Effect of Olfactory Stimulus on the Flight Course of a Honeybee, Apis mellifera, in a Wind Tunnel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Honeybee
2.2. Wind Tunnel
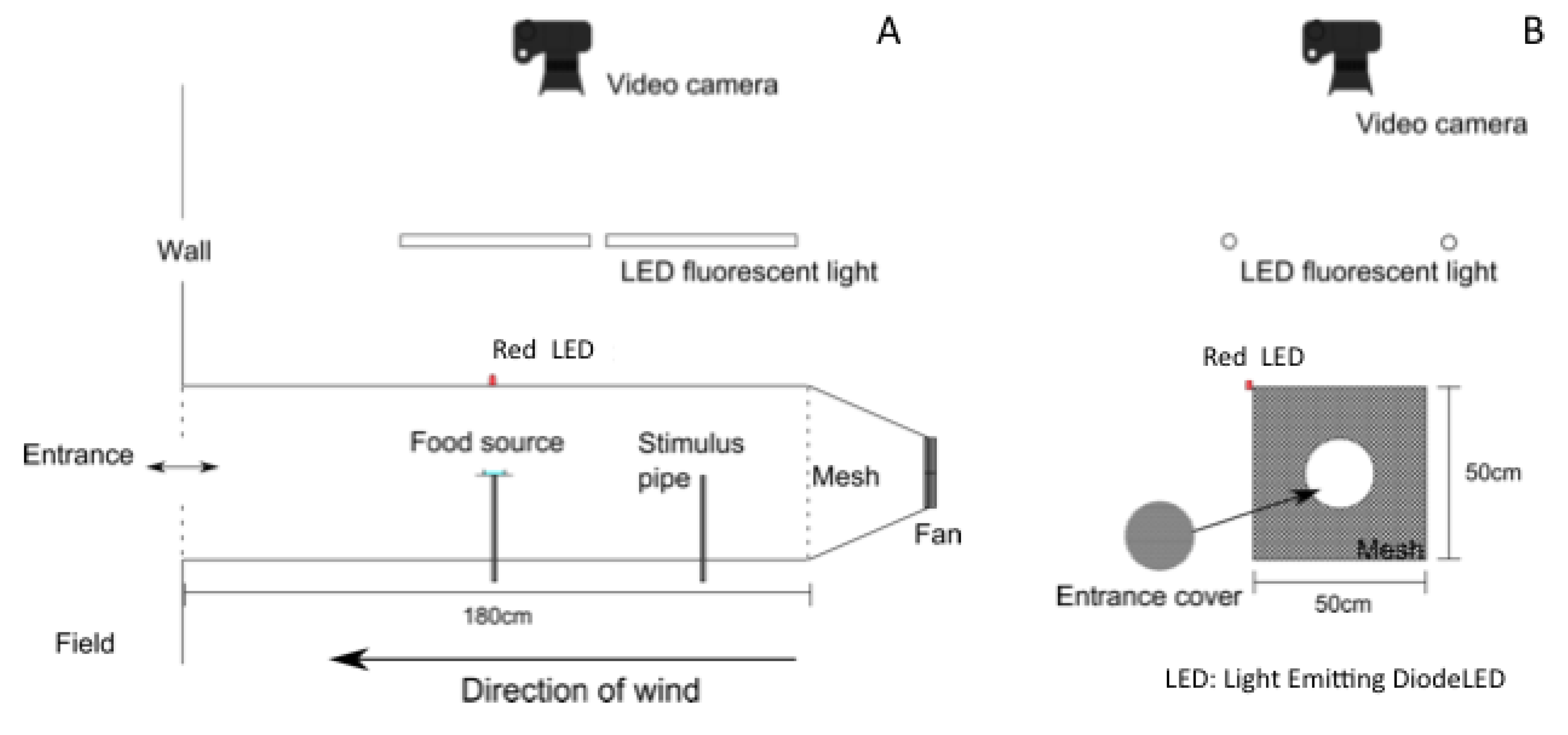
2.3. Odor Stimuli
2.4. Measurements
2.5. Data Analysis
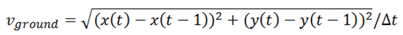
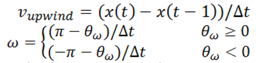
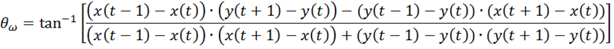
3. Results and Discussion
3.1. Results
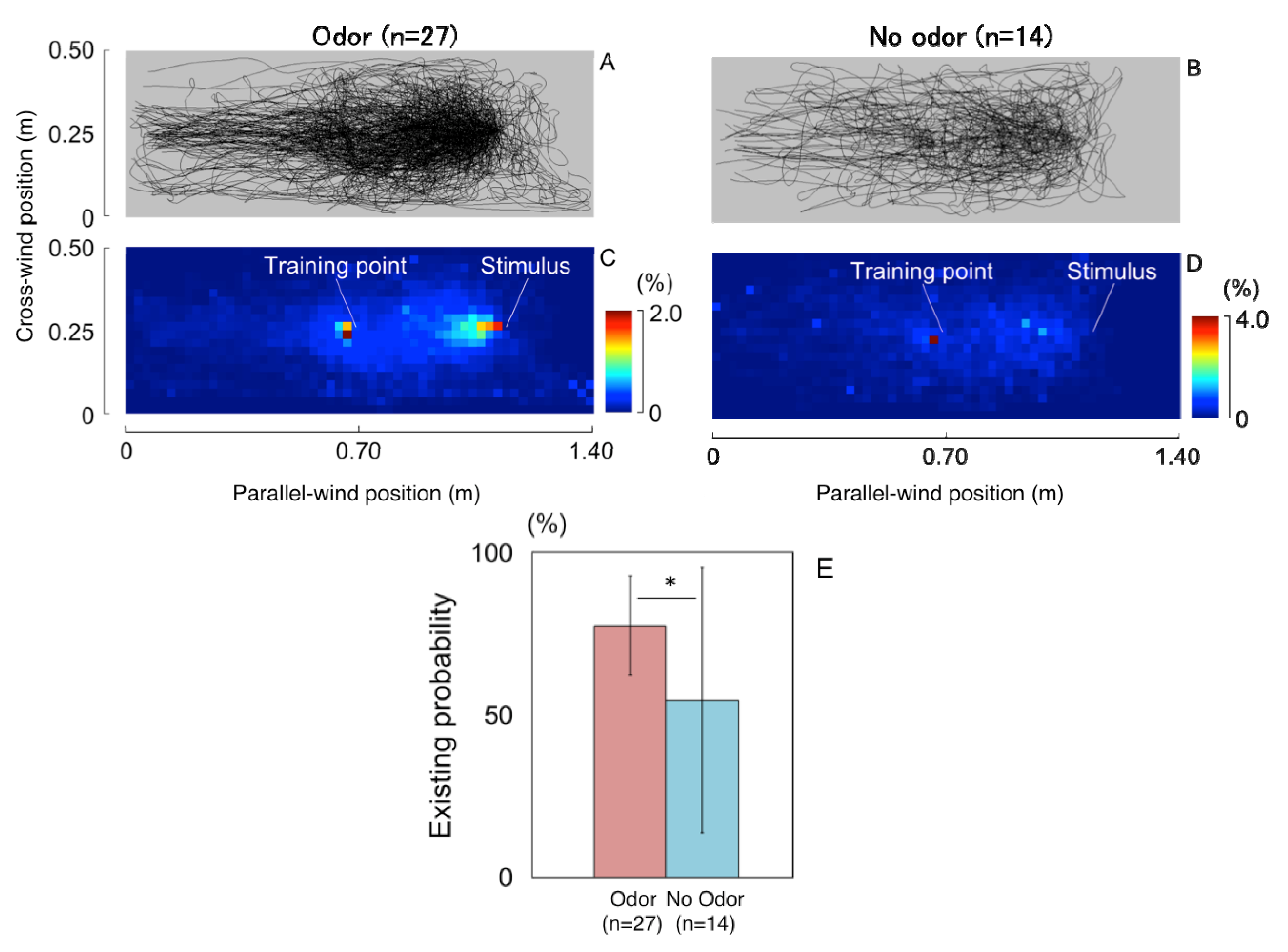
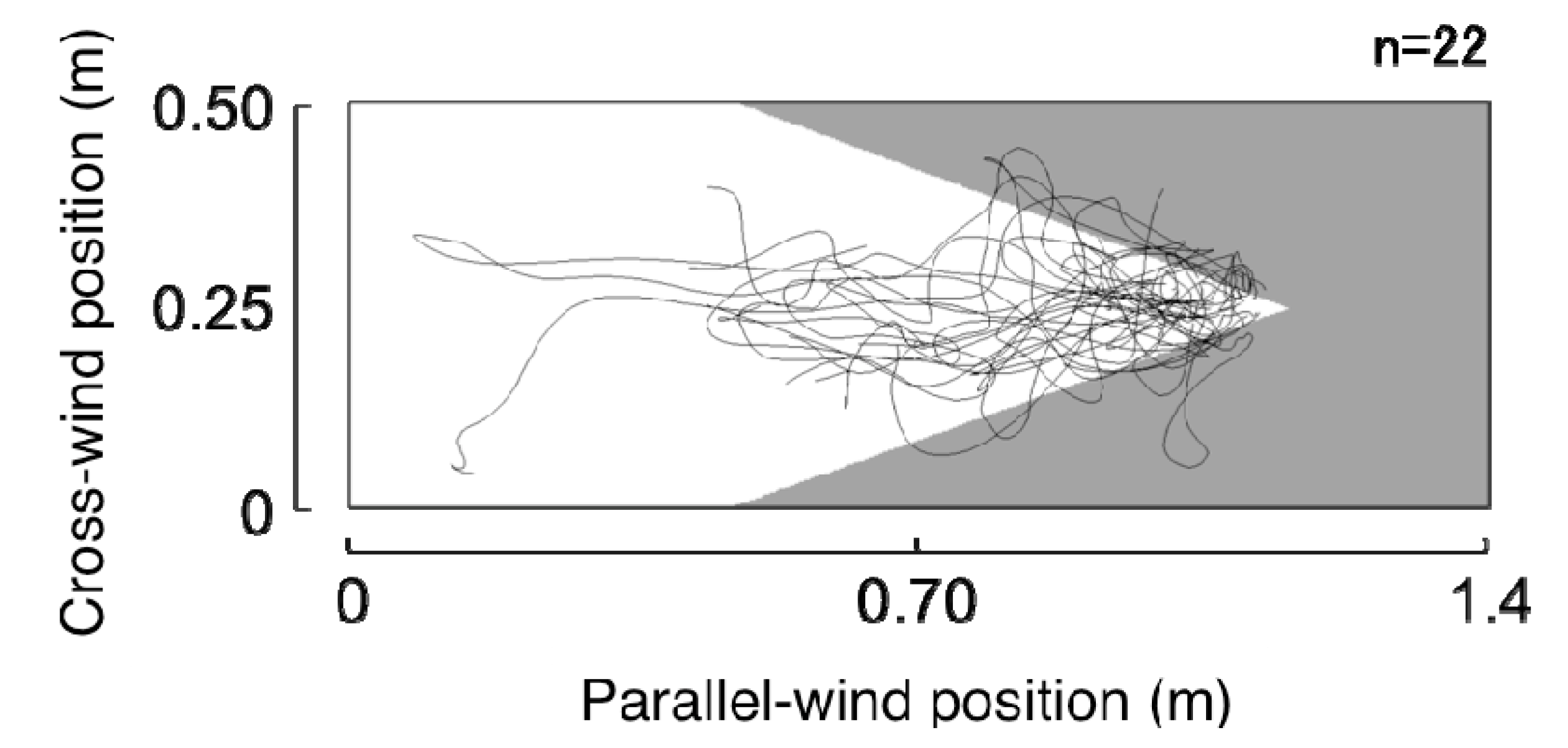
3.1.1. Searching Area

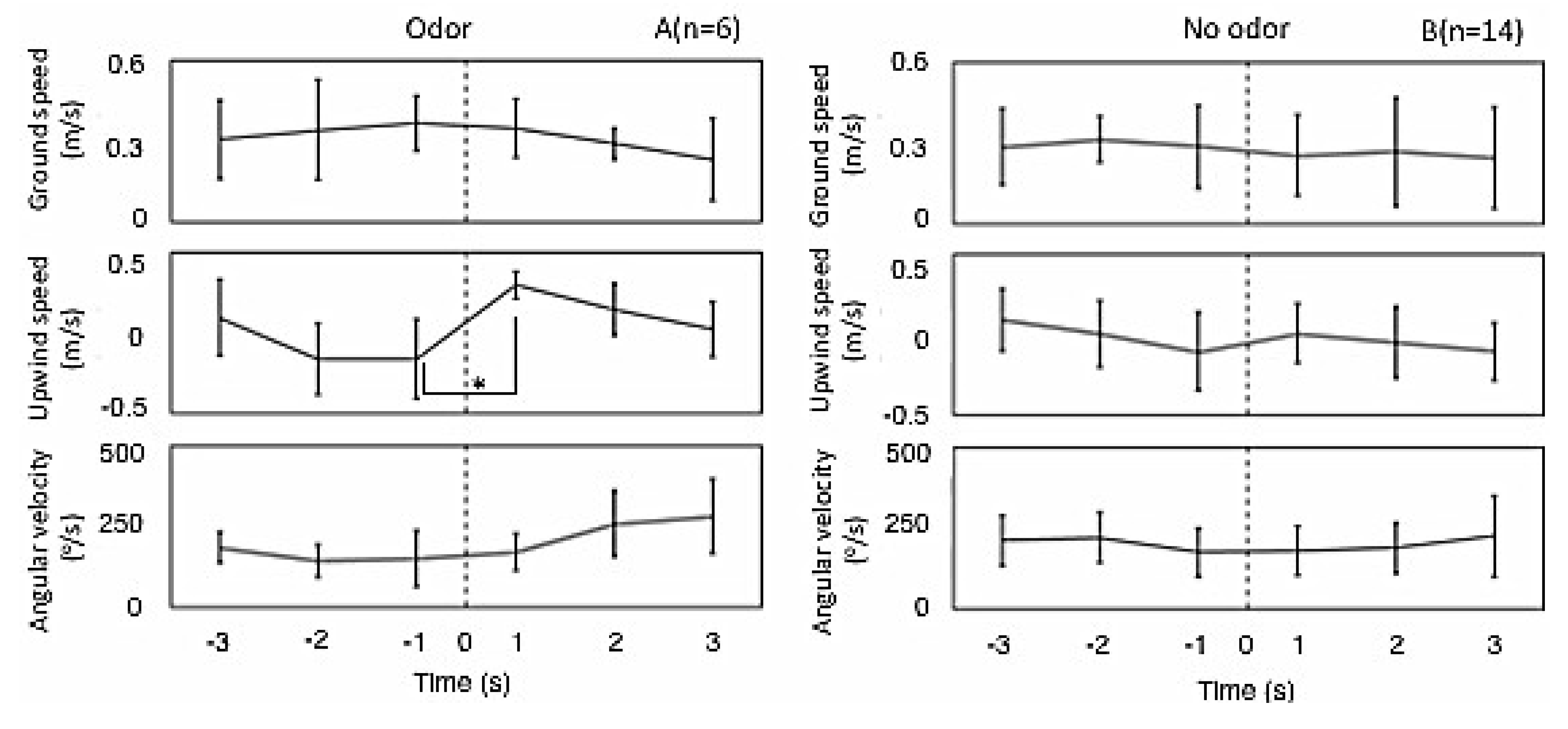
3.1.2. Flight Properties
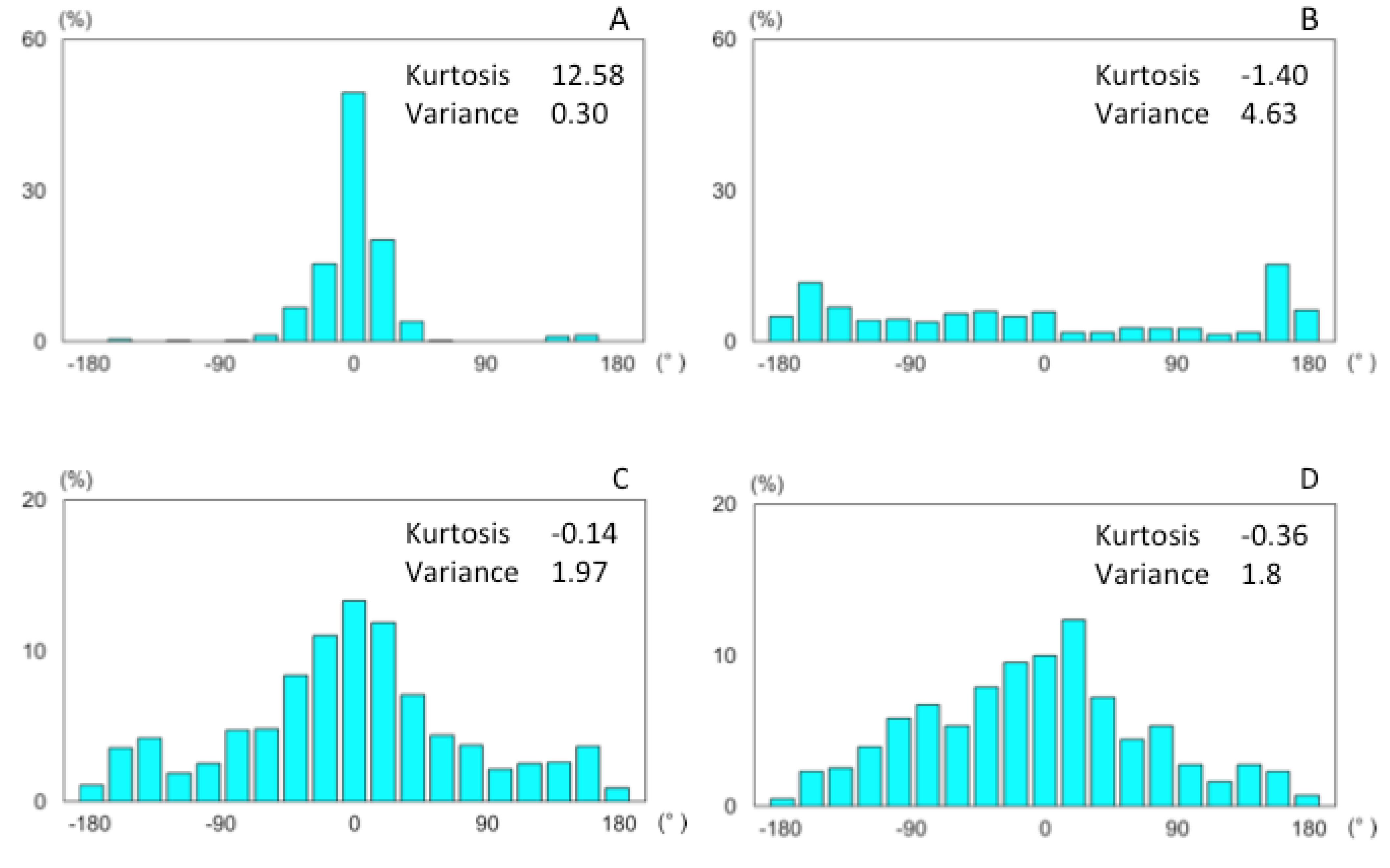
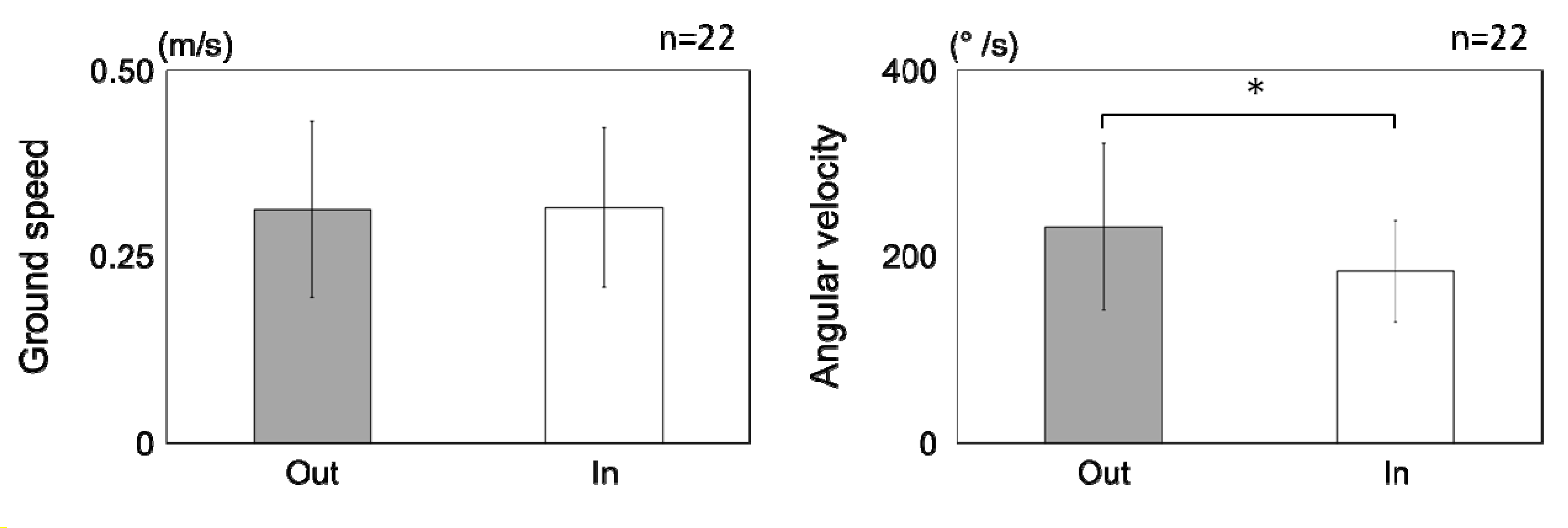
3.2. Discussion
3.2.1. Search Area
3.2.2. Behavioral Properties
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Wenner, A.M.; Wells, P.H.; Johnson, D.L. Honey bee recruitment to food sources: Olfaction or language? Science 1969, 164, 84–86. [Google Scholar]
- Valdusich, T.; Hemmi, J.M.; Zeil, J. Honeybee odometry and scent guidance. J. Exp. Biol. 2006, 209, 1367–1375. [Google Scholar] [CrossRef]
