Wear Performance of UHMWPE and Reinforced UHMWPE Composites in Arthroplasty Applications: A Review
Abstract
:1. Introduction
2. Mechanical Properties and Manufacturing Process of UHMWPE
3. Improvements on the Wear Resistance of UHMWPE
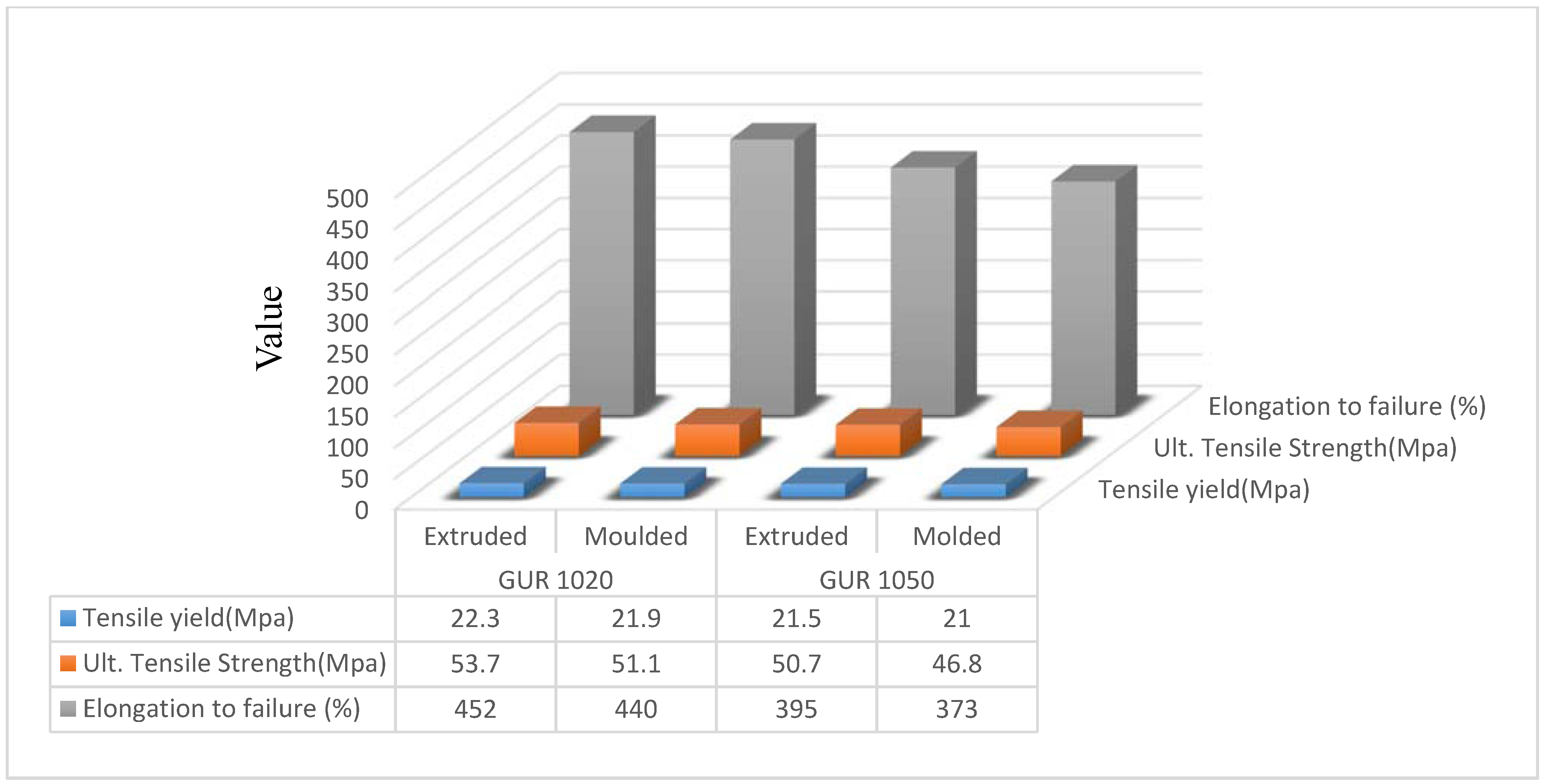
3.1. Crystallinity of UHMWPE
3.2. Cross-Linked UHMWPE Using Gamma Irradiation

3.3. Vitamin-E-Stabilised UHMWPE
| Commercial Names | Irradiation Technique | Irradiation Temp | Total Radiation Dose (kGy) | Post-Irradiation Thermal Treatment | Yield Strength (Mpa) (±SD) | Ultimate Tensile Strength (Mpa) (±SD) | True Elongation to Break (%) |
|---|---|---|---|---|---|---|---|
| UHMWPE unirradiated | - | - | - | - | 21 | 50 | 340 |
| Longevity a (Zimmer) b | E-beam | 40 °C | 100 | Melted | 21 ± 1.0 | 43 ± 9.8 | 240 ± 35 |
| Durasul (Zimmer) | 125 °C | 95 | Melted | 20 ± 0.7 | 30 ± 7.1 | 280 ± 74 | |
| Crossfire (Stryker Howmedica) | Gamma | RT | 105 | 130 °C | 24 ± 1.3 | 48 ± 7.2 | 280 ± 37 |
| Marathon (DePuy/J and J) | RT | 50 | Melted | 21 ± 1.5 | 56 ± 5.7 | 290 ± 14 | |
| XLPE (Smith & Nephew) | RT | 100 | Melted | 20 ± 1.3 | 56 ± 7.1 | 300 ± 20 |
3.3.1. Methods of Vitamin E Blending
3.3.2. Effect of Vitamin E on Tribological and Mechanical Properties of UHMWPE
3.3.3. Limitations and Challenges
3.4. Fillers (CNFs, CNTs, Graphene and Hard Particles)
| Resin | VE Concentration (wt%) | Tensile Strength Increase (%) | Strain Break Increase (%) | Yield Strength Increase (%) | Impact Strength Increase (%) | Elastic Modulus Increase (%) | Elongation Increase (%) | Fatigue Strength Increase (%) | Ref |
|---|---|---|---|---|---|---|---|---|---|
| UHMWPE GUR 1020 and 1050 | 0.01–0.05 | No significant change | No significant change | - | - | - | - | - | [51] |
| UHMWPE GUR 1020 | 0.1–0.4 | No significant change | - | - | No significant change | No significant change | No significant change | - | [58] |
| UHMWPE GUR 1020 | 0.8 | −20 | - | - | No significant change | −20 | No significant change | - | [58] |
| UHMWPE GUR 1050 | - | - | - | - | - | - | - | 58 | [60] |
| UHMWPE GUR 1050 | 0.1–0.3 | 51 | - | 19 | - | - | −19 | - | [52] |
| UHMWPE GUR 1050 | - | 36 | - | 4 | - | - | 32 | 35 | [25] |
| UHMWPE | 0.1–0.3 | - | - | - | - | - | - | 10–20 | [59] |
3.4.1. Carbon Nanofibres (CNFs)
| Fillers | Percentage of Inclusion | Improved Properties | Reduction in Wear Rate |
|---|---|---|---|
| Carbon nanofibers (CNF) [26,58,59,62,65] | 0.5%–5% | Tensile strength | 56%–58% |
| Carbon nanotube (CNTs) [66–69] | 0.1%–5% | Tensile strength Young’s modulus Toughness | 26%–86% |
