Biological Control of Tephritid Fruit Flies in Argentina: Historical Review, Current Status, and Future Trends for Developing a Parasitoid Mass-Release Program
Abstract
:1. Introduction
2. Current Fruit Fly Control Strategies
3. Fruit Fly Biological Control
3.1. Historical Overview
3.1.1. Native Parasitoids
| Parasitoid species and families | Parasitism modes 4 | Feeder types 5 | Host stage attacked 6 | Host fruit fly species |
|---|---|---|---|---|
| BRACONIDAE | ||||
| Asobara anastrephae (Muessebeck) 1 | S, K | En | L3 | Af |
| Doryctobracon areolatus (Szépligeti) 1 | S, K | En | L2–L3 | Af, Cc 8 |
| Doryctobracon brasiliensis (Szépligeti) 1 | S, K | En | L3 | Af, Cc 8 |
| Doryctobracon crawfordi (Viereck) 1 | S, K | En | L3 | Af |
| Opius bellus Gahan 1 | S K | En | L3 | Af |
| Utetes anastrephae (Viereck) 1 | S, K | En | L3 | Af, Cc 8 |
| FIGITIDAE | ||||
| Aganaspis pelleranoi (Brèthes) 1 | S, K | En | L3 | Af, Cc |
| Dicerataspis grenadensis Ashmead 1 | S, K | En | L3 | Af 8 |
| Lopheucoila anastrephae (Rohwer) 1 | S, K | En | L3 | Af |
| Rhoptromeris haywardi (Blanchard) 1 | S, K | En | L(?) 7 | Af 8, Cc 8 |
| Odontosema anastrephae Borgmeier 1 | S, K | En | L3 | Af |
| DIAPRIIDAE | ||||
| Coptera haywardi Loiácono 1 | S, I | En | P | Af, Cc |
| Tricopria anastrephae Costa Lima 1 | S, I | En | P | Af, Cc 8 |
| PTEROMALIDAE | ||||
| Pachycrepoideus vindemmiae (Rondani) 2 | S, I | Ec | P | Af, Cc |
| Pachyneuron sp. 3 | S, I | Ec | P | Cc |
| Spalangia sp. 3 | S, I | Ec | P | Cc |
3.1.2. Exotic Parasitoids
| Introduction year(s) | Parasitoid species | Source 1 (institution and country) | Target host(s) | Successfully laboratory Reared | Parasitoid status | Releaseyear(s) | Totalnumber released | ||
|---|---|---|---|---|---|---|---|---|---|
| Released | Recovered | Established | |||||||
| 1947 | Tetrastichus giffardianus | IBSP, Brasil | C. capitata | No | Yes | Yes | No | 1947 | ? |
| 1961 | Aceratoneuromyia indica | DGSV, Mexico | A. fraterculus | Yes | Yes | Yes | Yes | 1966–1969 | 700,000 |
| C. capitata | 1973–1977 | 100,000 | |||||||
| Diachasmimorpha longicaudata | DGSV, Mexico | A. fraterculus | Yes | Yes | Yes | Yes | 1966–1969 | 10,000 | |
| C. capitata | 1973–977 | 29,700 | |||||||
| Pachycrepoideusvindemmiae2 | DGSV, Mexico | A. fraterculus | Yes | Yes | Yes | Yes | 1966–1969 | 96,000 | |
| C. capitata | 1973–1977 | 259,200 | |||||||
| 1966 | Fopius arisanus | DGSV, Mexico | C. capitata | No | Yes | No | No | 1966–1967 | ? |
| Doryctobracon crawfordi | DGSV, Mexico | A. fraterculus | No | Yes | No | No | 1966–1967 | ? | |
| 1977 | A. indica | DGSV, Mexico | A. fraterculus | Yes | No | --- | --- | --- | --- |
| C. capitata | |||||||||
| 1986 | A. indica | OIRSA, Costa Rica | A. fraterculus | Yes | Yes | No | No | 1986–1988 | --- |
| C. capitata | |||||||||
| D. longicaudata | OIRSA, Costa Rica | A. fraterculus | Yes | Yes | No | No | 1986–1988 | 8,000 | |
| C. capitata | |||||||||
| P. vindemmiae 2 | OIRSA, Costa Rica | A. fraterculus | Yes | Yes | Yes | --- | 1986–1988 | 3,000 | |
| C. capitata | |||||||||
| 1999 | Diachasmimorpha tryoni | DGSV-Mexico | C. capitata | Yes | No | --- | --- | --- | --- |
