Solid Lubrication with MoS2: A Review
Abstract
:1. Introduction
2. Structure and Synthesis
3. Tribological Applications of MoS2
4. Mechanisms of Low Friction and Wear
- (1)
- The establishment of a transfer film on the counter-surface as it slides against the MoS2-coated component.
- (2)
- The shear-induced orientation of the basal planes of MoS2 (in the original coating, the transfer film and eventually in third bodies/wear particles) in the sliding direction.
5. Environment and Temperature Dependence
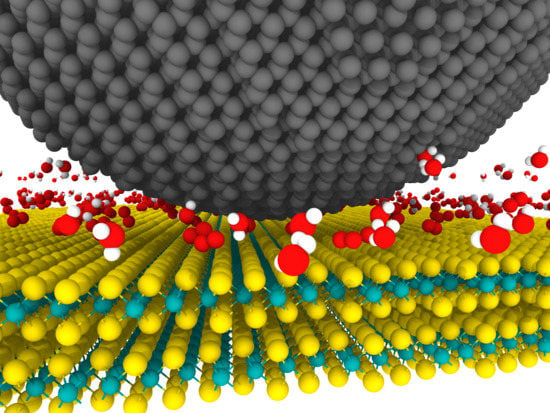
- Physisorption of water molecules at the surface which deteriorates the tribological behavior of MoS2 via disruption of the easy shear of lamellae [131,133,134,135,136,137], adhesion enhancement [138,139], hydrogen bonding between basal planes of MoS2 [140,141] and restriction of the growth and reorientation of the tribofilm [123].
6. Structure and Tribological Properties of Doped MoS2
6.1. Structure of Doped MoS2
6.2. Tribological Properties of Doped MoS2
7. Conclusions and Outlook
Funding
Conflicts of Interest
References
- Jost, H.P. Tribology—Origin and future. Wear 1990, 136, 1–17. [Google Scholar] [CrossRef]
- Bhushan, B. Introduction to Tribology; Wiley: New York, NY, USA, 2013. [Google Scholar]
- Holmberg, K.; Erdemir, A. Influence of tribology on global energy consumption, costs and emissions. Friction 2017, 5, 263–284. [Google Scholar] [CrossRef]
- Mang, T.; Bobzin, K.; Bartels, T. Industrial Tribology: Tribosystems, Friction, Wear and Surface Engineering, Lubrication; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Donnet, C.; Erdemir, A. Historical developments and new trends in tribological and solid lubricant coatings. Surf. Coat. Technol. 2004, 180, 76–84. [Google Scholar] [CrossRef]
- Roberts, E.W. Thin solid lubricant films in space. Tribol. Int. 1990, 23, 95–104. [Google Scholar] [CrossRef]
- Roberts, E.W. Space tribology: Its role in spacecraft mechanisms. J. Phys. D-Appl. Phys. 2012, 45, 503001. [Google Scholar] [CrossRef]
- Savan, A.; Pflüger, E.; Voumard, P.; Shröer, A.; Simmonds, M. Modern Solid Lubrication: Recent Developments and Applications of MoS2. Lubr. Sci. 2000, 12, 185. [Google Scholar] [CrossRef]
- Song, I.; Park, C.; Choi, H.C. Synthesis and properties of molybdenum disulphide: From bulk to atomic layers. RSC Adv. 2015, 5, 7495–7514. [Google Scholar] [CrossRef]
- Koehler, W. Antifriction and Antiabrasive Metal. U.S. Patent 1714564A, 28 May 1929. [Google Scholar]
- Donnet, C.; Martin, J.M.; LeMogne, T.; Belin, M. Super-low friction of MoS2 coatings in various environments. Tribol. Int. 1996, 29, 123–128. [Google Scholar] [CrossRef]
- Winer, W.O. Molybdenum disulfide as a lubricant: A review of the fundamental knowledge. Wear 1967, 10, 422–452. [Google Scholar] [CrossRef] [Green Version]
- Lansdown, A.R. Molybdenum Disulphide Lubrication; Elsevier: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Voevodin, A.A.; Zabinski, J.S. Nanocomposite and nanostructured tribological materials for space applications. Compos. Sci. Technol. 2005, 65, 741–748. [Google Scholar] [CrossRef]
- Lince, J.R. Doped MoS2 Coatings and Their Tribology. In Encyclopedia of Tribology; Wang, Q.J., Chung, Y.W., Eds.; Springer: Boston, MA, USA, 2013. [Google Scholar]
- Novoselov, K.S.; Mishchenko, A.; Carvalho, A.; Castro Neto, A.H. 2D materials and van der Waals heterostructures. Science 2016, 353, 461. [Google Scholar] [CrossRef] [PubMed]
- Peelaers, H.; Van de Walle, C.G. Elastic Constants and Pressure-Induced Effects in MoS2. J. Phys. Chem. C 2014, 118, 12073–12076. [Google Scholar] [CrossRef]
- Ganatra, R.; Zhang, Q. Few-Layer MoS2: A Promising Layered Semiconductor. ACS Nano 2014, 8, 4074–4099. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Li, R.; Dong, J.M. A new (2 x 1) dimerized structure of monolayer 1T-molybdenum disulfide, studied from first principles calculations. J. Chem. Phys. 2013, 139, 174702. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.F.; Nam, G.H.; He, Q.Y.; Wu, X.J.; Zhang, K.; Yang, Z.Z.; Chen, J.Z.; Ma, Q.L.; Zhao, M.T.; Liu, Z.Q.; et al. High phase-purity 1T’-MoS2- and 1T’-MoSe2- layered crystals. Nat. Chem. 2018, 10, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Wypych, F.; Schollhorn, R. 1T-MoS2, a New Metallic Modification of Molybdenum Disulfide. J. Chem. Soc. -Chem. Commun. 1992, 19, 1386–1388. [Google Scholar] [CrossRef]
- Jain, A.; Ong, S.P.; Hautier, G.; Chen, W.; Richards, W.D.; Dacek, S.; Cholia, S.; Gunter, D.; Skinner, D.; Ceder, G.; et al. Commentary: The Materials Project: A materials genome approach to accelerating materials innovation. APL Mater. 2013, 1, 011002. [Google Scholar] [CrossRef] [Green Version]
- Materials Project, mp-2815. Available online: https://materialsproject.org/materials/mp-2815 (accessed on 13 June 2019).
- Materials Project, mp-1434. Available online: https://materialsproject.org/materials/mp-1434 (accessed on 13 June 2019).