- Kirchner, W.H.; Grasser, A. The significance of odor cues and dance language information for the food search behavior of honeybees (Hymenoptera: Apidae). J. Insect Behav. 1998, 11, 169–178. [Google Scholar] [CrossRef]
- Gil, M.; Marco, R.J. Olfactory learning by means of trophallaxis in Apis mellifera. J. Exp. Biol. 2005, 208, 671–680. [Google Scholar] [CrossRef]
- Gruter, C.; Acosta, L.E.; Farina, W.M. Propagation of olfactory information within the honeybee hive. Behav. Ecol. Sociobiol. 2006, 60, 707–715. [Google Scholar] [CrossRef]
- Kellog, F.E.; Frizel, D.F.; Wright, R.H. The olfactory guidance of flying insects. IV Drosophila. Can. Entomol. 1962, 94, 884–888. [Google Scholar]
- Baker, T.C.; Willis, M.A.; Phelan, P.L. Optomotor anemotaxis polarizes self-steered zigzagging in flying moths. Physiol. Entomol. 1984, 9, 365–376. [Google Scholar] [CrossRef]
- Baker, T.C.; Willis, M.A.; Haynes, K.F.; Phelan, P.L. A pulsed cloud of sex pheromone elicits upwind flight in male moths. Physiol. Entomol. 1985, 10, 257–265. [Google Scholar] [CrossRef]