| D. longicaudata | DGSV-Mexico | A. fraterculus | Yes | Yes | Yes | --- | 2012 to present | ca. 520,000 | |
| C. capitata | |||||||||
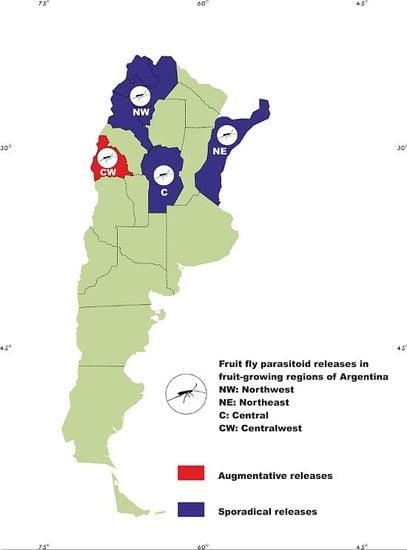
3.2. Current Status
3.3. Future Trends
4. Conclusions
Acknowledgments
References
- Aruani, R.; Ceresa, A.; Granados, J.C.; Taret, G.; Peruzzotti, P.; Ortiz, G. Advances in the National Fruit Fly Control and Eradication Program in Argentina. In Fruit Fly Pests: A World Assessment of Their Biology and Management; McPheron, B.A., Steck, G.J., Eds.; St. Lucie Press: DelRay Beach, FL, USA, 1996; pp. 521–530. [Google Scholar]
- Guillén, D.; Sánchez, R. Expansion of the National Fruit Fly Control Programme in Argentina. In Area-wide Control of Insects Pests: From Research to Field Implementation; Vreysen, M.J.B., Robinson, A.S., Hendrichs, J., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 653–660. [Google Scholar]
- Copeland, R.S.; Wharton, R.; Luke, Q.; de Meyer, M. Indigenous host of Ceratitis capitata (Diptera: Tephritidae) in Kenya. Ann. Entomol. Soc. Am. 2002, 95, 672–694. [Google Scholar] [CrossRef]
- White, I.M.; Elson-Harris, M.M. Fruit Flies of Economic Significance: Their Identification and Bionomics; CABI Publishing: Wallingford, UK, 1992; p. 601. [Google Scholar]
- Ovruski, S.M.; Schliserman, P.; Aluja, M. Native and introduced host plants of Anastrepha fraterculus and Ceratitis capitata (Diptera: Tephritidae) in Northwestern Argentina. J. Econ. Entomol. 2003, 96, 1108–1118. [Google Scholar] [CrossRef]
- Segura, D.F.; Vera, M.T.; Cagnotti, C.L.; Vaccaro, N.; DeColl, O.R.; Ovruski, S.M.; Cladera, J.L. Relative abundance of Ceratitis capitata and Anastrepha fraterculus (Diptera: Tephritidae) in diverse host species and localities of Argentina. Ann. Entomol. Soc. Am. 2006, 99, 70–83. [Google Scholar] [CrossRef]
- Aluja, M.; Pérez-Staples, D.; Macías-Ordoñez, R.; Piñero, J.; McPheron, B.; Hernández-Ortiz, V. Nonhost status of Citrus sinensis cultivar valencia and C. paradisi cultivar riby red to Mexican Anastrepha fraterculus (Diptera: Tephritidae). J. Econom. Entomol. 2003, 96, 1693–1703. [Google Scholar] [CrossRef]
- Vera, M.T.; Cáceres, C.; Wornoayporn, V.; Islam, A.; Robinson, A.S.; de la Veja, M.H.; Hendrichs, J.; Cayol, J.P. Mating incompatibility among populations of the South American fruit fly Anastrepha fraterculus (Wied.) (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 2006, 99, 387–397. [Google Scholar] [CrossRef]
- Oroño, L.E.; Ovruski, S.M.; Norrbom, A.L.; Schliserman, P.; Colin, C.; Martin, C.B. Two new native host plant records for Anastrepha fraterculus (Diptera: Tephritidae) in Argentina. Florida Entomol. 2005, 88, 228–232. [Google Scholar] [CrossRef]
- Putruele, G.; Abbiati, N.N.; Vaccaro, N.C. Soybean Protein Hydrolysate Bait for Medfly Control. In Fruit Flies: Biology and Management; Aluja, M., Liedo, P., Eds.; Springer-Verlag: New York, NY, USA, 1993; pp. 369–373. [Google Scholar]
- Turica, A.; Vergani, A.R.; Quintanilla, R.H.; Zerbino, M.C.; Ceruso, H.E. Las moscas de los frutos. INTA Ser. Form. Téc. Agríc. (Argentina) 1971, 7, 1–17. [Google Scholar]