- Chen, X.B.; Chen, Z.L.; Li, J. Critical electronic structures controlling phase transitions induced by lithium ion intercalation in molybdenum disulphide. Chin. Sci. Bull. 2013, 58, 1632–1641. [Google Scholar] [CrossRef] [Green Version]
- Gaur, A.P.S.; Sahoo, S.; Ahmadi, M.; Dash, S.P.; Guinel, M.J.F.; Katiyar, R.S. Surface Energy Engineering for Tunable Wettability through Controlled Synthesis of MoS2. Nano Lett. 2014, 14, 4314–4321. [Google Scholar] [CrossRef]
- Komsa, H.P.; Krasheninnikov, A.V. Native defects in bulk and monolayer MoS2 from first principles. Phys. Rev. B 2015, 91, 125304. [Google Scholar] [CrossRef]
- Escalera-Lopez, D.; Niu, Y.B.; Yin, J.L.; Cooke, K.; Rees, N.V.; Palmer, R.E. Enhancement of the Hydrogen Evolution Reaction from Ni-MoS2 Hybrid Nanoclusters. ACS Catal. 2016, 6, 6008–6017. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.Y.; Li, J.Q.; An, C.H.; Feng, J.; Chi, Y.H.; Liu, J.X.; Zhang, J.; Sun, Y.G. Ultrathin Co(Ni)-doped MoS2 nanosheets as catalytic promoters enabling efficient solar hydrogen production. Nano Res. 2016, 9, 2284–2293. [Google Scholar] [CrossRef]
- Feldman, Y.; Wasserman, E.; Srolovitz, D.J.; Tenne, R. High-Rate, Gas-Phase Growth of MoS2 Nested Inorganic Fullerenes and Nanotubes. Science 1995, 267, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Z.; Zhang, X.Y.; Shen, Y.L.; Wu, Z.Z. Ni-doped MoS2 nanoparticles as highly active hydrogen evolution electrocatalysts. RSC Adv. 2016, 6, 16656–16661. [Google Scholar] [CrossRef]
- Miki, Y.; Nakazato, D.; Ikuta, H.; Uchida, T.; Wakihara, M. Amorphous MoS2 as the cathode of lithium secondary batteries. J. Power Sources 1995, 54, 508–510. [Google Scholar] [CrossRef]
- Suzuki, R.; Sakano, M.; Zhang, Y.J.; Akashi, R.; Morikawa, D.; Harasawa, A.; Yaji, K.; Kuroda, K.; Miyamoto, K.; Okuda, T.; et al. Valley-dependent spin polarization in bulk MoS2 with broken inversion symmetry. Nat. Nanotechnol. 2014, 9, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Jellinek, F.; Brauer, G.; Muller, H. Molybdenum and Niobium Sulphides. Nature 1960, 185, 376–377. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Jiang, D.; Schedin, F.; Booth, T.J.; Khotkevich, V.V.; Morozov, S.V.; Geim, A.K. Two-dimensional atomic crystals. Proc. Natl. Acad. Sci. USA 2005, 102, 10451–10453. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, G.; Lotya, M.; Cucinotta, C.S.; Sanvito, S.; Bergin, S.D.; Menzel, R.; Shaffer, M.S.P.; Coleman, J.N. Solvent Exfoliation of Transition Metal Dichalcogenides: Dispersibility of Exfoliated Nanosheets Varies Only Weakly between Compounds. ACS Nano 2012, 6, 3468–3480. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Kim, P.; Kim, J.H.; Ye, J.H.; Kim, S.; Lee, C.J. Large-Area Atomically Thin MoS2 Nanosheets Prepared Using Electrochemical Exfoliation. ACS Nano 2014, 8, 6902–6910. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.J.; Liu, Z.; Najmaei, S.; Ajayan, P.M.; Lou, J. Large-Area Vapor-Phase Growth and Characterization of MoS2 Atomic Layers on a SiO2 Substrate. Small 2012, 8, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, J.; Bark, H.; Oh, I.K.; Ryu, G.H.; Lee, Z.; Kim, H.; Cho, J.H.; Ahn, J.H.; Lee, C. Synthesis of wafer-scale uniform molybdenum disulfide films with control over the layer number using a gas phase sulfur precursor. Nanoscale 2014, 6, 2821–2826. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Zhang, X.Q.; Zhang, W.J.; Chang, M.T.; Lin, C.T.; Chang, K.D.; Yu, Y.C.; Wang, J.T.W.; Chang, C.S.; Li, L.J.; et al. Synthesis of Large-Area MoS2 Atomic Layers with Chemical Vapor Deposition. Adv. Mater. 2012, 24, 2320–2325. [Google Scholar] [CrossRef] [PubMed]
- Dumcenco, D.; Ovchinnikov, D.; Marinov, K.; Lazic, P.; Gibertini, M.; Marzari, N.; Sanchez, O.L.; Kung, Y.C.; Krasnozhon, D.; Chen, M.W.; et al. Large-Area Epitaxial Mono layer MoS2. ACS Nano 2015, 9, 4611–4620. [Google Scholar] [CrossRef]
- Song, I.; Park, C.; Hong, M.; Baik, J.; Shin, H.J.; Choi, H.C. Patternable Large-Scale Molybdenium Disulfide Atomic Layers Grown by Gold-Assisted Chemical Vapor Deposition. Angew. Chem. Int. Ed. 2014, 53, 1266–1269. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Liao, M.Z.; Zhao, W.J.; Liu, G.D.; Zhou, X.J.; Wei, Z.; Xu, X.Z.; Liu, K.H.; Hu, Z.H.; Deng, K.; et al. Wafer-Scale Growth and Transfer of Highly-Oriented Monolayer MoS2 Continuous Films. ACS Nano 2017, 11, 12001–12007. [Google Scholar] [CrossRef]
- George, A.S.; Mutlu, Z.; Ionescu, R.; Wu, R.J.; Jeong, J.S.; Bay, H.H.; Chai, Y.; Mkhoyan, K.A.; Ozkan, M.; Ozkan, C.S. Wafer Scale Synthesis and High Resolution Structural Characterization of Atomically Thin MoS2 Layers. Adv. Funct. Mater. 2014, 24, 7461–7466. [Google Scholar] [CrossRef]
- Liu, K.K.; Zhang, W.J.; Lee, Y.H.; Lin, Y.C.; Chang, M.T.; Su, C.; Chang, C.S.; Li, H.; Shi, Y.M.; Zhang, H.; et al. Growth of Large-Area and Highly Crystalline MoS2 Thin Layers on Insulating Substrates. Nano Lett. 2012, 12, 1538–1544. [Google Scholar] [CrossRef]
- Yang, J.; Gu, Y.; Lee, E.; Lee, H.; Park, S.H.; Cho, M.H.; Kim, Y.H.; Kim, H. Wafer-scale synthesis of thickness-controllable MoS2 films via solution-processing using a dimethylformamide/n-butylamine/2-aminoethanol solvent system. Nanoscale 2015, 7, 9311–9319. [Google Scholar] [CrossRef]
- Fleischauer, P.D. Effects of Crystallite Orientation on Environmental Stability and Lubrication Properties of Sputtered MoS2 Thin Films. ASLE Trans. 1984, 27, 82–88. [Google Scholar] [CrossRef]
- Martin, J.M.; Donnet, C.; Lemogne, T.; Epicier, T. Superlubricity of Molybdenum Disulfide. Phys. Rev. B 1993, 48, 10583–10586. [Google Scholar] [CrossRef] [PubMed]
- Krause, O.; Muller, F.; Birkmann, S.; Bohm, A.; Ebert, M.; Grozinger, U.; Henning, T.; Hofferbert, R.; Huber, A.; Lemke, D.; et al. High-precision cryogenic wheel mechanisms of the JWST/MIRI instrument: Performance of the flight models. Proc. SPIE 2010, 7739, 773918. [Google Scholar] [CrossRef]
- Spalvins, T. Morphological and frictional behavior of sputtered MoS2 films. Thin Solid Films 1982, 96, 17–24. [Google Scholar] [CrossRef]
- Bichsel, R.; Buffat, P.; Levy, F. Correlation between process conditions, chemical composition and morphology of MoS2 films prepared by RF planar magnetron sputtering. J. Phys. D Appl. Phys. 1986, 19, 1575–1585. [Google Scholar] [CrossRef]
- Arslan, E.; Totik, Y.; Efeoglu, I. Comparison of structure and tribological properties of MoS2-Ti films deposited by biased-dc and pulsed-dc. Prog. Org. Coat. 2012, 74, 772–776. [Google Scholar] [CrossRef]