| Graphene [70–75] | 0.1%–1.0% | Lubrication, tensile strength Yield strength Reducing friction coefficient | 2.5–4.5 times (depending on load) |
| Hard particles [76–79] | 10%–20% | Bearing loading capacity | 36%–60% |
3.4.2. Carbon Nanotube (CNTs)
3.4.3. Graphene
3.4.4. Hard Particles
3.4.5. Limitations and Challenges
3.5. Surface Modification of UHMWPE
3.5.1. Surface Coating
3.5.2. Ion Beam Surface Modification
3.5.3. Photolithography and Nanoimprint Lithography
3.5.4. Laser Surface Texturing
3.5.5. Limitations and Challenges
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Kanaga Karuppiah, K.S.; Bruck, A.L.; Sundararajan, S.; Wang, J.; Lin, Z.; Xu, Z.-H.; Li, X. Friction and wear behavior of ultra-high molecular weight polyethylene as a function of polymer crystallinity. Acta Biomater. 2008, 4, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Garellick, G.; Maichou, H.; Herberts, P. Specific or general health outcome measures in the evaluation of total hip replacement. J. Bone Joint Surg. 1998, 80, 600–606. [Google Scholar] [CrossRef]
- Wayne, G. A National Public Health Agenda for Osteoarthritis; Centers for Disease Control and Prevention: DeKalb County, GA, USA, 2010. [Google Scholar]
- Delport, H.P.; Banks, S.A.; De Schepper, J.; Bellemans, J. A kinematic comparison of fixed- and mobile-bearing knee replacements. J. Bone Joint Surg. Br. 2006, 88, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.A. Wear and wear particles—Some fundamentals. Tribol. Int. 2005, 38, 863–870. [Google Scholar] [CrossRef]
- Jin, Z.; Fisher, J. 2—Tribology in joint replacement. In Joint Replacement Technology; Revell, P.A., Ed.; Woodhead Publishing: Sawston, UK, 2008; pp. 31–55. [Google Scholar]
- Bono, J.V.; Scott, R.D. Revision Total Knee Arthroplasty; Springer: Berlin/Heidelberg, Germany, 2005; p. 292. [Google Scholar]
- Bhushan, B. Adhesion and stiction: Mechanisms, measurement techniques, and methods for reduction. J. Vac. Sci. Technol. B 2003, 21. [Google Scholar] [CrossRef]
- Jacobs, J.J.; Skipor, A.K.; Patterson, L.M.; Hallab, N.J.; Paprosky, W.G.; Black, J.; Galante, J.O. Metal release in patients who have had a primary total hip arthroplasty. A prospective, controlled, longitudinal study. J. Bone Joint Surg. Am. 1998, 80, 1447–1458. [Google Scholar] [PubMed]
- Shi, W.; Li, X.Y.; Dong, H. Preliminary investigation into the load bearing capacity of ion beam surface modified UHMWPE. J. Mater. Sci. 2004, 39, 3183–3186. [Google Scholar] [CrossRef]
- Pearle, A.D.; Warren, R.F.; Rodeo, S.A. Basic science of articular cartilage and osteoarthritis. Clin. Sports Med. 2005, 24, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Shi, W.; Bell, T. Potential of improving tribological performance of UHMWPE by engineering the Ti6Al4V counterfaces. Wear 1999, 225–229, 146–153. [Google Scholar] [CrossRef]