- Harris, E.J.; Bautista, R.C.; Spencer, J.P. Utilisation of the Egg-larval Parasitoid, Fopius arisanus, for Augmentative Biological Control of Tephritid Fruit Flies. In Area-Wide Control of Fruit Flies and Other Insect Pests; Tan, K.H., Ed.; Penerbit Universiti Sains: Penang, Malaysia, 2000; pp. 725–732. [Google Scholar]
- Montoya, P.; Cancino, J.; Zenil, M.; Santiago, G.; Gutierrez, J.M. The augmentative Biological Control Component in the Mexican National Campaign against Anastrepha spp. Fruit Flies. In Area-wide Control of Insects Pests: From Research to Field Implementation; Vreysen, M.J.B., Robinson, A.S., Hendrichs, J., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 661–670. [Google Scholar]
- Montoya, P.; Liedo, P.; Benrey, B.; Barrera, J.F.; Cancino, J.; Sivinski, J.; Aluja, M. Biological control of Anastrepha spp. (Diptera: Tephritidae) in mango orchards through augmentative releases of Diachasmimorpha longicaudata (Ashmead) (Hymenoptera: Braconidae). Biol. Control 2000, 18, 212–224. [Google Scholar]
- Purcell, M.F. Contribution of biological control to integrated pest management of tephritid fruit flies in the tropics and subtropics. Int. Pest Manag. Rev. 1998, 3, 63–83. [Google Scholar] [CrossRef]
- Sivinski, J.M.; Calkins, C.O.; Baranowski, R.; Harris, D.; Brambila, J.; Diaz, J.; Burns, R.E.; Holler, T.; Dodson, G. Suppression of a Caribbean fruit fly (Anastrepha suspensa (Loew) (Diptera: Tephritidae) population through augmentative releases of the parasitoid Diachasmimorpha longicaudata (Ashmead) (Hymenoptera: Braconidae). Biol. Control 1996, 6, 177–185. [Google Scholar] [CrossRef]
- Vargas, R.I.; Peck, S.L.; McQuate, G.T.; Jackson, C.G.; Stark, J.D.; Armstrong, J.W. Potential for areawide integrated management of mediterranean fruit fly (Diptera: Tephritidae) with a braconid parasitoid and a novel bait spray. J. Econ. Entomol. 2001, 94, 817–825. [Google Scholar] [CrossRef]
- López, M.; Sivinski, J.; Rendón, P.; Holler, T.; Bloem, K.; Copeland, R.: Trostle, M.; Aluja, M. Colonization of Fopius ceratitivorus, a newly discovered African eggpupal parasitoid (Hymenoptera: Braconidae) of Ceratitis capitata (Diptera: Tephritidae). Fla. Entomol. 2003, 86, 53–60. [Google Scholar] [CrossRef]
- Wang, X.G.; Bokonon-Ganta, A.H.; Ramadan, M.M.; Messing, R.H. Egg-larval Opiine parasitoids (Hymenoptera: Braconidae) of tephritid fruit fly pests do not attack the flowerhead-feeder Trupanea dubautiae (Diptera: Tephritidae). J. Appl. Entomol. 2004, 128, 716–722. [Google Scholar] [CrossRef]
- Wharton, R.A.; Trostle, M.K.; Messing, R.H.; Copeland, R.S.; Kimani-Njogu, S.W.; Lux, S.; Overholt, W.A.; Mohamed, S.; Sivinski, J. Parasitoids of medfly, Ceratitis capitata and related tephritids in Kenyan coffee: A predominantly koinobiont assemblage. Bull. Entomol. Res. 2000, 90, 517–526. [Google Scholar]
- Aluja, M. Future Trends in Fruit Fly Managemen. In Fruit Fly Pests: A World Assessment of Their Biology and Management; McPheron, B.A., Steck, G.J., Eds.; St. Lucie Press: DelRay Beach, FL, USA, 1996; pp. 309–320. [Google Scholar]
- Aluja, M. Fruit fly (Diptera: Tephritidae) research in Latin America: Myths, realities and dreams. Anais Soc. Entomol. Brasil 1999, 28, 565–594. [Google Scholar] [CrossRef]
