Effects of Banana Plantation Pesticides on the Immune Response of Lepidopteran Larvae and Their Parasitoid Natural Enemies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study System

| Location | Region (Cantón) | Area (Ha) | Pesticide Input |
|---|---|---|---|
| Penjamo | Sarapiquí | 148 | Moderate |
| Rebusca | Sarapiquí | 102 | Conventional |
| La Selva | Sarapiquí | 1,600 | None |
2.2. Immune Challenge
2.3. Statistics
2.4. Parasitism Survey
3. Results and Discussion
3.1. Immune Challenge


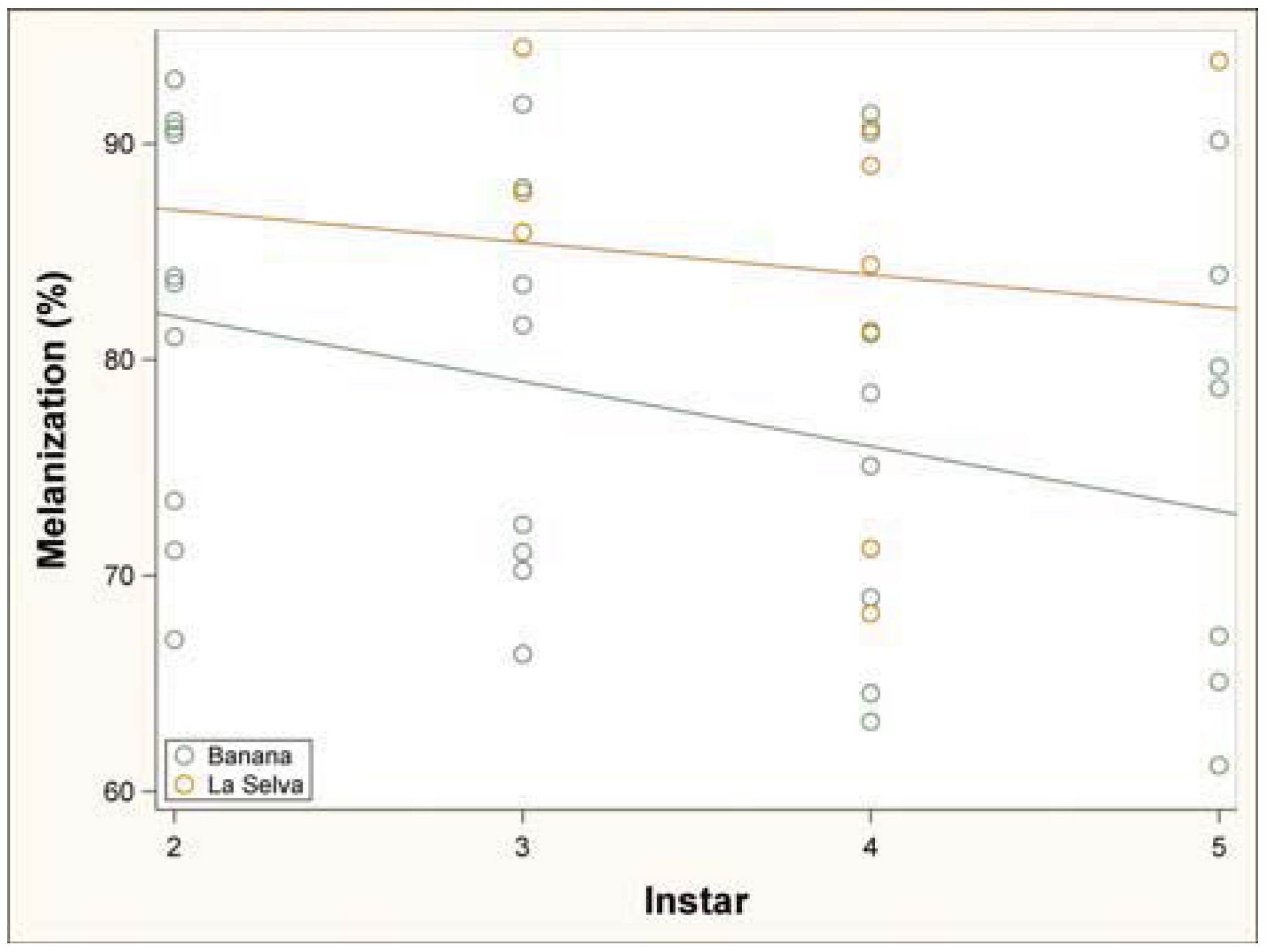
3.2. Parasitism Survey
| Location | Caterpillars Collected | Adult | Parasitism | Parasitoid ID | Parasitism by Taxa |
|---|---|---|---|---|---|
| La Selva | 542 | 276 | 26% | Tachinidae | 60% |
| Braconidae | 24% | ||||
| Ichneumonidae | 4% | ||||
| Chalcididae | 4% | ||||
| Eulophidae | 2% | ||||
| Hymenoptera | 3% | ||||
| Penjamo | 932 | 606 | 18% | Tachinidae | 78% |
| Hymenoptera | 22% | ||||
| Braconidae | 7% | ||||
| Rebusca | 1031 | 708 | 14% | Tachinidae | 81% |
| Hymenoptera | 8% | ||||
| Braconidae | 4% | ||||
| Ichneumonidae | 1% |
| Species | Caterpillars Collected | Adult | Parasitism |
|---|---|---|---|
| Caligo memnon (Brassolinae) | 1,211 | 808 | 12.2% |
| Antichloris viridis (Erebidae) | 863 | 564 | 20.8% |
| Opsiphanes tamarindi (Brassolinae) | 285 | 148 | 27.0% |
| Acharia nesea (Limacodidae) | 118 | 19 | 65% |
| Acharia hyperoche (Limacodidae) | 50 | 35 | 10.2% |
3.3. Discussion
4. Conclusions
Acknowledgments
References
- Stephens, C.S. Ecological upset and recuperation of natural control of insect pests in some Costa Rican banana plantations. Turrialba 1984, 34, 101–105. [Google Scholar]
- Thrupp, L.A. Entrapment and escape from fruitless insecticide use: Lessons from the banana sector of Costa Rica. Int. J. Environ. Stud. 1990, 36, 173–189. [Google Scholar] [CrossRef]
- van Driesche, R.; Bellows, T. Biological Control; Chapman and Hall: New York, NY, USA, 1996. [Google Scholar]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef]
- Matlock, R.B.; de la Cruz, R. An inventory of parasitic Hymenoptera in banana plantations under two pesticide regimes. Agr. Ecosyst. Environ. 2002, 93, 147–164. [Google Scholar] [CrossRef]
- Dyer, L.A.; Matlock, R.B.; Chehrezad, D.; O’Malley, R. Predicting caterpillar parasitism in banana plantations. Environ. Entomol. 2005, 34, 403–409. [Google Scholar] [CrossRef]
- Stireman, J.O., III; Dyer, L.; Matlock, R. Top-Down Forces in Managed Versus Unmanaged Habitats. In Ecology of Predator-Prey Interactions; Barbosa, P., Castellanos, I., Eds.; Oxford University Press: Oxford, UK, 2005; pp. 303–323. [Google Scholar]
- Godfray, H.C.J. Parasitoids: Behavioral and Evolutionary Ecology; Princeton University Press: Princeton, NJ, USA, 1994. [Google Scholar]