- Laing, K.; Hampshire, J.; Teer, D.; Chester, G. The effect of ion current density on the adhesion and structure of coatings deposited by magnetron sputter ion plating. Surf. Coat. Technol. 1999, 112, 177–180. [Google Scholar] [CrossRef]
- Zabinski, J.S.; Donley, M.S.; Dyhouse, V.J.; McDevitt, N.T. Chemical and tribological characterization of PbO-MoS2 films grown by pulsed laser deposition. Thin Solid Films 1992, 214, 156–163. [Google Scholar] [CrossRef]
- Miyoshi, K. Solid Lubricants and Coatings for Extreme Environments: State-of-the-Art Survey; NASA/TM: Washington, DC, USA, 2007. [Google Scholar]
- Miyoshi, K. Solid Lubricants. In Encyclopedia of Tribology; Wang, Q.J., Chung, Y.W., Eds.; Springer: Boston, MA, USA, 2013. [Google Scholar]
- Lince, J.R.; Fleischauer, P.D. Solid Lubricants. In Space Vehicle Mechanisms: Elements of Successful Design; Conley, P.L., Ed.; Wiley-Interscience: New York, NY, USA, 1998. [Google Scholar]
- Chen, Z.; He, X.; Xiao, C.; Kim, S.H. Effect of Humidity on Friction and Wear—A Critical Review. Lubricants 2018, 6, 74. [Google Scholar] [CrossRef]
- Sun, X. Solid Lubricants for Space Mechanisms. In Encyclopedia of Tribology; Wang, Q.J., Chung, Y.W., Eds.; Springer: Boston, MA, USA, 2013. [Google Scholar]
- Miyoshi, K. Aerospace mechanisms and tribology technology-Case study. Tribol. Int. 1999, 32, 673–685. [Google Scholar] [CrossRef]
- Weidlich, K.; Fischer, M.; Ellenrieder, M.M.; Gross, T.; Salvignol, J.C.; Barho, R.; Neugebauer, C.; Konigsreiter, G.; Trunz, M.; Muller, F.; et al. High-precision cryogenic wheel mechanisms for the JWST NIRSPEC instrument. In Proceedings of the International Conference on Advanced Optical and Mechanical Technologies in Telescopes and Instrumentation, Marseille, France, 23–28 June 2008. [Google Scholar]
- Gould, S.G.; Roberts, E.W. The in-vacuo torque performance of dry lubricated ball bearings at cryogenic temperatures. In Proceedings of the 23rd Aerospace Mechanisms Symp., Huntsville, AL, USA, 3–5 May 1989; NASA: Washington, DC, USA, 1989; p. 319. [Google Scholar]
- Neugebauer, C.; Supper, L.; Watters, R.; Roberts, E.W.; Demaret, C. Nirspec wheel support mechanism’s central duplex bearings cryogenic test results. In Proceedings of the 13th ESMATS, Vienna Austria, 23–25 September 2009. [Google Scholar]
- Renevier, N.M.; Fox, V.C.; Teer, D.G.; Hampshire, J. Coating characteristics and tribological properties of sputter-deposited MoS2/metal composite coatings deposited by closed field unbalanced magnetron sputter ion plating. Surf. Coat. Technol. 2000, 127, 24–37. [Google Scholar] [CrossRef]
- Fox, V.; Jones, A.; Renevier, N.M.; Teer, D.G. Hard lubricating coatings for cutting and forming tools and mechanical components. Surf. Coat. Technol. 2000, 125, 347–353. [Google Scholar] [CrossRef]
- Teer, D.G. New solid lubricant coatings. Wear 2001, 251, 1068–1074. [Google Scholar] [CrossRef]
- Mao, J.; Wang, Y.; Zheng, Z.L.; Deng, D.H. The rise of two-dimensional MoS2 for catalysis. Front. Phys. 2018, 13, 138118. [Google Scholar] [CrossRef]
- Bernardi, M.; Ataca, C.; Palummo, M.; Grossman, J.C. Optical and Electronic Properties of Two-Dimensional Layered Materials. Nanophotonics 2017, 6, 479–493. [Google Scholar] [CrossRef]
- Martin, J.M.; Pascal, H.; Donnet, C.; Lemogne, T.; Loubet, J.L.; Epicier, T. Superlubricity of MoS2: Crystal orientation mechanisms. Surf. Coat. Technol. 1994, 68, 427–432. [Google Scholar] [CrossRef]
- Fleischauer, P.D.; Bauer, R. Chemical and Structural Effects on the Lubrication Properties of Sputtered MoS2 Films. Tribol. Trans. 1988, 31, 239–250. [Google Scholar] [CrossRef]
- Baykara, M.Z.; Vazirisereshk, M.R.; Martini, A. Emerging superlubricity: A review of the state of the art and perspectives on future research. Appl. Phys. Rev. 2018, 5, 18. [Google Scholar] [CrossRef]
- Hirano, M.; Shinjo, K. Atomistic Locking and Friction. Phys. Rev. B 1990, 41, 11837–11851. [Google Scholar] [CrossRef]
- Sokoloff, J.B. Theory of energy dissipation in sliding crystal surfaces. Phys. Rev. B 1990, 42, 760–765. [Google Scholar] [CrossRef]
- Oviedo, J.P.; Santosh, K.C.; Lu, N.; Wang, J.G.; Cho, K.; Wallace, R.M.; Kim, M.J. In Situ TEM Characterization of Shear-Stress-Induced Interlayer Sliding in the Cross Section View of Molybdenum Disulfide. ACS Nano 2015, 9, 1543–1551. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, J.H.; Gao, S.; Chen, Q.; Peng, L.M.; Liu, K.H.; Wei, X.L. Superlubricity between MoS2 Monolayers. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Martin, J.M.; Erdemir, A. Superlubricity: Friction’s vanishing act. Phys. Today 2018, 71, 40–46. [Google Scholar] [CrossRef]
- Liu, Y.M.; Song, A.S.; Xu, Z.; Zong, R.L.; Zhang, J.; Yang, W.Y.; Wang, R.; Hu, Y.Z.; Luo, J.B.; Ma, T.B. Interlayer Friction and Superlubricity in Single-Crystalline Contact Enabled by Two-Dimensional Flake-Wrapped Atomic Force Microscope Tips. ACS Nano 2018, 12, 7638–7646. [Google Scholar] [CrossRef] [PubMed]
- Onodera, T.; Morita, Y.; Suzuki, A.; Koyama, M.; Tsuboi, H.; Hatakeyama, N.; Endou, A.; Takaba, H.; Kubo, M.; Dassenoy, F.; et al. A Computational Chemistry Study on Friction of h-MoS2. Part, I. Mechanism of Single Sheet Lubrication. J. Phys. Chem. B 2009, 113, 16526–16536. [Google Scholar] [CrossRef]
- Onodera, T.; Morita, Y.; Nagumo, R.; Miura, R.; Suzuki, A.; Tsuboi, H.; Hatakeyama, N.; Endou, A.; Takaba, H.; Dassenoy, F.; et al. A Computational Chemistry Study on Friction of h-MoS2. Part II. Friction Anisotropy. J. Phys. Chem. B 2010, 114, 15832–15838. [Google Scholar] [CrossRef] [PubMed]
- Levita, G.; Cavaleiro, A.; Molinari, E.; Polcar, T.; Righi, M.C. Sliding Properties of MoS2 Layers: Load and Interlayer Orientation Effects. J. Phys. Chem. C 2014, 118, 13809–13816. [Google Scholar] [CrossRef]
- Ye, Z.J.; Otero-De-La-Roza, A.; Johnson, E.R.; Martini, A. Oscillatory motion in layered materials: Graphene, boron nitride, and molybdenum disulfide. Nanotechnology 2015, 26, 165701. [Google Scholar] [CrossRef]
- Hao, R.; Tedstone, A.A.; Lewis, D.J.; Warrens, C.P.; West, K.R.; Howard, P.; Gaemers, S.; Dillon, S.J.; O’Brien, P. Property Self-Optimization During Wear of MoS2. ACS Appl. Mater. Interfaces 2017, 9, 1953–1958. [Google Scholar] [CrossRef]