- Aluja, M.; Rull, J. Managing Pestiferous Fruit Flies (Diptera: Tephritidae) Through Environmental Manipulation. In Biorational Tree Fruit Pest Management; Aluja, M., Leskey, T.C., Vincent, C., Eds.; CABI: Oxfordshire, UK, 2009; pp. 171–213. [Google Scholar]
- Quiroga, D.; Ramirez, W.; Ruiz, C. National Fruit Fly Control and Eradication Program (PROCEM) Argentina. In Proceedings of the 8th International Symposium on Fruit Flies of Economic Importance, Valencia, Spain, 26 September–1 October 2010; pp. 100–101.
- Ovruski, S.M.; Schliserman, P.; Aluja, M. Indigenous parasitoids (Hymenoptera) attacking Anastrepha fraterculus and Ceratitis capitata (Diptera: Tephritidae) in native and exotic host plants in Northwestern Argentina. Biol. Control 2004, 29, 43–57. [Google Scholar] [CrossRef]
- Ovruski, S.M.; Wharton, R.A.; Schliserman, P.; Aluja, M. Abundance of Anastrepha fraterculus (Diptera: Tephritidae) and its associated native parasitoids (Hymenoptera) in “feral” guavas growing in the endangered northernmost Yungas forest of Argentina with an update on the taxonomic status of opiine parasitoids previously reported in this country. Environ. Entomol. 2005, 34, 807–818. [Google Scholar] [CrossRef]
- Schliserman, P.; Ovruski, S.; DeColl, O.R.; Wharton, R. Diversity and abundance of hymenopterous parasitoids associated with Anastrepha fraterculus (Diptera: Tephritidae) in native and exotic host plants in Misiones, Northeastern Argentina. Fla. Entomol. 2010, 93, 175–182. [Google Scholar] [CrossRef]
- Wharton, R.A.; Ovruski, S.M.; Gilstrap, F.E. Neotropical Eucoilidae (Cynipoidea) associated with fruit infesting Tephritidae, with new records from Argentina, Bolivia and Costa Rica. J. Hym. Res. 1998, 7, 102–115. [Google Scholar]
- Ogloblin, A. La protección de los enemigos naturales de la mosca de la fruta (Anastrepha fraterculus). Alm. Min. Agric. (Argentina) 1937, 3, 177–179. [Google Scholar]
- Hayward, K.J. Distribución de enemigos naturales de las moscas de las frutas para su control biológico. Rev. Ind. Agric. Tucumán 1940, 30, 136–138. [Google Scholar]
- Schultz, E.F. La lucha contra las moscas de las frutas en Tucumán. Rev. Ind. Agric. Tucumán 1938, 28, 171–172. [Google Scholar]
- Hayward, K.J. Lucha biológica contra las moscas de las frutas. Dispositivo que permite la salida de los parásitos beneficiosos del pozo donde se arroja la fruta atacada. Rev. Ind. Agric. Tucumán 1940, 30, 230–233. [Google Scholar]
- Aluja, M.; Sivinski, J.; Ovruski, S.M.; Guillén, L.; López, M.; Cancino, J.; Torres-Anaya, A.; Gallegos-Chan, G.; Ruíz, L. Colonization and domestication of seven species of native new world hymenopterous larval-prepupal and pupal fruit fly (Diptera: Tephritidae) parasitoids. Biocontrol. Sci. Technol. 2009, 19, 49–79. [Google Scholar] [CrossRef]
- Núñez-Campero, S.R. Estudios Demográficos de Himenopteros Parasitoides Neotropicales Asociados con Anastrepha fraterculus (Diptera: Tephritidae) Para su Aplicación en Modelos de Optimización de cría Masiva. Ph.D. Dissertation, Facultad de Ciencias Naturales e Instituto Miguel Lillo, Universidad Nacional de Tucumán, San Miguel de Tucumán, Argentina, 2011; p. 102. [Google Scholar]