- Deng, Y.; Xiong, D.; Wang, K. Biotribological properties of UHMWPE grafted with AA under lubrication as artificial joint. J. Mater. Sci. Mater. Med. 2013, 24, 2085–2091. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Hoffman, E.; Wimmer, M.; Fischer, A.; Jacobs, J.; Marks, L. CoCrMo metal-on-metal hip replacements. Phys. Chem. Chem. Phys. 2013, 15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-X.; Shin, Y.-J.; Lee, D.-H.; Yoon, K.-B. Preparation of ultra high molecular weight polyethylene with MgCl2/TiCl4 catalyst: Effect of internal and external donor on molecular weight and molecular weight distribution. Polymer Bull. 2011, 66, 627–635. [Google Scholar] [CrossRef]
- Kurtz, S.M. Chapter 1—A primer on uhmwpe. In UHMWPE Biomaterials Handbook, 2nd ed.; Kurtz, S.M., Ed.; Academic Press: Boston, MA, USA, 2009; pp. 1–6. [Google Scholar]
- Edidin, A.A.; Kurtz, S.M. Influence of mechanical behavior on the wear of 4 clinically relevant polymeric biomaterials in a HIP simulator. J. Arthroplast. 2000, 15, 321–331. [Google Scholar] [CrossRef]
- Brach del Prever, E.M.; Bistolfi, A.; Bracco, P.; Costa, L. UHMWPE for arthroplasty: Past or future? J. Orthop. Traumatol. 2009, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.M. Chapter 2—From ethylene gas to uhmwpe component: The process of producing orthopedic implants. In The UHMWPE Handbook; Kurtz, S.M., Ed.; Academic Press: San Diego, CA, USA, 2004; pp. 13–36. [Google Scholar]
- Lim, K.L.K.; Ishak, Z.A.M.; Ishiaku, U.S.; Fuad, A.M.Y.; Yusof, A.H.; Czigany, T.; Pukanszky, B.; Ogunniyi, D.S. High-density polyethylene/ultrahigh-molecular-weight polyethylene blend. I. The processing, thermal, and mechanical properties. J. Appl. Polymer Sci. 2005, 97, 413–425. [Google Scholar] [CrossRef]
- Kurtz, S.M. Chapter 2—From ethylene gas to UHMWPE component: The process of producing orthopedic implants. In UHMWPE Biomaterials Handbook, 2nd ed.; Kurtz, S.M., Ed.; Academic Press: Boston, MA, USA, 2009; pp. 7–19. [Google Scholar]
- Ayache, J.; Beaunier, L.; Boumendil, J.; Ehret, G.; Laub, D. Introduction to Materials; Springer: Berlin/Heidelberg, Germany, 2010; pp. 3–31. [Google Scholar]
- McKeen, L.W. 3—Plastics used in medical devices. In Handbook of Polymer Applications in Medicine and Medical Devices; Ebnesajjad, K.M., Ed.; William Andrew Publishing: Oxford, UK, 2014; pp. 21–53. [Google Scholar]