- Tedstone, A.A.; Lewis, D.J.; Hao, R.; Mao, S.M.; Bellon, P.; Averback, R.S.; Warrens, C.P.; West, K.R.; Howard, P.; Gaemers, S.; et al. Mechanical Properties of Molybdenum Disulfide and the Effect of Doping: An in Situ TEM Study. ACS Appl. Mater. Interfaces 2015, 7, 20829–20834. [Google Scholar] [CrossRef]
- Stupp, B.C. Synergistic Effects of Metals Co-Sputtered with MoS2. Metall. Prot. Coat. 1981, 84, 257–266. [Google Scholar] [CrossRef]
- Deleanu, L.; Cantaragiu, A.; Birsan, I.G.; Podaru, G.; Georgescu, C. Evaluation of the spread range of 3D parameters for coated surfaces. Tribol. Ind. 2011, 33, 72–78. [Google Scholar]
- Horovistiz, A.; Laranjeira, S.; Davim, J.P. 2. Effect of glass fiber reinforcement and the addition of MoS2 on the tribological behavior of PA66 under dry sliding conditions: A study of distribution of pixel intensity on the counterface. In Wear of Composite Materials; De Gruyter: Berlin, Germany, 2018; Volume 9. [Google Scholar]
- Soleimani, S.; Sukumaran, J.; Kumcu, A.; De Baets, P.; Philips, W. Quantifying abrasion and micro-pits in polymer wear using image processing techniques. Wear 2014, 319, 123–137. [Google Scholar] [CrossRef]
- Binnig, G.; Quate, C.F.; Gerber, C. Atomic Force Microscope. Phys. Rev. Lett. 1986, 56, 930–933. [Google Scholar] [CrossRef] [Green Version]
- Mate, C.M.; McClelland, G.M.; Erlandsson, R.; Chiang, S. Atomic-scale Friction of a Tungsten Tip on a Graphite Surface. Phys. Rev. Lett. 1987, 59, 1942–1945. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Spear, J.C.; Ewers, B.W.; Batteas, J.D. 2D-nanomaterials for controlling friction and wear at interfaces. Nano Today 2015, 10, 301–314. [Google Scholar] [CrossRef] [Green Version]
- Schumacher, A.; Kruse, N.; Prins, R.; Meyer, E.; Lüthi, R.; Howald, L.; Güntherodt, H.J.; Scandella, L. Influence of humidity on friction measurements of supported MoS2 single layers. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 1996, 14, 1264–1267. [Google Scholar] [CrossRef]
- Lee, C.; Li, Q.Y.; Kalb, W.; Liu, X.Z.; Berger, H.; Carpick, R.W.; Hone, J. Frictional Characteristics of Atomically Thin Sheets. Science 2010, 328, 76–80. [Google Scholar] [CrossRef] [Green Version]
- Filleter, T.; McChesney, J.L.; Bostwick, A.; Rotenberg, E.; Emtsev, K.V.; Seyller, T.; Horn, K.; Bennewitz, R. Friction and Dissipation in Epitaxial Graphene Films. Phys. Rev. Lett. 2009, 102, 086102. [Google Scholar] [CrossRef]
- Ye, Z.J.; Balkanci, A.; Martini, A.; Baykara, M.Z. Effect of roughness on the layer-dependent friction of few-layer graphene. Phys. Rev. B 2017, 96, 115401. [Google Scholar] [CrossRef] [Green Version]
- Lavini, F.; Calo, A.; Gao, Y.; Albisetti, E.; Li, T.D.; Cao, T.F.; Li, G.Q.; Cao, L.Y.; Aruta, C.; Riedo, E. Friction and work function oscillatory behavior for an even and odd number of layers in polycrystalline MoS2. Nanoscale 2018, 10, 8304–8312. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Liu, D.M.; Guo, Y.Z.; Liao, Z.M.; Luo, J.B.; Wen, S.Z. Thickness dependent friction on few-layer MoS2, WS2, and WSe2. Nanotechnology 2017, 28, 245703. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.A.; Gan, X.H.; Lang, H.J.; Yu, K.; Ding, S.Y.; Peng, Y.T.; Yi, W.M. Anisotropic nanofriction on MoS2 with different thicknesses. Tribol. Int. 2019, 134, 308–316. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, J.S.; Byun, I.S.; Lee, D.H.; Lee, M.J.; Park, B.H.; Lee, C.; Yoon, D.; Cheong, H.; Lee, K.H.; et al. Friction Anisotropy-Driven Domain Imaging on Exfoliated Monolayer Graphene. Science 2011, 333, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.H.; Jung, J.; Kim, C.; Lee, J.; Oh, S.D.; Kim, K.S.; Lee, C. Comparison of Frictional Properties of CVD-Grown MoS2 and Graphene Films under Dry Sliding Conditions. Nanomaterials 2019, 9, 293. [Google Scholar] [CrossRef] [PubMed]
- Vazirisereshk, M.R.; Ye, H.; Ye, Z.; Otero-de-la-Roza, A.; Zhao, M.; Gao, Z.; Johnson, A.T.C.; Johnson, E.R.; Carpick, R.W.; Martini, A. Origin of Nanoscale Friction Contrast between Supported Graphene, MoS2, and a Graphene/MoS2 Heterostructure. Nano Lett. 2019. under review. [Google Scholar]
- Peterson, M.B.; Johnson, R.L. Friction and Wear Investigation of Molybdenum Disulfide 1: Effect of Moisture; Technical Note 3055; NACA: Washington, DC, USA, 1953. [Google Scholar]
- Ross, S.; Sussman, A. Surface Oxidation of Molybdenum Disulfide. J. Phys. Chem. 1955, 59, 889–892. [Google Scholar] [CrossRef]
- Haltner, A.J.; Oliver, C.S. Effect of Water Vapor on Friction of Molybdenum Disulfide. Ind. Eng. Chem. Fundam. 1966, 5, 348–355. [Google Scholar] [CrossRef]
- Pardee, R.P. The Effect of Humidity on Low-Load Frictional Properties of a Bonded Solid Film Lubricant. ASLE Trans. 1972, 15, 130–142. [Google Scholar] [CrossRef]
- Panitz, J.K.G.; Pope, L.E.; Lyons, J.E.; Staley, D.J. The tribological properties of MoS2 coatings in vacuum, low relative humidity, and high relative humidity environments. J. Vac. Sci. Technol. A 1988, 6, 1166–1170. [Google Scholar] [CrossRef]
- Tagawa, M.; Yokota, K.; Ohmae, N.; Matsumoto, K.; Suzuki, M. Hyperthermal Atomic Oxygen Interaction with MoS2 Lubricants Relevance to Space Environmental Effects in Low Earth Orbit—Atomic Oxygen-Induced Oxidation. Tribol. Lett. 2004, 17, 859–865. [Google Scholar] [CrossRef]
- Khare, H.S.; Burris, D.L. The Effects of Environmental Water and Oxygen on the Temperature-Dependent Friction of Sputtered Molybdenum Disulfide. Tribol. Lett. 2013, 52, 485–493. [Google Scholar] [CrossRef]
- Tagawa, M.; Ikeda, J.; Kinoshita, H.; Umeno, M.; Ohmae, N. Effect of Atomic Oxygen Exposures on the Tribological Properties of Molybdenum Disulfide Lubricants. In Protection of Space Materials from the Space Environment; Springer: Dordrecht, The Netherlands, 2001; pp. 73–84. [Google Scholar]
- Miyoshi, K. Solid Lubrication Fundamentals and Applications; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Liang, T.; Sawyer, W.G.; Perry, S.S.; Sinnott, S.B.; Phillpot, S.R. Energetics of Oxidation in MoS2 Nanoparticles by Density Functional Theory. J. Phys. Chem. C 2011, 115, 10606–10616. [Google Scholar] [CrossRef]
- Johnson, M.R. The Galileo High Gain Antenna Deployment Anomaly. In Proceedings of the 28th Aerospace Mechanisms Symposium, NASA Lewis Research Center, Cleveland, OH, USA, 18–20 May 1994; pp. 359–377. [Google Scholar]
- Fusaro, R.L. Lubrication and Failure Mechanisms of Molybdenum Disulfide Films I-Effect of Atmosphere; Technical Note 1343; NASA: Washington, DC, USA, 1978.
