Molecular Mechanisms of Transcription Activation by Juvenile Hormone: A Critical Role for bHLH-PAS and Nuclear Receptor Proteins
Abstract
:1. Introduction
2. MET as a JH Receptor
| Homolog a | bHLH | PAS A motif | PAS B motif | PAC motif | PAS Domain 1 b | Pas Domain 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MET | GCE | MET | GCE | MET | GCE | MET | GCE | MET | GCE | MET | GCE | |
| DmGCE | 82 c | - | 71 | - | 86 | - | 76 | - | 69 | - | 80 | - |
| BmMET1 | 41 | 41 | 34 | 34 | 65 | 67 | 60 | 63 | 40 | 38 | 62 | 65 |
| BmMET2 | 62 | 62 | 46 | 38 | 55 | 55 | 51 | 51 | 45 | 45 | 53 | 53 |
| AaMET | 78 | 96 | 71 | 61 | 67 | 76 | 68 | 65 | 69 | 64 | 67 | 69 |
| AgMET | 78 | 92 | 75 | 61 | 72 | 83 | 69 | 66 | 72 | 63 | 70 | 73 |
| CpMET | 74 | 90 | 71 | 63 | 69 | 79 | 66 | 66 | 68 | 64 | 67 | 71 |
| TcMET | 60 | 64 | 55 | 44 | 67 | 72 | 67 | 62 | 49 | 45 | 67 | 67 |
| AmMET | 52 | 54 | 42 | 38 | 60 | 62 | 51 | 51 | 38 | 40 | 55 | 56 |
| PaMET d | 46 | 53 | 44 | 38 | 44 | 46 | 53 | 53 | 41 | 39 | 49 | 50 |
| RpMET d | 45 | 45 | 48 | 44 | 48 | 48 | 55 | 51 | 39 | 40 | 52 | 50 |
| TdMET d | 52 | 55 | 53 | 46 | 60 | 62 | 58 | 56 | 48 | 48 | 59 | 59 |
3. JH-Dependent Transcription Mediated by bHLH-PAS and Nuclear Receptor Proteins

4. Structural Elements of MET Involved in JH Reception and Function
4.1. Structure and Function of the PAS Domains.

4.2. Role of JH in MET Nuclear Localization.
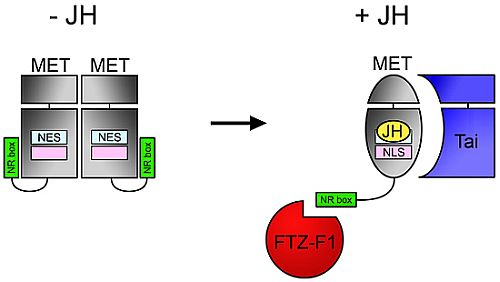
4.3. NR Box in MET C-Terminus Enables JH-Dependent Interaction with Nuclear Receptor FTZ-F1

5. Crosstalk between JH and Ecdysone
6. Conclusions
Acknowledgments
References
- Riddiford, L.M.; Cherbas, P.; Truman, J.W. Ecdysone receptors and their biological actions. Vitam. Horm. 2000, 60, 1–73. [Google Scholar] [CrossRef]
- Riddiford, L.M. Juvenile hormone action: A 2007 perspective. J. Insect Physiol. 2008, 54, 895–901. [Google Scholar] [CrossRef]
- Tan, A.; Tanaka, H.; Tamura, T.; Shiotsuki, T. Precocious metamorphosis in transgenic silkworms overexpressing juvenile hormone esterase. Proc. Natl. Acad. Sci. USA 2005, 102, 11751–11756. [Google Scholar]
- Konopova, B.; Jindra, M. Juvenile hormone resistance gene methoprene-tolerant controls entry into metamorphosis in the beetle tribolium castaneum. Proc. Natl. Acad. Sci. USA 2007, 104, 10488–10493. [Google Scholar] [CrossRef]
- Parthasarathy, R.; Tan, A.; Palli, S.R. bHLH-PAS family transcription factor methoprene-tolerant plays a key role in JH action in preventing the premature development of adult structures during larval-pupal metamorphosis. Mech. Dev. 2008, 125, 601–616. [Google Scholar] [CrossRef]
- Kremen, C.; Nijhout, H.F. Control of pupal commitment in the imaginal disks of precis coenia (lepidoptera: Nymphalidae). J. Insect Physiol. 1998, 44, 287–296. [Google Scholar] [CrossRef]
- Minakuchi, C.; Zhou, X.; Riddiford, L.M. Krüppel homolog 1 (kr-h1) mediates juvenile hormone action during metamorphosis of drosophila melanogaster. Mech. Dev. 2008, 125, 91–105. [Google Scholar] [CrossRef]