- Smilanich, A.M.; Dyer, L.A.; Gentry, G.L. The insect immune response and other putative defenses as effective predictors of parasitism. Ecology 2009, 90, 1434–1440. [Google Scholar] [CrossRef]
- Stanley, D. Eicosanoids: Progress towards manipulating insect immunity. J. Appl. Entomol. 2011, 135, 534–545. [Google Scholar] [CrossRef]
- Dyer, L.A.; Gentry, G. Predicting natural-enemy responses to herbivores in natural and managed systems. Ecol. Appl. 1999, 9, 402–408. [Google Scholar] [CrossRef]
- Cotter, S.C.; Simpson, S.J.; Raubenheimer, D.; Wilson, K. Macronutrient balance mediates trade-offs between immune function and life history traits. Funct. Ecol. 2011, 25, 186–198. [Google Scholar] [CrossRef]
- Smilanich, A.M.; Dyer, L.A.; Chambers, J.Q.; Bowers, M.D. Immunological cost of chemical defence and the evolution of herbivore diet breadth. Ecol. Lett. 2009, 12, 612–621. [Google Scholar] [CrossRef]
- Hawkins, B.A.; Cornell, H.V.; Hochberg, M.E. Predators, parasitoids, and pathogens as mortality agents in phytophagous insect populations. Ecology 1997, 78, 2145–2152. [Google Scholar] [CrossRef]
- Greeney, H.F.; Dyer, L.A.; Smilanich, A.M. Feeding by lepidopteran larvae is dangerous: A review of caterpillars’ chemical, physiological, morphological, and behavioral defenses against natural enemies. Invert. Surv. J. 2012, 9, 7–34. [Google Scholar]
- Cornell, J.C.; Stamp, N.E.; Bowers, M.D. Developmental change in aggregation, defense and escape behavior of buckmoth caterpillars, Hemileuca lucina (Saturniidae). Behav. Ecol. Sociobiol. 1987, 20, 383–388. [Google Scholar] [CrossRef]
- Gross, P. Insect behavioral and morphological defenses against parasitoids. Annu. Rev. Entomol. 1993, 38, 251–273. [Google Scholar] [CrossRef]
- Gauld, I.D.; Gaston, K.J.; Hawkins, B.A.; Sheehan, W. The Taste of Enemy-Free Space: Parasitoids and Nasty Hosts. In Parasitoid Community Ecology; Hawkins, B.A., Sheehan, W., Eds.; Oxford University Press: New York, NY, USA, 1994; pp. 279–299. [Google Scholar]
- Gentry, G.; Dyer, L.A. On the conditional nature of neotropical caterpillar defenses against their natural enemies. Ecology 2002, 83, 3108–3119. [Google Scholar] [CrossRef]
- Beckage, N.E. Insect Immunology; Academic Press: Oxford, UK, 2008. [Google Scholar]
- Strand, M.R. The insect cellular immune response. Insect. Sci. 2008, 15, 1–14. [Google Scholar] [CrossRef]
- Schmid-Hempel, P. Evolutionary ecology of insect immune defenses. Annu. Rev. Entomol. 2005, 50, 529–551. [Google Scholar] [CrossRef]
- Moret, Y.; Schmid-Hempel, P. Survival for immunity: The price of immune system activation for bumblebee workers. Science 2002, 290, 1166–1168. [Google Scholar]