- Muratore, C.; Bultman, J.E.; Aouadi, S.M.; Voevodin, A.A. In situ Raman spectroscopy for examination of high temperature tribological processes. Wear 2011, 270, 140–145. [Google Scholar] [CrossRef]
- Spychalski, W.L.; Pisarek, M.; Szoszkiewicz, R. Microscale Insight into Oxidation of Single MoS2 Crystals in Air. J. Phys. Chem. C 2017, 121, 26027–26033. [Google Scholar] [CrossRef]
- Windom, B.C.; Sawyer, W.G.; Hahn, D.W. A Raman Spectroscopic Study of MoS2 and MoO3: Applications to Tribological Systems. Tribol. Lett. 2011, 42, 301–310. [Google Scholar] [CrossRef]
- Kubart, T.; Polcar, T.; Kopecký, L.; Novák, R.; Nováková, D. Temperature dependence of tribological properties of MoS2 and MoSe2 coatings. Surf. Coat. Technol. 2005, 193, 230–233. [Google Scholar] [CrossRef]
- Sliney, H.E. High Temperature Solid Lubricants: When and Where to Use Them; Technical Memorandum 68201; NASA: Washington, DC, USA, 1973.
- Yamamoto, M.; Einstein, T.L.; Fuhrer, M.S.; Cullen, W.G. Anisotropic Etching of Atomically Thin MoS2. J. Phys. Chem. C 2013, 117, 25643–25649. [Google Scholar] [CrossRef]
- Zhang, X.; Qiao, L.; Chai, L.; Xu, J.; Shi, L.; Wang, P. Structural, mechanical and tribological properties of Mo–S–N solid lubricant films. Surf. Coat. Technol. 2016, 296, 185–191. [Google Scholar] [CrossRef]
- Wang, P.; Qiao, L.; Xu, J.; Li, W.; Liu, W. Erosion Mechanism of MoS2-Based Films Exposed to Atomic Oxygen Environments. ACS Appl. Mater. Interfaces 2015, 7, 12943–12950. [Google Scholar] [CrossRef] [PubMed]
- Curry, J.F.; Argibay, N.; Babuska, T.; Nation, B.; Martini, A.; Strandwitz, N.C.; Dugger, M.T.; Krick, B.A. Highly Oriented MoS2 Coatings: Tribology and Environmental Stability. Tribol. Lett. 2016, 64, 11. [Google Scholar] [CrossRef]
- Curry, J.F.; Wilson, M.A.; Luftman, H.S.; Strandwitz, N.C.; Argibay, N.; Chandross, M.; Sidebottom, M.A.; Krick, B.A. Impact of Microstructure on MoS2 Oxidation and Friction. ACS Appl. Mater. Interfaces 2017, 9, 28019–28026. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Fu, Y.; Jiang, D.; Wang, D.; Weng, L.; Yang, J.; Sun, J.; Hu, M. Responses of TMDs-metals composite films to atomic oxygen exposure. J. Alloy. Compd. 2018, 765, 854–861. [Google Scholar] [CrossRef]
- Tagawa, M.; Yokota, K.; Ochi, K.; Akiyama, M.; Matsumoto, K.; Suzuki, M. Comparison of Macro and Microtribological Property of Molybdenum Disulfide Film Exposed to LEO Space Environment. Tribol. Lett. 2012, 45, 349–356. [Google Scholar] [CrossRef]
- Tagawa, M.; Muromoto, M.; Hachiue, S.; Yokota, K.; Ohmae, N.; Matsumoto, K.; Suzuki, M. Hyperthermal atomic oxygen interaction with MoS2 lubricants and relevance to space environmental effects in low earth orbit–effects on friction coefficient and wear-life. Tribol. Lett. 2005, 18, 437–443. [Google Scholar] [CrossRef]
- Wei, R.; Wilbur, P.J.; Buchholz, B.W.; Kustas, F.M. In Situ Tribological Evaluation of Greases and Solid Lubricants in a Simulated Atomic Oxygen Environment. Tribol. Trans. 1995, 38, 950–958. [Google Scholar] [CrossRef]
- Gao, X.; Hu, M.; Sun, J.; Fu, Y.; Yang, J.; Liu, W.; Weng, L. Changes in the composition, structure and friction property of sputtered MoS2 films by LEO environment exposure. Appl. Surf. Sci. 2015, 330, 30–38. [Google Scholar] [CrossRef]
- Tagawa, M.; Yokota, K.; Matsumoto, K.; Suzuki, M.; Teraoka, Y.; Kitamura, A.; Belin, M.; Fontaine, J.; Martin, J.M. Space environmental effects on MoS2 and diamond-like carbon lubricating films: Atomic oxygen-induced erosion and its effect on tribological properties. Surf. Coat. Technol. 2007, 202, 1003–1010. [Google Scholar] [CrossRef]
- Khare, H.S.; Burris, D.L. Surface and Subsurface Contributions of Oxidation and Moisture to Room Temperature Friction of Molybdenum Disulfide. Tribol. Lett. 2014, 53, 329–336. [Google Scholar] [CrossRef]
- Stewart, T.B.; Fleischauer, P.D. Chemistry of sputtered molybdenum disulfide films. Inorg. Chem. 1982, 21, 2426–2431. [Google Scholar] [CrossRef]
- Serpini, E.; Rota, A.; Ballestrazzi, A.; Marchetto, D.; Gualtieri, E.; Valeri, S. The role of humidity and oxygen on MoS2 thin films deposited by RF PVD magnetron sputtering. Surf. Coat. Technol. 2017, 319, 345–352. [Google Scholar] [CrossRef]
- Pritchard, C.; Midgley, J.W. The effect of humidity on the friction and life of unbonded molybdenum disulphide films. Wear 1969, 13, 39–50. [Google Scholar] [CrossRef]
- Johnston, R.R.M.; Moore, A.J.W. Water Adsorption on Molybdenum Disulfide Containing Surface Contaminants. J. Phys. Chem. 1964, 68, 3399–3406. [Google Scholar] [CrossRef]
- Levita, G.; Righi, M.C. Effects of Water Intercalation and Tribochemistry on MoS2 Lubricity: An Ab Initio Molecular Dynamics Investigation. Chem. Phys. Chem. 2017, 18, 1475–1480. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Jeong, H.; Suh, J.; Doh, W.H.; Baik, J.; Shin, H.J.; Ko, J.H.; Wu, J.; Kim, Y.H.; Park, J.Y. Nanoscale Friction on Confined Water Layers Intercalated between MoS2 Flakes and Silica. J. Phys. Chem. C 2019, 123, 8827–8835. [Google Scholar] [CrossRef]
- Uemura, M.; Saito, K.; Nakao, K. A Mechanism of Vapor Effect on Friction Coefficient of Molybdenum Disulfide. Tribol. Trans. 1990, 33, 551–556. [Google Scholar] [CrossRef]
- Lancaster, J.K. A review of the influence of environmental humidity and water on friction, lubrication and wear. Tribol. Int. 1990, 23, 371–389. [Google Scholar] [CrossRef]
- Holinski, R.; Gänsheimer, J. A study of the lubricating mechanism of molybdenum disulfide. Wear 1972, 19, 329–342. [Google Scholar] [CrossRef]
- Zhao, X.; Perry, S.S. The Role of Water in Modifying Friction within MoS2 Sliding Interfaces. ACS Appl. Mater. Interfaces 2010, 2, 1444–1448. [Google Scholar] [CrossRef]
- Ataca, C.; Ciraci, S. Dissociation of H2O at the vacancies of single-layer MoS2. Phys. Rev. B 2012, 85, 195410. [Google Scholar] [CrossRef]
