson, J. An aphid sex attractant II. Histological, ethological and comparative studies. Entomol. Scand. 1971, 2, 81–93. [Google Scholar]
- Marsh, D. Sex pheromone in the aphid Megoura viciae. Nature 1972, 238, 31–32. [Google Scholar] [CrossRef] [PubMed]
- Losel, P.M.; Lindemann, M.; Scherkenbeck, J.; Campbell, C.A.M.; Hardie, J.; Pickett, J.A.; Wadhams, L.J. Effect of primary host kairomones on the attractiveness of the hop aphid sex pheromone to Phorodon humuli males and gynoparae. Entomol. Exp. Appl. 1996, 80, 79–82. [Google Scholar] [CrossRef]
- Nottingham, S.F.; Hardie, J.; Dawson, G.; Hick, A.; Pickett, J.A.; Wadhams, L.J.; Woodcock, C.M. Behavioral and electrophysiological responses of aphids to host and nonhost plant volatiles. J. Chem. Ecol. 1991, 17, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Lilley, R.; Hardie, J. Cereal aphid responses to sex pheromones and host-plant odours in the laboratory. Physiol. Entomol. 1996, 21, 304–308. [Google Scholar] [CrossRef]
- Goldansaz, S.H.; McNeil, J.N. Effect of wind speed on the pheromone-mediated behaviour of sexual morphs of the potato aphid, Macrosiphum euphoribae (Thomas) under laboratory and field conditions. J. Chem. Ecol. 2006, 32, 1719–1729. [Google Scholar] [CrossRef] [PubMed]
- Goldansaz, S.H.; McNeil, J.N. Calling behaviour of the potato aphid Macrosiphum euphorbiae (Thomas) (Homoptera: Aphididae) oviparae under laboratory and field conditions. Ecol. Entomol. 2003, 28, 291–298. [Google Scholar] [CrossRef]
- Dixon, A.F.G.; Howard, M.T. Dispersal in aphids, a problem in resource allocation. In Insect Flight, Dispersal and Migration; Danthanaryana, W., Ed.; Springer: Berlin, Germany, 1986; pp. 145–151. [Google Scholar]
- Ward, S.A.; Leather, S.R.; Pickett, J.; Harrington, R. Mortality during dispersal and the cost of host-specificity in parasites: How many aphids find hosts? J. Anim. Ecol. 1998, 67, 763–773. [Google Scholar] [CrossRef]
- Hardie, J. Spectral specificity for targeted flight in the black bean aphid, Aphis fabae. J. Insect Physiol. 1989, 35, 619–626. [Google Scholar] [CrossRef]
- Hardie, J.; Storer, R.S.; Cook, F.J.; Campbell, C.A.; Wadhams, L.J.; Lilley, R.; Peace, L. Sex pheromone and visual trap interactions in mate location strategies and aggregation by host-alternating aphids in the field. Physiol. Entomol. 1996, 21, 97–106. [Google Scholar] [CrossRef]
- Powell, G.; Hardie, J. The chemical ecology of aphid host alternation: How do return migrants find the primary host plant? Appl. Entomol. Zool. 2001, 36, 259–267. [Google Scholar] [CrossRef]
- Storer, J.R.; Powell, G.; Hardie, J. Settling responses of aphids in air permeated with non-host plant volatiles. Entomol. Exp. Appl. 1996, 80, 76–78. [Google Scholar] [CrossRef]
- Powell, G.; Hardie, J. A potent, morph-specific parturition stimulant in the overwintering host plant of the black bean aphid, Aphis fabae. Physiol. Entomol. 2001, 26, 194–201. [Google Scholar] [CrossRef]
- Hurley, J. The importance of primary host plant volatiles in the reproductive biology of the potato aphid, Macrosiphum euphorbiae. M.Sc. Thesis, University of Western Ontario, London, ON, Canada, 2009. [Google Scholar]
- Vinson, S.B. Host selection by insect parasitoids. Annu. Rev. Entomol. 1976, 21, 109–133. [Google Scholar] [CrossRef]
- Kennedy, J.S. Zigzagging and casting as a programmed response to wind-borne odour: A review. Phsiol. Entomol. 1983, 8, 109–120. [Google Scholar] [CrossRef]
- Kennedy, J.S.; Ludlow, A.R.; Sanders, C.J. Guidance system used in moth sex attraction. Nature 1980, 288, 475–477. [Google Scholar] [CrossRef]
- Kennedy, J.S.; Ludlow, A.R.; Sanders, C.J. Guidance of flying male moths by wind-borne sex pheromone. Physiol. Entomol. 1981, 6, 395–412. [Google Scholar] [CrossRef]
- Cardé, R.T. Chemo-orientation in flying insects. In Chemical Ecology of Insects; Bell, W.J., Cardé, R.T., Eds.; Chapman and Hall: London, UK, 1984; pp. 111–124. [Google Scholar]
- Dawson, G.W.; Griffiths, D.C.; Merritt, L.A.; Mudd, A.; Pickett, J.A.; Wadhams, L.J.; Woodcock, C.M. Aphid semiochemicals—A review, and recent advances on the sex pheromone. J. Chem. Ecol. 1990, 16, 3019–3030. [Google Scholar] [CrossRef]
- Hardie, J.; Holyoak, M.; Nicholas, J.; Nottingham, S.F.; Pickett, J.A.; Wadhams, L.J.; Woodcock, C.M. Aphid sex pheromone components: Age-dependent release by females and species-specific male response. Chemoecology 1990, 1, 63–68. [Google Scholar] [CrossRef]
- Boo, K.S.; Choi, M.Y.; Chung, I.B.; Eastop, V.F.; Pickett, J.A.; Wadhams, L.J.; Woodcock, C.M. Sex pheromone of the peach aphid, Tuberocephalus momonis and optimal blends for trapping males and females in the field. J. Chem. Ecol. 2000, 26, 601–609. [Google Scholar] [CrossRef]
- Stewart-Jones, A.; Dewhirst, S.Y.; Durrant, L.; Fitzgerald, J.D.; Hardie, J.; Hooper, A.M.; Pickett, J.A.; Poppy, G.M. Structure, ratios and patterns of release in the sex pheromone of an aphid, Dysaphis plantaginea. J. Exp. Biol. 2007, 210, 4335–4344. [Google Scholar] [CrossRef] [PubMed]
- Foster, S.P.; Denholm, I.; Thompson, R. Variation in response to neonicotinoid insecticides in peach-potato aphids, Myzus persicae (Hemiptera: Aphididae). Pest Manag. Sci. 2003, 59, 166–173. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hurley, J.; Takemoto, H.; Takabayashi, J.; McNeil, J.N. Host Plant Volatiles and the Sexual Reproduction of the Potato Aphid, Macrosiphum euphorbiae. Insects 2014, 5, 783-792. https://doi.org/10.3390/insects5040783
Hurley J, Takemoto H, Takabayashi J, McNeil JN. Host Plant Volatiles and the Sexual Reproduction of the Potato Aphid, Macrosiphum euphorbiae. Insects. 2014; 5(4):783-792. https://doi.org/10.3390/insects5040783
Chicago/Turabian StyleHurley, Jessica, Hiroyuki Takemoto, Junji Takabayashi, and Jeremy N. McNeil. 2014. "Host Plant Volatiles and the Sexual Reproduction of the Potato Aphid, Macrosiphum euphorbiae" Insects 5, no. 4: 783-792. https://doi.org/10.3390/insects5040783