- Rolff, J.; Kraaijeveld, A.R. Selection for parasitoid resistance alters mating success in Drosophila. Proc. Roy. Soc. Biol. Sci. 2003, 270, S154–S155. [Google Scholar] [CrossRef]
- Rantala, M.J.; Roff, D.A. Analysis of the importance of genotypic variation, metabolic rate, morphology, sex and development time on immune function in the cricket, Gryllus firmus. J. Evol. Biol. 2006, 19, 834–843. [Google Scholar] [CrossRef]
- Freitak, D.; Heckel, D.G.; Vogel, H. Bacterial feeding induces changes in immune-related gene expression and has trans-generational impacts in the cabbage looper (Trichoplusia ni). Front. Zool. 2009, 6, 7. [Google Scholar] [CrossRef]
- Haviola, S.; Kapari, L.; Ossipov, V.; Rantala, M.J.; Ruuhola, T.; Haukioja, E. Foliar phenolics are differently associated with Epirrita autumnata growth and immunocompetence. J. Chem. Ecol. 2007, 33, 1013–1023. [Google Scholar] [CrossRef]
- Klemola, N.; Klemola, T.; Rantala, M.J.; Ruuhola, T. Natural host-plant quality affects immune defence of an insect herbivore. Entomol. Exp. Appl. 2007, 123, 167–176. [Google Scholar] [CrossRef]
- Plapp, F.W.; Vinson, S.B. Comparative toxicities of some insecticides to tobacco budworm (Lepidoptera-Noctuidae) and its ichneumonid parasite, Campoletis sonorensis (Hymendoptera - Ichneumonidae). Environ. Entomol. 1997, 6, 381–384. [Google Scholar]
- Rajakulendran, S.V.; Plapp, F.W. Comparative toxicities of 5 synthetic pyrethroids to the tobacco budworm (Lepidoptera, Noctuidae), an ichneumonid parasite, Campoletis-sonorensis, (Hymenoptera, Ichneumonidae) and a predator, Chrysopa-carnea (Neuroptera Chrysopidae). J. Econ. Entomol. 1982, 75, 769–772. [Google Scholar]
- Croft, B.A. Management of pesticide resistance in arthropod pests - research and policy issues. ACS Symp. Ser. 1990, 421, 149–168. [Google Scholar]
- Chiang, F.M.; Sun, C.N. Detoxifying enzymes and susceptibility to several insecticides of Apanteles-plutellae (Hymenoptera, Braconidae) and Diadegma-semiclausum (Hymenoptera, Ichneumonidae), parasitoids of diamondback moth (Lepidoptera, Plutellidae) larvae. Environ. Entomol. 1991, 20, 1687–1690. [Google Scholar]
- Scott, J.G.; Dong, K.; Geden, C.J.; Rutz, D.A. Evaluation of the biochemical basis of insecticide selectivity between host and parasitoid species. Environ. Entomol. 1990, 19, 1722–1725. [Google Scholar]
- Geden, C.J.; Rutz, D.A.; Scott, J.G.; Long, S.J. Susceptibility of house-flies (Diptera, Muscidae) and 5 pupal parasitoids (Hymenoptera, Pteromalidae) to abamectin and 7 commercial insecticides. J. Econ. Entomol. 1992, 85, 435–440. [Google Scholar]
- Idris, A.B.; Grafius, E. Pesticides affect immature stages of diadegma-insulare (Hymenoptera, Ichneumonidae) and its host, the diamondback moth (Lepidoptera, Plutellidae). J. Econ. Entomol. 1992, 86, 1203–1212. [Google Scholar]