- Ovruski, S.M.; Aluja, M. Mating behavior of Aganaspis pelleranoi (Brèthes) (Hymenoptera: Figitidae, Eucoilinae), a fruit fly (Diptera: Tephritidae) larval-parasitoid. J. Insect Behav. 2002, 15, 139–151. [Google Scholar] [CrossRef]
- Núñez-Campero, S.R.; Ovruski, S.M.; Aluja, M. Survival analysis and demographic parameters of the pupal parasitoid Coptera haywardi (Hymenoptera: Diapriidae), reared on Anastrepha fraterculus (Diptera: Tephritidae). Biol. Control 2012, 61, 40–46. [Google Scholar] [CrossRef]
- Ovruski, S.M.; Aluja, M.; Sivinski, J.; Wharton, R.A. Hymenopteran parasitoids on fruit-infesting tephritidae (Diptera) in Latin America and the Southern United States: Diversity, distribution, taxonomic status and their use in fruit fly biological control. Int. Pest Manag. Rev. 2000, 5, 81–107. [Google Scholar] [CrossRef]
- Turica, A. Lucha biológica como medio de control de las moscas de los frutos. Rev. IDIA (Argentina) 1968, 241, 29–38. [Google Scholar]
- Ovruski, S.M.; Cancino, J.L.; Fidalgo, P.; Liedo, P. Nuevas perspectivas para la aplicación del control biológico contra moscas de la fruta (Diptera: Tephritidae) en Argentina. Rev. Manejo Int. Plagas (Costa Rica) 1999, 54, 1–12. [Google Scholar]
- Gassmann, A. Europe as a source of biological control agents of exotic invasive weeds: Status and implications. Bull. Soc. Entomol. Suisse 1995, 68, 313–322. [Google Scholar]
- Aluja, M.; Montoya, P.; Cancino, J.; Guillén, L.; Ramírez-Romero, R. Moscas de la Fruta, Anastrepha spp. (Diptera: Tephritidae). In Casos de Control Biológico en México; Casos de Control Biológico en, México, Ed.; Mundi Prensa: México, CA, USA, 2008; pp. 193–222. [Google Scholar]
- Nasca, A.J. Parásitos de moscas de los frutos establecidos en algunas zonas de Tucumán. Rev. Agric. Noroeste Argent. 1973, 10, 31–43. [Google Scholar]
- Ovruski, S.M.; Schliserman, P.; DeColl, O.R.; Peñaloza, C.; Oroño, L.; Colin, C. The establishment of Aceratoneuromyia indica (Hymenoptera: Eulophidae) in three biogeographical regions of Argentina. Fla. Entomol. 2006, 89, 270–273. [Google Scholar] [CrossRef]
- Schliserman, P.; Ovruski, S.M.; DeColl, O.R. The recovery and permanent establishment of Diachasmimorpha longicaudata (Hymenoptera: Braconidae) in Misiones, northeastern Argentina. Fla. Entomol. 2003, 86, 491–492. [Google Scholar] [CrossRef]
- Oroño, L.E.; Ovruski, S.M. Presence of Diachasmimorpha longicaudata (Hymenoptera: Braconidae) in a guild of parasitoids attacking Anastrepha fraterculus (Diptera: Tephritidae) in northwestern Argentina. Fla. Entomol. 2007, 90, 410–412. [Google Scholar] [CrossRef]
- Ovruski, S.M. New records of fruit fly parasitoids from La Rioja province Northwestern Argentina. Proc. Entomol. Soc. Wash. 2002, 104, 1054–1056. [Google Scholar]
- Wang, X.G.; Messing, R.H. The ectoparasitic pupal parasitoid, Pachycrepoideus vindemmiae (Hymenoptera: Pteromlidae), attacks other primary tephritids fruit fly parasitoids: Host expansion and potential non-target impact. Biol. Control 2004, 31, 227–236. [Google Scholar] [CrossRef]