- Ghuman, K.K.; Yadav, S.; Singh, C.V. Adsorption and Dissociation of H2O on Monolayered MoS2 Edges: Energetics and Mechanism from ab Initio Simulations. J. Phys. Chem. C 2015, 119, 6518–6529. [Google Scholar] [CrossRef]
- Levita, G.; Restuccia, P.; Righi, M.C. Graphene and MoS2 interacting with water: A comparison by ab initio calculations. Carbon 2016, 107, 878–884. [Google Scholar] [CrossRef]
- Vierneusel, B.; Schneider, T.; Tremmel, S.; Wartzack, S.; Gradt, T. Humidity resistant MoS2 coatings deposited by unbalanced magnetron sputtering. Surf. Coat. Technol. 2013, 235, 97–107. [Google Scholar] [CrossRef]
- Muratore, C.; Voevodin, A.A. Control of molybdenum disulfide basal plane orientation during coating growth in pulsed magnetron sputtering discharges. Thin Solid Films 2009, 517, 5605–5610. [Google Scholar] [CrossRef]
- Gradt, T.; Schneider, T. Tribological Performance of MoS2 Coatings in Various Environments. Lubricants 2016, 4, 32. [Google Scholar] [CrossRef]
- Chhowalla, M.; Amaratunga, G.A.J. Thin films of fullerene-like MoS2 nanoparticles with ultra-low friction and wear. Nature 2000, 407, 164. [Google Scholar] [CrossRef]
- Kohli, A.K.; Prakash, B. Contact Pressure Dependency in Frictional Behavior of Burnished Molybdenum Disulphide Coatings. Tribol. Trans. 2001, 44, 147–151. [Google Scholar] [CrossRef]
- Sliney, H.E. Solid lubricant materials for high temperatures—A review. Tribol. Int. 1982, 15, 303–315. [Google Scholar] [CrossRef]
- Colbert, R.S.; Sawyer, W.G. Thermal dependence of the wear of molybdenum disulphide coatings. Wear 2010, 269, 719–723. [Google Scholar] [CrossRef]
- Dunckle, C.G.; Aggleton, M.; Glassman, J.; Taborek, P. Friction of molybdenum disulfide–titanium films under cryogenic vacuum conditions. Tribol. Int. 2011, 44, 1819–1826. [Google Scholar] [CrossRef]
- Zhao, X.; Phillpot, S.R.; Sawyer, W.G.; Sinnott, S.B.; Perry, S.S. Transition from Thermal to Athermal Friction under Cryogenic Conditions. Phys. Rev. Lett. 2009, 102, 186102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curry, J.F.; Hinkle, A.R.; Babuska, T.F.; Wilson, M.A.; Dugger, M.T.; Krick, B.A.; Argibay, N.; Chandross, M. Atomistic Origins of Temperature-Dependent Shear Strength in 2D Materials. ACS Appl. Nano Mater. 2018, 1, 5401–5407. [Google Scholar] [CrossRef]
- Curry, J.F.; Babuska, T.F.; Brumbach, M.T.; Argibay, N. Temperature-Dependent Friction and Wear of MoS2/Sb2O3/Au Nanocomposites. Tribol. Lett. 2016, 64, 18. [Google Scholar] [CrossRef]
- Babuska, T.F.; Pitenis, A.A.; Jones, M.R.; Nation, B.L.; Sawyer, W.G.; Argibay, N. Temperature-Dependent Friction and Wear Behavior of PTFE and MoS2. Tribol. Lett. 2016, 63, 15. [Google Scholar] [CrossRef]
- Hamilton, M.A.; Alvarez, L.A.; Mauntler, N.A.; Argibay, N.; Colbert, R.; Burris, D.L.; Muratore, C.; Voevodin, A.A.; Perry, S.S.; Sawyer, W.G. A Possible Link Between Macroscopic Wear and Temperature Dependent Friction Behaviors of MoS2 Coatings. Tribol. Lett. 2008, 32, 91–98. [Google Scholar] [CrossRef]
- Seitzman, L.E.; Bolster, R.N.; Singer, I.L. Effects of temperature and ion-to-atom ratio on the orientation of IBAD MoS2 coatings. Thin Solid Films 1995, 260, 143–147. [Google Scholar] [CrossRef]
- Moser, J.; Lévy, F.; Bussy, F. Composition and growth mode of MoSx sputtered films. J. Vac. Sci. Technol. A 1994, 12, 494–500. [Google Scholar] [CrossRef]
- Zabinski, J.S.; Day, A.E.; Donley, M.S.; Dellacorte, C.; McDevitt, N.T. Synthesis and characterization of a high-temperature oxide lubricant. J. Mater. Sci. 1994, 29, 5875–5879. [Google Scholar] [CrossRef]
- Muratore, C.; Voevodin, A.A. Molybdenum disulfide as a lubricant and catalyst in adaptive nanocomposite coatings. Surf. Coat. Technol. 2006, 201, 4125–4130. [Google Scholar] [CrossRef]
- Aouadi, S.M.; Paudel, Y.; Simonson, W.J.; Ge, Q.; Kohli, P.; Muratore, C.; Voevodin, A.A. Tribological investigation of adaptive Mo2N/MoS2/Ag coatings with high sulfur content. Surf. Coat. Technol. 2009, 203, 1304–1309. [Google Scholar] [CrossRef]
- Chen, F.; Feng, Y.; Shao, H.; Zhang, X.; Chen, J.; Chen, N. Friction and Wear Behaviors of Ag/MoS2/G Composite in Different Atmospheres and at Different Temperatures. Tribol. Lett. 2012, 47, 139–148. [Google Scholar] [CrossRef]
- Voevodin, A.A.; Muratore, C.; Aouadi, S.M. Hard coatings with high temperature adaptive lubrication and contact thermal management: Review. Surf. Coat. Technol. 2014, 257, 247–265. [Google Scholar] [CrossRef]
- Yang, J.-F.; Jiang, Y.; Hardell, J.; Prakash, B.; Fang, Q.-F. Influence of service temperature on tribological characteristics of self-lubricant coatings: A review. Front. Mater. Sci. 2013, 7, 28–39. [Google Scholar] [CrossRef]
- Aouadi, S.M.; Gao, H.; Martini, A.; Scharf, T.W.; Muratore, C. Lubricious oxide coatings for extreme temperature applications: A review. Surf. Coat. Technol. 2014, 257, 266–277. [Google Scholar] [CrossRef]
- Aouadi, S.M.; Luster, B.; Kohli, P.; Muratore, C.; Voevodin, A.A. Progress in the development of adaptive nitride-based coatings for high temperature tribological applications. Surf. Coat. Technol. 2009, 204, 962–968. [Google Scholar] [CrossRef]
- Muratore, C.; Voevodin, A.A. Chameleon Coatings: Adaptive Surfaces to Reduce Friction and Wear in Extreme Environments. Annu. Rev. Mater. Res. 2009, 39, 297–324. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Luster, B.; Church, A.; Muratore, C.; Voevodin, A.A.; Kohli, P.; Aouadi, S.; Talapatra, S. Carbon Nanotube−MoS2 Composites as Solid Lubricants. ACS Appl. Mater. Interfaces 2009, 1, 735–739. [Google Scholar] [CrossRef]
- Voevodin, A.A.; Fitz, T.A.; Hu, J.J.; Zabinski, J.S. Nanocomposite tribological coatings with “chameleon” surface adaptation. J. Vac. Sci. Technol. A 2002, 20, 1434–1444. [Google Scholar] [CrossRef]
- Baker, C.C.; Chromik, R.R.; Wahl, K.J.; Hu, J.J.; Voevodin, A.A. Preparation of chameleon coatings for space and ambient environments. Thin Solid Films 2007, 515, 6737–6743. [Google Scholar] [CrossRef]