- Liu, Y.; Sheng, Z.; Liu, H.; Wen, D.; He, Q.; Wang, S.; Shao, W.; Jiang, R.J.; An, S.; Sun, Y.; et al. Juvenile hormone counteracts the bHLH-PAS transcription factors MET and GCE to prevent caspase-dependent programmed cell death in drosophila. Development 2009, 136, 2015–2025. [Google Scholar]
- Flatt, T.; Tu, M.-P.; Tatar, M. Hormonal pleiotropy and the juvenile hormone regulation of drosophila development and life history. BioEssays 2005, 27, 999–1010. [Google Scholar] [CrossRef]
- Dubrovsky, E.B.; Dubrovskaya, V.A.; Bilderback, A.L.; Berger, E.M. The isolation of two juvenile hormone-inducible genes in drosophila melanogaster. Dev. Biol. 2000, 224, 486–495. [Google Scholar] [CrossRef]
- Beckstead, R.B.; Lam, G.; Thummel, C.S. Specific transcriptional responses to juvenile hormone and ecdysone in drosophila. Insect Biochem. Mol. Biol. 2007, 37, 570–578. [Google Scholar] [CrossRef]
- Willis, D.K.; Wang, J.; Lindholm, J.R.; Orth, A.; Goodman, W.G. Microarray analysis of juvenile hormone response in drosophila melanogaster S2 cells. J. Insect Sci. 2010, 10. art. no. 66.. [Google Scholar]
- Wheeler, D.E.; Nijhout, H.F. A perspective for understanding the modes of juvenile hormone action as a lipid signaling system. BioEssays 2003, 25, 994–1001. [Google Scholar] [CrossRef]
- Davey, K. From insect ovaries to sheep red blood cells: A tale of two hormones. J. Insect Physiol. 2007, 53, 1–10. [Google Scholar] [CrossRef]
- Wilson, T.G.; Fabian, J. A drosophila melanogaster mutant resistant to a chemical analog of juvenile hormone. Dev. Biol. 1986, 118, 190–201. [Google Scholar] [CrossRef]
- Li, Z.-Q.; Cheng, D.-J.; Wei, L.; Zhao, P.; Shu, X.; Tang, L.; Xiang, Z.-H.; Xia, Q.-Y. The silkworm homolog of methoprene-tolerant (met) gene reveals sequence conservation but function divergence. Insect Sci. 2010, 17, 313–324. [Google Scholar]
- Konopova, B.; Smykal, V.; Jindra, M. Common and distinct roles of juvenile hormone signaling genes in metamorphosis of holometabolous and hemimetabolous insects. PLoS One 2011, 6, e28728. [Google Scholar]
- Riddiford, L.M.; Truman, J.W.; Mirth, C.K.; Shen, Y.-C. A role for juvenile hormone in the prepupal development of drosophila melanogaster. Development 2010, 137, 1117–1126. [Google Scholar] [CrossRef]
- Baumann, A.; Barry, J.; Wang, S.; Fujiwara, Y.; Wilson, T.G. Paralogous genes involved in juvenile hormone action in drosophila melanogaster. Genetics 2010, 185, 1327–1336. [Google Scholar] [CrossRef]
- Moore, A.W.; Barbel, S.; Jan, L.Y.; Jan, Y.N. A genomewide survey of basic helix-loop-helix factors in drosophila. Proc. Natl. Acad. Sci. USA 2000, 97, 10436–10441. [Google Scholar]
- Miura, K.; Oda, M.; Makita, S.; Chinzei, Y. Characterization of the drosophila methoprene-tolerant gene product: Juvenile hormone binding and ligand-dependent gene regulation. FEBS J. 2005, 272, 1169–1178. [Google Scholar]
- Charles, J.-P.; Iwema, T.; Epa, V.C.; Takaki, K.; Rynes, J.; Jindra, M. Ligand-binding properties of a juvenile hormone receptor. Proc. Natl. Acad. Sci. USA 2011, 108, 21128–21133. [Google Scholar]