- Wang, S.; Ge, S. The mechanical property and tribological behavior of UHMWPE: Effect of molding pressure. Wear 2007, 263, 949–956. [Google Scholar] [CrossRef]
- Oral, E.; Christensen, S.D.; Malhi, A.S.; Wannomae, K.K.; Muratoglu, O.K. Wear resistance and mechanical properties of highly cross-linked, ultrahigh-molecular weight polyethylene doped with vitamin E. J. Arthroplasty 2006, 21, 580–591. [Google Scholar] [CrossRef] [PubMed]
- Sui, G.; Zhong, W.H.; Ren, X.; Wang, X.Q.; Yang, X.P. Structure, mechanical properties and friction behavior of UHMWPE/HDPE/carbon nanofibers. Mater. Chem. Phys. 2009, 115, 404–412. [Google Scholar] [CrossRef]
- Medel, F.J.; Puertolas, J.A. Wear resistance of highly cross-linked and remelted polyethylenes after ion implantation and accelerated ageing. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2008, 222, 877–885. [Google Scholar] [CrossRef]
- Oral, E.; Godleski Beckos, C.A.; Lozynsky, A.J.; Malhi, A.S.; Muratoglu, O.K. Improved resistance to wear and fatigue fracture in high pressure crystallized vitamin E-containing ultra-high molecular weight polyethylene. Biomaterials 2009, 30, 1870–1880. [Google Scholar] [CrossRef] [PubMed]
- Jahan, M.S. Chapter 29—Esr insights into macroradicals in uhmwpe. In Uhmwpe Biomaterials Handbook, 2nd ed.; Kurtz, S.M., Ed.; Academic Press: Boston, MA, USA, 2009; pp. 433–450. [Google Scholar]
- Green, T.R.; Fisher, J.; Stone, M.H.; Wroblewski, B.M.; Ingham, E. Polyethylene particles of a 'critical size' are necessary for the induction of cytokines by macrophages in vitro. Biomaterials 1998, 19, 2297–2302. [Google Scholar] [CrossRef] [PubMed]
- Muratoglu, O.K. Chapter 13—Highly crosslinked and melted UHMWPE. In UHMWPE Biomaterials Handbook, 2nd ed.; Kurtz, S.M., Ed.; Academic Press: Boston, MA, USA, 2009; pp. 197–204. [Google Scholar]
- McKellop, H.; Shen, F.W.; Lu, B.; Campbell, P.; Salovey, R. Development of an extremely wear-resistant ultra high molecular weight polyethylene for total hip replacements. J. Orthop. Res. 1999, 17, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Furmanski, J.; Anderson, M.; Bal, S.; Greenwald, A.S.; Halley, D.; Penenberg, B.; Ries, M.; Pruitt, L. Clinical fracture of cross-linked UHMWPE acetabular liners. Biomaterials 2009, 30, 5572–5582. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.; Luda, M.P.; Trossarelli, L. Ultra-high molecular weight polyethylene: I. Mechano-oxidative degradation. Polymer Degrad. Stab. 1997, 55, 329–338. [Google Scholar] [CrossRef]
- Costa, L.; Luda, M.P.; Trossarelli, L.; Brach del Prever, E.M.; Crova, M.; Gallinaro, P. In vivo uhmwpe biodegradation of retrieved prosthesis. Biomaterials 1998, 19, 1371–1385. [Google Scholar] [CrossRef] [PubMed]