- Wharton, R.A. Classical Biological Control of fruit-infesting Tephritidae. In World Crop Pests. Fruit Flies, Their Biology, Natural Enemies and Control; Robinson, A.S., Hooper, G., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1989; Volume 3B, pp. 303–313. [Google Scholar]
- Morgan, P.B. Mass culturing microhymenopteran pupal parasites (Hymenoptera: Pteromalidae) of filth breeding flies. Misc. Publ. Entomol. Soc. Am. 1986, 61, 77–87. [Google Scholar]
- DeSantis, L. Catálogo de los Himenópteros Argentinos de la serie Parasitica, Incluyendo Bethyloidea; Publ. Comis. Invest. Cient. Prov. Bs. As.: Buenos Aires, Argentina, 1967; p. 337. [Google Scholar]
- DeSantis, L.; Fidalgo, P. Catálogo de los Himenópteros Calcidoideos de América al sur de los Estdos Unidos; Supl. Acad. Nac. Agr. Vet. Bs. As.: Buenos Aires, Argentina, 1979; p. 154. [Google Scholar]
- Cancino, J.L.; Ruiz, L.; Gomez, Y.; Toledo, J. Irradiación de larvas de Anastrepha ludens (Loew) (Diptera: Tephritidae) para inhibir la emergencia de moscas en la cría del parasitoide Diachasmimorpha longicaudata (Ashmead) (Hymenoptera: Braconidae). Folia Entomol. Mex. 2002, 41, 195–208. [Google Scholar]
- Ovruski, S.M.; Colin, C.; Soria, A.; Oroño, L.E.; Schliserman, P. Introducción y establecimiento en laboratorio de Diachasmimorpha tryoni y Diachasmimorpha longicaudata (Hymenoptera: Braconidae, Opiinae) para el control biológico de Ceratitis capitata (Diptera: Tephritidae, Dacinae) en la Argentina. Rev. Soc. Entomol. Argent. 2003, 62, 49–59. [Google Scholar]
- Segura, D.F.; Viscarret, M.M.; Carabajal-Paladino, L.Z.; Ovruski, S.M.; Cladera, J.L. Role of visual information and learning in habitat selection by a generalist parasitoid foraging for concealed hosts. Anim. Behav. 2007, 74, 131–142. [Google Scholar] [CrossRef]
- Viscarret, M.M.; La Rossa, R.; Segura, D.F.; Ovruski, S.M.; Cladera, J. Evaluation of the parasitoid Diachasmimorpha longicaudata (Ashmead) (Hymenoptera: Braconidae) reared on a genetic sexing strain of Ceratitis capitata (Wied.) (Diptera: Tephritidae). Biol. Control 2006, 36, 147–153. [Google Scholar] [CrossRef]
- Cladera, J.L. Self sexing strain of Ceratitis capitata (Diptera: Tephritidae) based on a gene that affects the rate of development. Ann. Entomol. Soc. Am. 1995, 88, 353–356. [Google Scholar]
- Suárez, L.; Bezdjian, L.; Beorchia Nigris, V.; Lara, N.; Murua, F.; Escobar, J.; van Nieuwenhove, G.; Nuñez-Campero, S.R.; Schliserman, P.; Cancino-Diaz, J.; Ovruski, S.M. Establishment of a Fruit Fly Parasitoids Mass-Rearing Facility in the Province of San Juan, Argentina. In 7ma Reunión del Grupo de Trabajo en Moscas de la Fruta del Hemisferio Occidental; IAEA, Programa Moscamed-Moscafrut México, SAGARPA-SENASICA: Mazatlán, Sinaloa, México, 2008. [Google Scholar]
- Vargas, R.I.; Ramadan, M.; Hussain, T.; Mochizuki, N.; Bautista, R.C.; Stark, J.D. Comparative demography of six fruit fly (Diptera: Tephritidae) parasitoids (Hymenoptera: Braconidae). Biol. Control 2002, 25, 30–40. [Google Scholar]
- Montoya, P.; Cancino, J.; Pérez-Lachaud, G.; Liedo, P. Host Size, Superparasitism and sex ratio in mass-reared Diachasmimorpha longicaudata, a fruit fly parasitoid. Biol. Control 2011, 56, 11–17. [Google Scholar]