- Byers, J.A. Upwind flight orientation to pheromone in western pine beetle tested with rotating windvane traps. J. Chem. Ecol. 1988, 14, 189–198. [Google Scholar] [CrossRef]
- Willis, M.A.; Arbas, E.A. Odor-modulated upwind flight of the phinx moth, Manduca sexta L. J. Comp. Physiol. A 1991, 169, 427–440. [Google Scholar]
- Barata, E.N.; Araujo, J. Olfactory orientation responses of the eucalyptus woodborer, Phoracantha semipunctata, to host plant in a wind tunnel. Physiol. Entomol. 2001, 26, 26–37. [Google Scholar] [CrossRef]
- Frye, M.A.; Tarsitano, M.; Dickinson, M.H. Odor localization requires visual feedback during free flight in Drosophila melanogaster. J. Exp. Biol. 2002, 206, 843–855. [Google Scholar]
- Budick, S.A.; Dickinson, M.H. Free-flight responses of Drosophila melanogaster to attractive odor. J. Exp. Biol. 2006, 209, 3001–3017. [Google Scholar] [CrossRef]
- Becher, P.G.; Bengtsson, M.; Hansson, B.S.; Witzgall, P. Flying the fly: Long-range flight behavior of Drosophila melanogaster to attractive odors. J. Chem. Ecol. 2010, 36, 599–607. [Google Scholar] [CrossRef]