- Premnath, V.; Harris, W.H.; Jasty, M.; Merrill, E.W. Gamma sterilization of UHMWPE articular implants: An analysis of the oxidation problem. Biomaterials 1996, 17, 1741–1753. [Google Scholar] [CrossRef] [PubMed]
- Stea, S.; Antonietti, B.; Baruffaldi, F.; Visentin, M.; Bordini, B.; Sudanese, A.; Toni, A. Behavior of hylamer polyethylene in hip arthroplasty: Comparison of two gamma sterilization techniques. Int. Orthop. 2006, 30, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Oral, E.; Malhi, A.S.; Muratoglu, O.K. Mechanisms of decrease in fatigue crack propagation resistance in irradiated and melted UHMWPE. Biomaterials 2006, 27, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Bracco, P.; Oral, E. Vitamin E-stabilized UHMWPE for total joint implants: A review. Clin. Orthop. Relat. Res. 2011, 469, 2286–2293. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.M. Chapter 16 vitamin-E-blended UHMWPE biomaterials. In UHMWPE Biomaterials Handbook, 2nd ed.; Academic Press: Boston, MA, USA, 2009; pp. 1–6. [Google Scholar]
- Bracco, P.; Brunella, V.; Zanetti, M.; Luda, M.P.; Costa, L. Stabilisation of ultra-high molecular weight polyethylene with vitamin E. Polymer Degrad. Stab. 2007, 92, 2155–2162. [Google Scholar] [CrossRef]
- Shibata, N.; Tomita, N.; Onmori, N.; Kato, K.; Ikeuchi, K. Defect initiation at subsurface grain boundary as a precursor of delamination in ultrahigh molecular weight polyethylene. J. Biomed. Mater. Res. Part A 2003, 67A, 276–284. [Google Scholar] [CrossRef]
- Kiyose, C.; Ueda, T. Vitamin E distribution and metabolism of tocopherols and tocotrienols in vivo. J. Clin. Biochem. Nutr. 2004, 35, 47–52. [Google Scholar] [CrossRef]
- Oral, E.; Wannomae, K.K.; Hawkins, N.; Harris, W.H.; Muratoglu, O.K. Α-tocopherol-doped irradiated UHMWPE for high fatigue resistance and low wear. Biomaterials 2004, 25, 5515–5522. [Google Scholar] [CrossRef] [PubMed]
- Oral, E.; Wannomae, K.K.; Rowell, S.L.; Muratoglu, O.K. Diffusion of vitamin E in ultra-high molecular weight polyethylene. Biomaterials 2007, 28, 5225–5237. [Google Scholar] [CrossRef] [PubMed]
- Oral, E.; Muratoglu, O. Vitamin E diffused, highly crosslinked UHMWPE: A review. Int. Orthop. (SICOT) 2011, 35, 215–223. [Google Scholar] [CrossRef]
- Oral, E.; Rowell, S.L.; Muratoglu, O.K. The effect of α-tocopherol on the oxidation and free radical decay in irradiated uhmwpe. Biomaterials 2006, 27, 5580–5587. [Google Scholar] [CrossRef] [PubMed]