- García-Medel, D.; Sivinski, J.; Díaz-Fleischer, F.; Ramirez-Romero, R.; Aluja, M. Foraging behavior by six fruit fly parasitoids (Hymenoptera: Braconidae) released as single- or multiple-species cohorts in field cages: Influence of fruit location and host density. Biol. Control 2007, 43, 12–22. [Google Scholar] [CrossRef]
- Cancino, J.L.; López, E.; Aguilar, C.E. Liberaciones Inundativas de Parasitoides Como Método Alternativo de Control de Ceratitis capitata en Fincas Cafetaleras en el Soconusco, Chiapas, México. In Actas de la Primera Conferencia Internacional Sobre Café Orgánico en México; IFOAM-AMAE-Universidad Autónoma de Chapingo: México, CA, USA, 1995; pp. 51–53. [Google Scholar]
- López, M.; Aluja, M.; Sivinski, J. Hymenopterous larval-pupal and pupal parasitoids of Anastrepha flies (Diptera: Tephritidae) in Mexico. Biol. Control 1999, 15, 119–129. [Google Scholar] [CrossRef]
- Sivinski, J.; Piñero, J.; Aluja, M. The distributions of parasitoids (Hymenoptera) of Anastrepha fruit flies (Diptera: Tephritidae) along an altitudinal gradient in Veracruz, Mexico. Biol. Control 2000, 18, 258–269. [Google Scholar] [CrossRef]
- Sivinski, J.; Vulinec, K.; Aluja, M. Ovipositor length in a guild of parasitoids (Hymenoptera: Braconidae) attacking Anastrepha spp. fruit flies (Diptera: Tephritidae) in southern Mexico. Ann. Entomol. Soc. Am. 2001, 94, 886–895. [Google Scholar] [CrossRef]
- Burns, R.E.; Diaz, J.D.; Holler, T.C. Inundative Release of Parasitoid Diachasmimorpha longicaudata for the Control of the Caribbean Fruit Fly, Anastrepha suspensa. In Fruit Fly Pests: A World Assessment of Their Biology and Management; McPheron, B.A., Steck, G.J., Eds.; St. Lucie Press: DelRay Beach, FL, USA, 1996; pp. 377–381. [Google Scholar]
- Ovruski, S.M.; Bezdjian, L.P.; van Nieuwenhove, G.A.; Albornoz-Medina, P.; Schliserman, P. Host preference by Diachasmimorpha longicaudata (Hymneoptera: Braconidae) reared on larvae of Anastrepha fraterculus and Ceratitis capitata (Diptera: Tephritidae). Fla. Entomol. 2011, 94, 195–200. [Google Scholar] [CrossRef]
- Ovruski, S.M.; van Nieuwenhove, G.A.; Bezdjian, L.P.; Albornoz-Medina, P.; Schliserman, P. Evaluation of Diachasmimorpha longicaudata (Hymenoptera: Braconidae) as a mortality factor of Ceratitis capitata (Diptera: Tephritidae) infesting Citrus species under laboratory and field-cage conditions. Biocontrol. Sci. Technol. 2012, 22, 187–202. [Google Scholar] [CrossRef]
- Bokonon-Ganta, A.H.; Ramadan, M.M.; Wang, X.G.; Messing, R.H. Biological performance and potential of Fopius ceratitivorus (Hymenoptera: Braconidae), an egg-larval parasitoid of tephritid fruit flies newly imported to Hawaii. Biol. Control 2005, 33, 238–247. [Google Scholar] [CrossRef]
- Bautista, C.B.; Mochizuki, N.; Spencer, J.P.; Harris, E.J.; Ichimura, D.M. Mass rearing of the teprhitid fruit fly parasitoid Fopius arisanus (Hymenoptera: Braconidae). Biol. Control 1999, 15, 137–144. [Google Scholar] [CrossRef]
- Wang, X.G.; Messing, R.H. Intra- and interspecific competition by Fopius arisanus and Diachasmimorpha tryoni (Hymenoptera: Braconidae), parasitoids of tephritid fruit flies. Biol. Control 2003, 27, 251–259. [Google Scholar] [CrossRef]