- Minakuchi, C.; Namiki, T.; Shinoda, T. Krüppel homolog 1, an early juvenile hormone-response gene downstream of methoprene-tolerant, mediates its anti-metamorphic action in the red flour beetle tribolium castaneum. Dev. Biol. 2009, 325, 341–350. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, J.; Sheng, Z.; Sui, Y.; Palli, S.R. Steroid receptor co-activator is required for juvenile hormone signal transduction through a bHLH-PAS transcription factor, methoprene tolerant. J. Biol. Chem. 2011, 286, 8437–8447. [Google Scholar]
- Li, M.; Mead, E.A.; Zhu, J. Heterodimer of two bHLH-PAS proteins mediates juvenile hormone-induced gene expression. Proc. Natl. Acad. Sci. USA 2011, 108, 638–643. [Google Scholar] [CrossRef]
- Dubrovsky, E.; Dubrovskaya, V.A.; Bernardo, T.J.; Otte, V.; DiFillipo, R.; Bryan, H. The Drosophila FTZ-F1 nuclear receptor mediates juvenile hormone activation of E75A gene expression through an intracellular pathway. J. Biol. Chem. 2011, 286, 33689–33700. [Google Scholar]
- Wang, S.; Baumann, A.; Wilson, T.G. Drosophila melanogaster methoprene-tolerant (met) gene homologs from three mosquito species: Members of PAS transcriptional factor family. J. Insect Physiol. 2007, 53, 246–253. [Google Scholar] [CrossRef]
- Baumann, A.; Fujiwara, Y.; Wilson, T.G. Evolutionary divergence of the paralogs methoprene tolerant (met) and germ cell expressed (gce) within the genus drosophila. J. Insect Physiol. 2010, 56, 1445–1455. [Google Scholar]
- Bernardo, T.J.; Dubrovsky, E.B. The Drosophila juvenile hormone receptor candidates Methoprene-tolerant (MET) and Germ cell-expressed (GCE) utilize a conserved LIxxL motif to bind the FTZ-F1 nuclear receptor. J. Biol. Chem. 2012, 287, 7821–7833. [Google Scholar]
- Abdou, M.A.; He, Q.; Wen, D.; Zyaan, O.; Wang, J.; Xu, J.; Baumann, A.A.; Joseph, J.; Wilson, T.G.; Li, S.; et al. Drosophila met and gce are partially redundant in transducing juvenile hormone action. Insect Biochem. Mol. Biol. 2011, 41, 938–945. [Google Scholar] [CrossRef]
- Zhu, J.; Busche, J.M.; Zhang, X. Identification of juvenile hormone target genes in the adult female mosquitoes. Insect Biochem. Mol. Biol. 2010, 40, 23–29. [Google Scholar] [CrossRef]
- Wang, H.-B.; Ali, S.M.; Moriyama, M.; Iwanaga, M.; Kawasaki, H. 20-hydroxyecdysone and juvenile hormone analog prevent precocious metamorphosis in recessive trimolter mutants of bombyx mori. Insect Biochem. Mol. Biol. 2011, 42, 102–108. [Google Scholar]
- Lozano, J.; Belles, X. Conserved repressive function of Krüppel homolog 1 on insect metamorphosis in hemimetabolous and holometabolous species. Sci. Rep. 2011, 1. art. no. 163. [Google Scholar] [Green Version]
- Dubrovsky, E.B.; Dubrovskaya, V.A.; Berger, E.M. Hormonal regulation and functional role of drosophila E75A orphan nuclear receptor in the juvenile hormone signaling pathway. Dev. Biol. 2004, 268, 258–270. [Google Scholar] [CrossRef]