- Jacobs, L.F. From chemotaxis to the cognitive map: The function of olfaction. Proc. Natl. Acad. Sci. USA 2012, 109, 10693–10700. [Google Scholar] [CrossRef]
- Kanzaki, R.; Sugi, N.; Shibuya, T. Self-generated zigzag turning of Bombyx mori males during pheromone-mediated upwind walking. Zool. Sci. 1992, 9, 515–527. [Google Scholar]
- Reynolds, A.M.; Swain, J.L.; Smith, A.D.; Martin, A.P.; Osborne, J.L. Honeybees use a Lévy flight search strategy and odour-mediated anemotaxis to relocate food sources. Behav. Ecol. Sociobiol. 2009, 64, 115–123. [Google Scholar] [CrossRef]
- Menzel, R.; Greggers, U. Guidance by odors in honeybee navigation. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2013, 199, 867–873. [Google Scholar] [CrossRef]
- Virtual DubMod Homepage. Available online: http://virtualdubmod.sourceforge.net/ (accessed on 31 October 2013).
- Wehner, R.; Rossel, S. The bee’s celestial compass-a case study in behavioral neurobiology. In Experimental Behavioral Ecology and Sociobiology; Holldobler, B., Lindauer, M., Eds.; Sinauer Associates: Sunderland, CT, USA, 1985; pp. 11–53. [Google Scholar]