- Bayoun, I.M.; Plapp, F.W.; Gilstrap, F.E.; Michels, G.J. Toxicity of selected insecticides to Diuraphis-noxia (Homoptera, Aphididae) and its natural enemies. J. Econ. Entomol. 1995, 88, 1177–1185. [Google Scholar]
- Blanco-Munoz, J.; Morales, M.M.; Lacasana, M.; Aguilar-Garduno, C.; Bassol, S.; Cebian, M.E. Exposure to organophosphate pesticides and male hormone profile in floriculturist of the state of Morelos, Mexico. Hum. Reprod. 2010, 25, 1787–1795. [Google Scholar] [CrossRef]
- Seidler, F.J.; Slotkin, T.A. Developmental neurotoxicity targeting hepatic and cardiac sympathetic innervation: Effects of organophosphates are distinct from those of glucocorticoids. Brain Res. Bull. 2011, 85, 225–230. [Google Scholar] [CrossRef]
- Viswanath, G.; Chatterjee, S.; Dabral, S.; Nanguneri, S.R.; Divya, G.; Roy, P. Anti-androgenic endocrine disrupting activities of chlorpyrifos and piperophos. J. Steroid Biochem. Molec. Biol. 2010, 120, 22–29. [Google Scholar] [CrossRef]
- Soto, M. World situation and advances of banana production and technology. Rev. Bras. De Frutic. 2011, 33, 13–28. [Google Scholar] [CrossRef]
- Lavine, M.D.; Beckage, N.E. Temporal pattern of parasitism-induced immunosuppression in Manduca sexta larvae parasitized by Cotesia congregata. J. Insect. Physiol. 1996, 42, 41–51. [Google Scholar] [CrossRef]
- Forister, M.L.; Gompert, Z.; Fordyce, J.A.; Nice, C.C. After 60 years, an answer to the question: What is the Karner blue butterfly? Biol. Lett. 2011, 7, 399–402. [Google Scholar] [CrossRef]
- Fordyce, J.A.; Gompert, Z.; Forister, M.L.; Nice, C.C. A hierarchical bayesian approach to ecological count data: A flexible tool for ecologists. PLoS One 2011, 6, 11. [Google Scholar]
- Dyer, L.A.; Singer, M.S.; Lill, J.T.; Stireman, J.O.; Gentry, G.L.; Marquis, R.J.; Ricklefs, R.E.; Greeney, H.F.; Wagner, D.L.; Morais, H.C.; et al. Host specificity of Lepidoptera in tropical and temperate forests. Nature 2007, 448, 696–699. [Google Scholar]
- James, R.R.; Xu, J. Mechanisms by which pesticides affect insect immunity. J. Invert. Pathol. 2012, 109, 175–182. [Google Scholar] [CrossRef]
- Richards, L.A.; Dyer, L.A.; Smilanich, A.M.; Dodson, C.D. Synergistic effects of amides from two Piper species on generalist and specialist herbivores. J. Chem. Ecol. 2012, 36, 1105–1113. [Google Scholar]
- Dyer, L.A.; Dodson, C.D.; Stireman, J.O.; Tobler, M.A.; Smilanich, A.M.; Fincher, R.M.; Letourneau, D.K. Synergistic effects of three Piper amides on generalist and specialist herbivores. J. Chem. Ecol. 2003, 29, 2499–2514. [Google Scholar] [CrossRef]
- Jones, C.G.; Firn, R.D. On the evolution of plant secondary chemical diversity. Philos. Trans. Roy. Soc. Biol. 1991, 333, 273–280. [Google Scholar] [CrossRef]