- Bernardo, T.J.; Dubrovskaya, V.A.; Jannat, H.; Maughan, B.; Dubrovsky, E.B. Hormonal regulation of the E75 gene in drosophila: Identifying functional regulatory elements through computational and biological analysis. J. Mol. Biol. 2009, 387, 794–808. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, L.; Sun, G.; Raikhel, A.S. The competence factor βFtz-F1 potentiates ecdysone receptor activity via recruiting a p160/SRC coactivator. Mol. Cell. Biol. 2006, 26, 9402–9412. [Google Scholar] [CrossRef]
- Kewley, R.J.; Whitelaw, M.L.; Chapman-Smith, A. The mammalian basic helix–loop–helix/PAS family of transcriptional regulators. Int. J. Biochem. Cell Biol. 2004, 36, 189–204. [Google Scholar] [CrossRef]
- Godlewski, J.; Wang, S.; Wilson, T.G. Interaction of bHLH-PAS proteins involved in juvenile hormone reception in Drosophila. Biochem. Biophys. Res. Commun. 2006, 342, 1305–1311. [Google Scholar]
- Hefti, M.H.; Françoijs, K.J.; De Vries, S.C.; Dixon, R.; Vervoort, J. The PAS fold: A redefinition of the PAS domain based upon structural prediction. Eur. J. Biochem. 2004, 271, 1198–1208. [Google Scholar] [CrossRef]
- Zoltowski, B.D.; Schwerdtfeger, C.; Widom, J.; Loros, J.J.; Bilwes, A.M.; Dunlap, J.C.; Crane, B.R. Conformational switching in the fungal light sensor vivid. Science 2007, 316, 1054–1057. [Google Scholar]
- Henry, J.T.; Crosson, S. Ligand-binding PAS domains in a genomic, cellular, and structural context. Annu. Rev. Microbiol. 2011, 65, 261–286. [Google Scholar] [CrossRef]
- Kumar, S.; Saradhi, M.; Chaturvedi, N.K.; Tyagi, R.K. Intracellular localization and nucleocytoplasmic trafficking of steroid receptors: An overview. Mol. Cell. Endocrinol. 2006, 246, 147–156. [Google Scholar] [CrossRef]
- Greb-Markiewicz, B.; Orlowski, M.; Dobrucki, J.; Ozyhar, A. Sequences that direct subcellular traffic of the drosophila methoprene-tolerant protein (MET) are located predominantly in the PAS domains. Mol. Cell. Endocrinol. 2011, 345, 16–26. [Google Scholar] [CrossRef]
- Pursley, S.; Ashok, M.; Wilson, T.G. Intracellular localization and tissue specificity of the methoprene-tolerant (met) gene product in drosophila melanogaster. Insect Biochem. Mol. Biol. 2000, 30, 839–845. [Google Scholar] [CrossRef]
- Partch, C.L.; Gardner, K.H. Coactivator recruitment: A new role for PAS domains in transcriptional regulation by the bHLH-PAS family. J. Cell. Physiol. 2010, 223, 553–557. [Google Scholar]
- Leo, C.; Chen, J.D. The SRC family of nuclear receptor coactivators. Gene 2000, 245, 1–11. [Google Scholar] [CrossRef]
- Savkur, R.S.; Burris, T.P. The coactivator LXXLL nuclear receptor recognition motif. J. Pept. Res. 2004, 63, 207–212. [Google Scholar] [CrossRef]
- Hong, H.; Kohli, K.; Garabedian, M.J.; Stallcup, M.R. GRIP1, a transcriptional coactivator for the AF-2 transactivation domain of steroid, thyroid, retinoid, and vitamin D receptors. Mol. Cell Biol. 1997, 17, 2735–2744. [Google Scholar]
- Onate, S.A.; Boonyaratanakornkit, V.; Spencer, T.E.; Tsai, S.Y.; Tsai, M.-J.; Edwards, D.P.; O’Malley, B.W. The steroid receptor coactivator-1 contains multiple receptor interacting and activation domains that cooperatively enhance the activation function 1 (AF1) and AF2 domains of steroid receptors. J. Biol. Chem. 1998, 273, 12101–12108. [Google Scholar]