- Von Frisch, K. Der Farbensinn und Formensinn der Bienen. In Abteilung für Allgemeine Zoologie und Physiologie der Tiere; Verlag von Gustav Fischer: Jena, Germany, 1914; Volume 35, pp. 1–188. [Google Scholar]
- Esch, H.E.; Burns, J.E. Honeybees use optic flow to measure the distance of a food source. Naturwissenshaften 1995, 82, 28–40. [Google Scholar] [CrossRef]
- Esch, H.E.; Burns, J.E. Distance estimation by foraging honeybees. J. Exp. Biol. 1996, 199, 155–162. [Google Scholar]
- Srinivasan, M.V.; Zhang, S.W.; Lehrer, M.; Collet, T.S. Honeybee navigation en route to the goal: Visual flight control and odometry. J. Exp. Biol. 1996, 199, 237–244. [Google Scholar]
- Srinivasan, M.V.; Zhang, S.W.; Bidwell, N. Visually mediated odometry in honebees. J. Exp. Biol. 1997, 200, 2513–2522. [Google Scholar]
- Srinivasan, M.V.; Zhang, S.W.; Altwein, M.; Tautz, J. Honeybee navigation: Nature and calibration of the ‘odometer’. Science 2000, 287, 851–853. [Google Scholar] [CrossRef]