- Wang, X.G.; Messing, R.H. Intra- and interspecific competition by Fopius arisanus and Diachasmimorpha tryoni (Hymenoptera: Braconidae), parasitoids of tephritid fruit flies. Biol. Control 2003, 27, 251–259. [Google Scholar] [CrossRef]
- Castillo, D.; Velasco-Hernandez, J.X. Coexistence in a competitive parasitoid-host system. J. Theor. Biol. 2003, 22, 161–77. [Google Scholar]
- Reitz, S.R.; Trumble, J.T. Competitive displacement among insects and arachnids. Annu. Rev. Entomol. 2002, 47, 435–465. [Google Scholar] [CrossRef]
- Hanson, P.E.; Gauld, I.D. The Hymenoptera of Costa Rica; Oxford University Press: Oxford, UK, 1995. [Google Scholar]
- Chalapathy, M.V.; Bidyapati, L.; Singh, N.I.; Prasad, B. New records of two Brachymeria species (Hymenoptera: Chalcididae) hyperparasitizing tachinid parasitoids of Antheraea proylei Jolly (Lepidoptera: Saturnidae) from north-eastern parts of India. J. Entomol. Res. 1998, 22, 291–292. [Google Scholar]
- White, E.B.; Bernal, J.S.; Gonzalez, D.; Triapitsyn, S.V. Facultative hyperparasitism in Brachymeria pomonae (Hymenoptera: Chalcididae). Eur. J. Entomol. 1998, 95, 359–366. [Google Scholar]
- McAuslane, H.J.; Bennett, F.D. Parasitoids and predators associated with Syntomeida-epilais (Lepidoptera, Arctiidae) on Oleander. Fla. Entomol. 1995, 78, 543–546. [Google Scholar] [CrossRef]
- Rossini, C.; Hoebeke, E.R.; Iyengar, V.K.; Conner, W.E.; Eisner, M.; Eisner, T. Alkaloid content of parasitoids reared from pupae of an alkaloid-sequestering arctiid moth (Utetheisa ornatrix). Entomol. News 2000, 111, 287–290. [Google Scholar]
- Kareiva, P. Contributions of ecology to biological control. Ecology 1996, 77, 1963–1964. [Google Scholar] [CrossRef]
- Murdoch, W.W.; Briggs, C.J. Theory for biological control: Recent developments. Ecology 1996, 77, 2001–2013. [Google Scholar] [CrossRef]
- Waage, J.K.; Ehler, L.H.; Roland, J. Ecological Theory and the Selection of Biological Control Agents. In Critical Issues in Biological Control; Intercept: Andover, MA, USA, 1990; pp. 135–158. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Smilanich, A.M.; Dyer, L.A. Effects of Banana Plantation Pesticides on the Immune Response of Lepidopteran Larvae and Their Parasitoid Natural Enemies. Insects 2012, 3, 616-628. https://doi.org/10.3390/insects3030616
Smilanich AM, Dyer LA. Effects of Banana Plantation Pesticides on the Immune Response of Lepidopteran Larvae and Their Parasitoid Natural Enemies. Insects. 2012; 3(3):616-628. https://doi.org/10.3390/insects3030616
Chicago/Turabian StyleSmilanich, Angela M., and Lee A. Dyer. 2012. "Effects of Banana Plantation Pesticides on the Immune Response of Lepidopteran Larvae and Their Parasitoid Natural Enemies" Insects 3, no. 3: 616-628. https://doi.org/10.3390/insects3030616