- Voegel, J.J.; Heine, M.J.S.; Tini, M.; Vivat, V.; Chambon, P.; Gronemeyer, H. The coactivator TIF2 contains three nuclear receptor-binding motifs and mediates transactivation through CBP binding-dependent and -independent pathways. EMBO J. 1998, 17, 507–519. [Google Scholar] [CrossRef]
- Lazennec, G.; Ediger, T.R.; Petz, L.N.; Nardulli, A.M.; Katzenellenbogen, B.S. Mechanistic aspects of estrogen receptor activation probed with constitutively active estrogen receptors: Correlations with DNA and coregulator interactions and receptor conformational changes. Mol. Endocrinol. 1997, 11, 1375–1386. [Google Scholar]
- White, R.; Sjöberg, M.; Kalkhoven, E.; Parker, M.G. Ligand-independent activation of the oestrogen receptor by mutation of a conserved tyrosine. EMBO J. 1997, 16, 1427–1435. [Google Scholar] [CrossRef]
- Ito, M.; Yu, R.N.; Jameson, J.L. Steroidogenic factor-1 contains a carboxy-terminal transcriptional activation domain that interacts with steroid receptor coactivator-1. Mol. Endocrinol. 1998, 12, 290–301. [Google Scholar] [CrossRef]
- Xu, P.L.; Liu, Y.-Q.; Shan, S.-F.; Kong, Y.-Y.; Zhou, Q.; Li, M.; Ding, J.-P.; Xie, Y.-H.; Wang, Y. Molecular mechanism for the potentiation of the transcriptional activity of human liver receptor homolog 1 by steroid receptor coactivator-1. Mol. Endocrinol. 2004, 18, 1887–1905. [Google Scholar] [CrossRef]
- Jones, D.; Jones, G.; Teal, P.; Hammac, C.; Messmer, L.; Osborne, K.; Belgacem, Y.; Martin, J.-R. Suppressed production of methyl farnesoid hormones yields developmental defects and lethality in drosophila larvae. Gen. Comp. Endocrinol. 2010, 165, 244–254. [Google Scholar] [CrossRef]
- Jones, G.; Jones, D.; Li, X.; Tang, L.; Ye, L.; Teal, P.; Riddiford, L.; Sandifer, C.; Borovsky, D.; Martin, J.-R. Activities of natural methyl farnesoids on pupariation and metamorphosis of drosophila melanogaster. J. Insect Physiol. 2010, 56, 1456–1464. [Google Scholar] [CrossRef]
- Edgar, B.A. How flies get their size: Genetics meets physiology. Nat. Rev. Genet. 2006, 7, 907–916. [Google Scholar] [CrossRef]
- Abdou, M.; Peng, C.; Huang, J.; Zyaan, O.; Wang, S.; Li, S.; Wang, J. Wnt signaling cross-talks with JH signaling by suppressing met and gce expression. PLoS One 2011, 6, e26772. [Google Scholar]
- Huang, J.; Tian, L.; Peng, C.; Abdou, M.; Wen, D.; Wang, Y.; Li, S.; Wang, J. DPP-mediated TGFβ signaling regulates juvenile hormone biosynthesis by activating the expression of juvenile hormone acid methyltransferase. Development 2011, 138, 2283–2291. [Google Scholar] [CrossRef]
- Riddiford, L.M.; Hiruma, K.; Zhou, X.; Nelson, C.A. Insights into the molecular basis of the hormonal control of molting and metamorphosis from manduca sexta and drosophila melanogaster. Insect Biochem. Mol. Biol. 2003, 33, 1327–1338. [Google Scholar] [CrossRef]
- Konopova, B.; Jindra, M. Broad-complex acts downstream of met in juvenile hormone signaling to coordinate primitive holometabolan metamorphosis. Development 2008, 135, 559–568. [Google Scholar] [CrossRef]