- Esch, H.E.; Zhang, S.; Srinivasan, M.V.; Tautz, J. Honeybee dances communicate distances measured by optic flow. Nature 2001, 411, 581–583. [Google Scholar] [CrossRef]
- Tautz, J.; Zhang, S.W.; Spaethe, J.; Brockmann, A.; Si, A.; Srinivasan, M. Honeybee odometry: Performance in varying natural terrain. PLoS Biol. 2004, 2, 915–923. [Google Scholar]
- Weber, A.A.; Portelli, G.; Benard, B.; Dyer, A.; Giurfa, M. Configural processing enables discrimination and categorization of face-like stimuli in honeybees. J. Exp. Biol. 2010, 213, 593–601. [Google Scholar] [CrossRef]
- Franceschini, N.; Ruffier, F.; Serres, J.; Viollet, S. Optic flow based visual guidance: From flying insects to miniature aerial vehicles. In Aerial Vehicles; Lam, T.M., Ed.; InTech: Rijeka, Croatia, 2009; pp. 747–770. [Google Scholar]
- Chaffiol, A.; Laloi, D.; Pham-Delegue, M.-H. Prior classical olfactory conditioning improves odour-cued flight orientation of honey bees in a wind tunnel. J. Exp. Biol. 2005, 208, 3731–3737. [Google Scholar] [CrossRef]