- Zenil, M.; Liedo, P.; Williams, T.; Valle, J.; Cancino, J.; Liedo, P. Reproductive biology of Fopius arisanus (Hymenoptera: Braconidae) on Ceratitis capitata and Anastrepha spp. (Diptera: Tephritidae). Biol. Control 2004, 29, 169–178. [Google Scholar] [CrossRef]
- Rendón, P.; Sivinski, J.; Holler, T.; Bloem, K.; López, M.; Martínez, A.; Aluja, M. The effects of sterile males and two braconid parasitoids, Fopius arisanus (Sonan) and Diachasmimorpha krausii (Fullaway) (Hymenoptera), on caged populations of Mediterranean fruit flies, Ceratitis capitata (Wied.) (Diptera: Tephritidae) at various sites in Guatemala. Biol. Control 2006, 36, 224–231. [Google Scholar] [CrossRef]
- Aluja, M.; Ovruski, S.M.; Guillén, L.; Oroño, L.E.; Sivinski, J. Comparison of the Host searching and oviposition behaviors of the tephritid (Diptera) parasitoids Aganaspis pelleranoi and Odontosema anastrephae (Hymenoptera: Figitidae, Eucoilinae). J. Insect Behav. 2009, 22, 423–451. [Google Scholar] [CrossRef]
- Sivinski, J.; Vulinec, K.; Menezes, E.; Aluja, M. The bionomics of Coptera haywardi (Oglobin) (Hymenotera: Diapriidae) and other pupal parasitoids of tephritid fruit .ies (Diptera). Biol. Control 1998, 11, 193–202. [Google Scholar] [CrossRef]
- Cancino, J.L.; Ruíz, L.; Sivinski, J.; Gálvez, F.O.; Aluja, M. Rearing of five hymenopterous larval-prepupal (Braconidae, Figitidae) and three pupal (Diapriidae, Chalcidoidea, Eurytomidae) native parasitoids of the genus Anastrepha (Diptera: Tephritidae) on irradiated A. Ludens larvae and pupae. Biocontrol. Sci. Technol. 2009, 19, 193–209. [Google Scholar] [CrossRef]
- Guillén, L.; Aluja, M.; Equihua, M.; Sivinski, J. Performance of two fruit fly (Diptera: Tephritidae) pupal parasitoids (Coptera haywardi (Hymenoptera: Diapriidae) and Pachycrepoideus vindemmiae (Hymenoptera: Pteromalidae)) under different environmental soil conditions. Biol. Control 2002, 23, 219–227. [Google Scholar] [CrossRef]
- Aluja, M.; Rull, J.; Sivinski, J.; Norrbom, A.; Wharton, R.; Macías-Ordoñez, R.; Díaz-Fleischer, F.; López, M. Fruit flies of the genus Anastrepha (Diptera: Tephritidae) and associated native parasitoids (Hymenoptera) in the tropical rainforest biosphere reserve of Montes Azules, Chiapas, México. Environ. Entomol. 2003, 32, 1377–1385. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ovruski, S.M.; Schliserman, P. Biological Control of Tephritid Fruit Flies in Argentina: Historical Review, Current Status, and Future Trends for Developing a Parasitoid Mass-Release Program. Insects 2012, 3, 870-888. https://doi.org/10.3390/insects3030870
Ovruski SM, Schliserman P. Biological Control of Tephritid Fruit Flies in Argentina: Historical Review, Current Status, and Future Trends for Developing a Parasitoid Mass-Release Program. Insects. 2012; 3(3):870-888. https://doi.org/10.3390/insects3030870
Chicago/Turabian StyleOvruski, Sergio M., and Pablo Schliserman. 2012. "Biological Control of Tephritid Fruit Flies in Argentina: Historical Review, Current Status, and Future Trends for Developing a Parasitoid Mass-Release Program" Insects 3, no. 3: 870-888. https://doi.org/10.3390/insects3030870