- Suzuki, Y.; Truman, J.W.; Riddiford, L.M. The role of broad in the development of tribolium castaneum: Implications for the evolution of the holometabolous insect pupa. Development 2008, 135, 569–577. [Google Scholar] [CrossRef]
- Henrich, V.C.; Burns, E.; Yelverton, D.P.; Christensen, E.; Weinberger, C. Juvenile hormone potentiates ecdysone receptor-dependent transcription in a mammalian cell culture system. Insect Biochem. Mol. Biol. 2003, 33, 1239–1247. [Google Scholar] [CrossRef]
- Maki, A.; Sawatsubashi, S.; Ito, S.; Shirode, Y.; Suzuki, E.; Zhao, Y.; Yamagata, K.; Kouzmenko, A.; Takeyama, K.-I.; Kato, S. Juvenile hormones antagonize ecdysone actions through co-repressor recruitment to EcR/USP heterodimers. Biochem. Biophys. Res. Commun. 2004, 320, 262–267. [Google Scholar] [CrossRef]
- Jones, G.; Jones, D.; Fang, F.; Xu, Y.; New, D.; Wu, W.-H. Juvenile hormone action through a defined enhancer motif to modulate ecdysteroid-activation of natural core promoters. Comp. Biochem. Physiol. B 2011, 161, 219–225. [Google Scholar]
- Bai, J.; Uehara, Y.; Montell, D.J. Regulation of invasive cell behavior by taiman, a drosophila protein related to AIB1, a steroid receptor coactivator amplified in breast cancer. Cell 2000, 10, 1047–1058. [Google Scholar]
- Bitra, K.; Tan, A.; Dowling, A.; Palli, S.R. Functional characterization of PAS and HES family bHLH transcription factors during the metamorphosis of the red flour beetle, tribolium castaneum. Gene 2009, 448, 74–87. [Google Scholar] [CrossRef]
- Swedenborg, E.; Rüegg, J.; Mäkelä, S.; Pongratz, I. Endocrine disruptive chemicals: Mechanisms of action and involvement in metabolic disorders. J. Mol. Endocrinol. 2009, 43, 1–10. [Google Scholar] [CrossRef]
- Yamada, M.-A.; Murata, T.; Hirose, S.; Lavorgna, G.; Suzuki, E.; Ueda, H. Temporally restricted expression of transcription and factor βFTZ-F1: Significance for embryogenesis, molting and metamorphosis in drosophila melanogaster. Development 2000, 127, 5083–5092. [Google Scholar]
- Broadus, J.; McCabe, J.R.; Endrizzi, B.; Thummel, C.S.; Woodard, C.T. The drosophila βFTZ-F1 orphan nuclear receptor provides competence for stage-specific responses to the steroid hormone ecdysone. Mol. Cell 1999, 3, 143–149. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bernardo, T.J.; Dubrovsky, E.B. Molecular Mechanisms of Transcription Activation by Juvenile Hormone: A Critical Role for bHLH-PAS and Nuclear Receptor Proteins. Insects 2012, 3, 324-338. https://doi.org/10.3390/insects3010324
Bernardo TJ, Dubrovsky EB. Molecular Mechanisms of Transcription Activation by Juvenile Hormone: A Critical Role for bHLH-PAS and Nuclear Receptor Proteins. Insects. 2012; 3(1):324-338. https://doi.org/10.3390/insects3010324
Chicago/Turabian StyleBernardo, Travis J., and Edward B. Dubrovsky. 2012. "Molecular Mechanisms of Transcription Activation by Juvenile Hormone: A Critical Role for bHLH-PAS and Nuclear Receptor Proteins" Insects 3, no. 1: 324-338. https://doi.org/10.3390/insects3010324