- Reinhard, J.; Srinivasan, M.V.; Guez, D.; Zhang, S.W. Floral scents induce recall of navigational and visual memories in honeybees. J. Exp. Biol. 2004, 207, 4371–4381. [Google Scholar] [CrossRef]
- Sigg, D.; Thompson, C.M.; Mercer, A.R. Activity-dependent changes to the brain and behavior of the honey bee, Apis mellifera (L.). J. Neurosci. 1997, 17, 7148–7156. [Google Scholar]
- Farris, S.M.; Robinson, G.E.; Farhbach, S.E. Experience- and age-related outgrowth of intrinsic neurons in the Mushroom bodies of the adult worker honeybee. J. Neurosci. 2001, 21, 6395–6404. [Google Scholar]
- Frye, M.A. Effects of stretch receptor ablation on the optomotor control of lift in the hawkmoth Manduca sexta. J. Exp. Biol. 2001, 204, 3683–3691. [Google Scholar]
- Tytell, E.D.; Ellington, C.P. How to perform measurements in a hovering animal’s wake: Physical modeling of the cortex wake of the hawkmoth, Manduca sexta. Philos. Trans. R. Soc. Lond. B 2003, 358, 1559–1566. [Google Scholar] [CrossRef]
- Bastlan, J.; Esch, H. The nervous control of the indirect flight muscles of the honey bee. Z. Vgl. Physiol. 1970, 67, 307–324. [Google Scholar] [CrossRef]
- Altshuler, D.L.; Dickson, W.B.; Vance, J.T.; Roverts, S.P.; Dickinson, M.H. Short-amplitude high-frequency wing strokes determine the aerodynamics of honeybee flight. Proc. Natl. Acad. Sci. USA 2005, 102, 18213–18218. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ikeno, H.; Akamatsu, T.; Hasegawa, Y.; Ai, H. Effect of Olfactory Stimulus on the Flight Course of a Honeybee, Apis mellifera, in a Wind Tunnel. Insects 2014, 5, 92-104. https://doi.org/10.3390/insects5010092
Ikeno H, Akamatsu T, Hasegawa Y, Ai H. Effect of Olfactory Stimulus on the Flight Course of a Honeybee, Apis mellifera, in a Wind Tunnel. Insects. 2014; 5(1):92-104. https://doi.org/10.3390/insects5010092
Chicago/Turabian StyleIkeno, Hidetoshi, Tadaaki Akamatsu, Yuji Hasegawa, and Hiroyuki Ai. 2014. "Effect of Olfactory Stimulus on the Flight Course of a Honeybee, Apis mellifera, in a Wind Tunnel" Insects 5, no. 1: 92-104. https://doi.org/10.3390/insects5010092