- Torres, H.; Rodríguez Ripoll, M.; Prakash, B. Tribological behaviour of self-lubricating materials at high temperatures. Int. Mater. Rev. 2018, 63, 309–340. [Google Scholar] [CrossRef]
- Liu, G.L.; Robertson, A.W.; Li, M.M.J.; Kuo, W.C.H.; Darby, M.T.; Muhieddine, M.H.; Lin, Y.C.; Suenaga, K.; Stamatakis, M.; Warner, J.H.; et al. MoS2 monolayer catalyst doped with isolated Co atoms for the hydrodeoxygenation reaction. Nat. Chem. 2017, 9, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Ivanovskaya, V.V.; Zobelli, A.; Gloter, A.; Brun, N.; Serin, V.; Colliex, C. Ab initio study of bilateral doping within the MoS2-NbS2 system. Phys. Rev. B 2008, 78, 134104. [Google Scholar] [CrossRef]
- Tedstone, A.A.; Lewis, D.J.; O’Brien, P. Synthesis, Properties, and Applications of Transition Metal-Doped Layered Transition Metal Dichalcogenides. Chem. Mater. 2016, 28, 1965–1974. [Google Scholar] [CrossRef]
- Kutana, A.; Penev, E.S.; Yakobson, B.I. Engineering electronic properties of layered transition-metal dichalcogenide compounds through alloying. Nanoscale 2014, 6, 5820–5825. [Google Scholar] [CrossRef] [PubMed]
- Dolui, K.; Rungger, I.; Das Pemmaraju, C.; Sanvito, S. Possible doping strategies for MoS2 monolayers: An ab initio study. Phys. Rev. B 2013, 88, 075420. [Google Scholar] [CrossRef]
- Deng, J.; Li, H.B.; Xiao, J.P.; Tu, Y.C.; Deng, D.H.; Yang, H.X.; Tian, H.F.; Li, J.Q.; Ren, P.J.; Bao, X.H. Triggering the electrocatalytic hydrogen evolution activity of the inert two-dimensional MoS2 surface via single-atom metal doping. Energy Environ. Sci. 2015, 8, 1594–1601. [Google Scholar] [CrossRef]
- Hakala, M.; Kronberg, R.; Laasonen, K. Hydrogen adsorption on doped MoS2 nanostructures. Sci. Rep. 2017, 7, 15243. [Google Scholar] [CrossRef]
- Benavente, E.; Santa Ana, M.A.; Mendizabal, F.; Gonzalez, G. Intercalation chemistry of molybdenum disulfide. Coord. Chem. Rev. 2002, 224, 87–109. [Google Scholar] [CrossRef]
- Suh, J.; Tan, T.L.; Zhao, W.J.; Park, J.; Lin, D.Y.; Park, T.E.; Kim, J.; Jin, C.H.; Saigal, N.; Ghosh, S.; et al. Reconfiguring crystal and electronic structures of MoS2 by substitutional doping. Nat. Commun. 2018, 9, 199. [Google Scholar] [CrossRef]
- Kondekar, N.; Boebinger, M.G.; Tian, M.; Kirmani, M.H.; McDowell, M.T. The Effect of Nickel on MoS2 Growth Revealed with in Situ Transmission Electron Microscopy. ACS Nano 2019. [Google Scholar] [CrossRef] [PubMed]
- Lince, J.R.; Hilton, M.R.; Bommannavar, A.S. Metal incorporation in sputter-deposited MoS2 films studied by extended x-ray absorption fine structure. J. Mater. Res. 1995, 10, 2091–2105. [Google Scholar] [CrossRef]
- Shi, Y.; Zhou, Y.; Yang, D.R.; Xu, W.X.; Wang, C.; Wang, F.B.; Xu, J.J.; Xia, X.H.; Chen, H.Y. Energy Level Engineering of MoS2 by Transition-Metal Doping for Accelerating Hydrogen Evolution Reaction. J. Am. Chem. Soc. 2017, 139, 15479–15485. [Google Scholar] [CrossRef] [PubMed]
- Hallam, T.; Monaghan, S.; Gity, F.; Ansari, L.; Schmidt, M.; Downing, C.; Cullen, C.P.; Nicolosi, V.; Hurley, P.K.; Duesberg, G.S. Rhenium-doped MoS2 films. Appl. Phys. Lett. 2017, 111, 203101. [Google Scholar] [CrossRef]
- Lin, Y.C.; Dumcenco, D.O.; Komsa, H.P.; Niimi, Y.; Krasheninnikov, A.V.; Huang, Y.S.; Suenaga, K. Properties of Individual Dopant Atoms in Single-Layer MoS2: Atomic Structure, Migration, and Enhanced Reactivity. Adv. Mater. 2014, 26, 2857–2861. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.; Park, T.E.; Lin, D.Y.; Fu, D.Y.; Park, J.; Jung, H.J.; Chen, Y.B.; Ko, C.; Jang, C.; Sun, Y.H.; et al. Doping against the Native Propensity of MoS2: Degenerate Hole Doping by Cation Substitution. Nano Lett. 2014, 14, 6976–6982. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Hautier, G.; Ong, S.P.; Moore, C.J.; Fischer, C.C.; Persson, K.A.; Ceder, G. Formation enthalpies by mixing GGA and GGA plus U calculations. Phys. Rev. B 2011, 84, 045115. [Google Scholar] [CrossRef]
- Lieber, C.M.; Kim, Y. Characterization of the structural, electronic and tribological properties of metal dichalcogenides by scanning probe microscopies. Thin Solid Films 1991, 206, 355–359. [Google Scholar] [CrossRef]
- Lauritsen, J.V.; Kibsgaard, J.; Olesen, G.H.; Moses, P.G.; Hinnemann, B.; Helveg, S.; Norskov, J.K.; Clausen, B.S.; Topsoe, H.; Laegsgaard, E.; et al. Location and coordination of promoter atoms in Co- and Ni-promoted MoS2-based hydrotreating catalysts. J. Catal. 2007, 249, 220–233. [Google Scholar] [CrossRef]
- Lewis, D.J.; Tedstone, A.A.; Zhong, X.L.; Lewis, E.A.; Rooney, A.; Savjani, N.; Brent, J.R.; Haigh, S.J.; Burke, M.G.; Muryn, C.A.; et al. Thin Films of Molybdenum Disulfide Doped with Chromium by Aerosol-Assisted Chemical Vapor Deposition (AACVD). Chem. Mater. 2015, 27, 1367–1374. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.F.; Zhang, J.J.; Li, S.; Grote, F.; Zhang, X.D.; Zhang, H.; Wang, R.X.; Lei, Y.; Pan, B.C.; Xie, Y. Controllable Disorder Engineering in Oxygen-Incorporated MoS2 Ultrathin Nanosheets for Efficient Hydrogen Evolution. J. Am. Chem. Soc. 2013, 135, 17881–17888. [Google Scholar] [CrossRef] [PubMed]
- Neal, A.T.; Pachter, R.; Mou, S. P-type conduction in two-dimensional MoS2 via oxygen incorporation. Appl. Phys. Lett. 2017, 110, 193103. [Google Scholar] [CrossRef]
- Yang, L.M.; Majumdar, K.; Liu, H.; Du, Y.C.; Wu, H.; Hatzistergos, M.; Hung, P.Y.; Tieckelmann, R.; Tsai, W.; Hobbs, C.; et al. Chloride Molecular Doping Technique on 2D Materials: WS2 and MoS2. Nano Lett. 2014, 14, 6275–6280. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.H.; Xia, W.J.; Wu, Z.J.; Huo, J.; Liu, D.D.; Wang, Q.; Wang, S.Y. The origin of the enhanced performance of nitrogen-doped MoS2 in lithium ion batteries. Nanotechnology 2016, 27, 175402. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Ko, C.; Kim, K.; Chen, Y.B.; Suh, J.; Ryu, S.G.; Wu, K.D.; Meng, X.Q.; Suslu, A.; Tongay, S.; et al. Site Selective Doping of Ultrathin Metal Dichalcogenides by Laser-Assisted Reaction. Adv. Mater. 2016, 28, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Zabinski, J.S.; Donley, M.S.; Walck, S.D.; Schneider, T.R.; McDevitt, N.T. The Effects of Dopants on the Chemistry and Tribology of Sputter-Deposited MoS2 Films. Tribol. Trans. 1995, 38, 894–904. [Google Scholar] [CrossRef]
- Simmonds, M.C.; Savan, A.; Pfluger, E.; Swygenhovena, H.V. Mechanical and tribological performance of MoS2 co-sputtered composites. Surf. Coat. Technol. 2000, 126, 15–24. [Google Scholar] [CrossRef]
- Nainaparampil, J.J.; Phani, A.R.; Krzanowski, J.E.; Zabinski, J.S. Pulsed laser-ablated MoS2-Al films: Friction and wear in humid conditions. Surf. Coat. Technol. 2004, 187, 326–335. [Google Scholar] [CrossRef]
- Ye, M.; Zhang, G.; Ba, Y.; Wang, T.; Wang, X.; Liu, Z. Microstructure and tribological properties of MoS2 +Zr composite coatings in high humidity environment. Appl. Surf. Sci. 2016, 367, 140–146. [Google Scholar] [CrossRef]
- Stoyanov, P.; Chromik, R.R.; Goldbaum, D.; Lince, J.R.; Zhang, X. Microtribological Performance of Au–MoS2 and Ti–MoS2 Coatings with Varying Contact Pressure. Tribol. Lett. 2010, 40, 199–211. [Google Scholar] [CrossRef]
- Paul, A.; Singh, H.; Mutyala, K.C.; Doll, G.L. An Improved Solid Lubricant for Bearings Operating in Space and Terrestrial Environments. In Proceedings of the 44th Aerospace Mechanisms Symposium, NASA Glenn Research Center, Cleveland, OH, USA, 16–18 May 2018. NASA/CP—2018-219887. [Google Scholar]
- Li, H.; Li, X.; Zhang, G.; Wang, L.; Wu, G. Exploring the Tribophysics and Tribochemistry of MoS2 by Sliding MoS2/Ti Composite Coating Under Different Humidity. Tribol. Lett. 2017, 65, 38. [Google Scholar] [CrossRef]
- Spalvins, T. Frictional and Morphological Properties of Au-MoS2 Films Sputtered from a Compact Target. Metall. Prot. Coat. 1984, 118, 375–384. [Google Scholar] [CrossRef]
- Scharf, T.W.; Kotula, P.G.; Prasad, S.V. Friction and wear mechanisms in MoS2/Sb2O3/Au nanocomposite coatings. Acta Mater. 2010, 58, 4100–4109. [Google Scholar] [CrossRef]
- Scharf, T.W.; Goeke, R.S.; Kotula, P.G.; Prasad, S.V. Synthesis of Au-MoS2 nanocomposites: Thermal and friction-induced changes to the structure. ACS Appl. Mater. Interfaces 2013, 5, 11762–11767. [Google Scholar] [CrossRef] [PubMed]
- Lince, J.R.; Kim, H.I.; Adams, P.M.; Dickrell, D.J.; Dugger, M.T. Nanostructural, electrical, and tribological properties of composite Au–MoS2 coatings. Thin Solid Films 2009, 517, 5516–5522. [Google Scholar] [CrossRef]
- Ding, X.Z.; Zeng, X.T.; He, X.Y.; Chen, Z. Tribological properties of Cr- and Ti-doped MoS2 composite coatings under different humidity atmosphere. Surf. Coat. Technol. 2010, 205, 224–231. [Google Scholar] [CrossRef]
- Singh, H.; Mutyala, K.C.; Mohseni, H.; Scharf, T.W.; Evans, R.D.; Doll, G.L. Tribological Performance and Coating Characteristics of Sputter-Deposited Ti-Doped MoS2in Rolling and Sliding Contact. Tribol. Trans. 2015, 58, 767–777. [Google Scholar] [CrossRef]
- Hsu, W.K.; Zhu, Y.Q.; Yao, N.; Firth, S.; Clark, R.J.H.; Kroto, H.W.; Walton, D.R.M. Titanium-doped molybdenum disulfide nanostructures. Adv. Funct. Mater. 2001, 11, 69–74. [Google Scholar] [CrossRef]
- Singh, H.; Mutyala, K.C.; Evans, R.D.; Doll, G.L. An atom probe tomography investigation of Ti–MoS2 and MoS2–Sb2O3–Au films. J. Mater. Res. 2017, 32, 1710–1717. [Google Scholar] [CrossRef]
- Li, H.; Zhang, G.; Wang, L. The role of tribo-pairs in modifying the tribological behavior of the MoS2/Ti composite coating. J. Phys. D Appl. Phys. 2016, 49, 095501. [Google Scholar] [CrossRef]
- Lince, J.R. Tribology of co-sputtered nanocomposite Au/MoS2 solid lubricant films over a wide contact stress range. Tribol. Lett. 2004, 17, 419–428. [Google Scholar] [CrossRef]
- Singh, H.; Mutyala, K.C.; Evans, R.D.; Doll, G.L. An investigation of material and tribological properties of Sb2O3/Au-doped MoS2 solid lubricant films under sliding and rolling contact in different environments. Surf. Coat. Technol. 2015, 284, 281–289. [Google Scholar] [CrossRef]











| Polytype | Space Group | Point Group | Atoms Per Conv. Cell | Stacking | XRD Lattice Parameters | Properties |
|---|---|---|---|---|---|---|
| 1T′ | Pm1 | D3d | 9 | AAAAAA | a = 5.60 Å, c = 5.99 Å [21] | metallic, metastable |
| 2H | P63/mmc | D6h | 6 | ABABAB | a = 3.16 Å, c = 12.29 Å [34] | semiconducting, naturally occurring |
| 3R | R3m | C3v | 9 | ABCABC | a = 3.17 Å, c = 18.38 Å [34] | semiconducting, naturally occurring |
| Space Mechanisms | Medical or Dental Equipment | Nuclear Reactors | Food Processing Equipment | Hard Disks, Microscopes, Cameras | Semiconductor Manufacturing | Furnaces/ Metalworking Equipment | Refrigeration/Liquid Nitrogen Pumps | Bridge, Plant or Building Supports | |
|---|---|---|---|---|---|---|---|---|---|
| High Temperature | x | x | |||||||
| Cryogenic Temperature | x | x | |||||||
| Radiation | x | x | |||||||
| Corrosive Gas | x | x | |||||||
| High Pressure/Load | x | x | x | ||||||
| Product Contamination Unacceptable | x | x | x | x | |||||
| Service Difficult or Impossible | x | x | |||||||
| Weight Limited Applications | x |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vazirisereshk, M.R.; Martini, A.; Strubbe, D.A.; Baykara, M.Z. Solid Lubrication with MoS2: A Review. Lubricants 2019, 7, 57. https://doi.org/10.3390/lubricants7070057
Vazirisereshk MR, Martini A, Strubbe DA, Baykara MZ. Solid Lubrication with MoS2: A Review. Lubricants. 2019; 7(7):57. https://doi.org/10.3390/lubricants7070057
Chicago/Turabian StyleVazirisereshk, Mohammad R., Ashlie Martini, David A. Strubbe, and Mehmet Z. Baykara. 2019. "Solid Lubrication with MoS2: A Review" Lubricants 7, no. 7: 57. https://doi.org/10.3390/lubricants7070057