- Wannomae, K.K.; Christensen, S.D.; Micheli, B.R.; Rowell, S.L.; Schroeder, D.W.; Muratoglu, O.K. Delamination and adhesive wear behavior of α-tocopherol-stabilized irradiated ultrahigh-molecular-weight polyethylene. J. Arthroplasty 2010, 25, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Haider, H.; Weisenburger, J.N.; Kurtz, S.M.; Rimnac, C.M.; Freedman, J.; Schroeder, D.W.; Garvin, K.L. Does vitamin E-stabilized ultrahigh-molecular-weight polyethylene address concerns of cross-linked polyethylene in total knee arthroplasty? J. Arthroplasty 2012, 27, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Lerf, R.; Zurbrügg, D.; Delfosse, D. Use of vitamin e to protect cross-linked uhmwpe from oxidation. Biomaterials 2010, 31, 3643–3648. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.M.; Dumbleton, J.; Siskey, R.S.; Wang, A.; Manley, M. Trace concentrations of vitamin E protect radiation crosslinked UHMWPE from oxidative degradation. J. Biomed. Mater. Res. Part A 2009, 90A, 549–563. [Google Scholar] [CrossRef]
- Oral, E.; Greenbaum, E.S.; Malhi, A.S.; Harris, W.H.; Muratoglu, O.K. Characterization of irradiated blends of alpha-tocopherol and UHMWPE. Biomaterials 2005, 26, 6657–6663. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Doshi, B.N.; Oral, E.; Muratoglu, O.K. High temperature melted, radiation cross-linked, vitamin E stabilized oxidation resistant UHMWPE with low wear and high impact strength. Polymer 2013, 54, 199–209. [Google Scholar] [CrossRef]
- Oral, E.; Godleski Beckos, C.; Malhi, A.S.; Muratoglu, O.K. The effects of high dose irradiation on the cross-linking of vitamin E-blended ultrahigh molecular weight polyethylene. Biomaterials 2008, 29, 3557–3560. [Google Scholar] [CrossRef] [PubMed]
- Muratoglu, O.K.; O’Connor, D.O.; Bragdon, C.R.; Delaney, J.; Jasty, M.; Harris, W.H.; Merrill, E.; Venugopalan, P. Gradient crosslinking of uhmwpe using irradiation in molten state for total joint arthroplasty. Biomaterials 2002, 23, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Oral, E.; Neils, A.; Muratoglu, O.K. High vitamin E content, impact resistant UHMWPE blend without loss of wear resistance. J. Biomed. Mater. Res. Part B 2015, 103, 790–797. [Google Scholar] [CrossRef]
- Teramura, S.; Sakoda, H.; Terao, T.; Endo, M.M.; Fujiwara, K.; Tomita, N. Reduction of wear volume from ultrahigh molecular weight polyethylene knee components by the addition of vitamin E. J. Orthop. Res. 2008, 26, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Wolf, C.; Krivec, T.; Lederer, K.; Schneider, W. Examination of the suitability of alpha-tocopherol as a stabilizer for ultra-high molecular weight polyethylene used for articulating surfaces in joint endoprostheses. J. Mater. Sci. Mater. Med. 2002, 13, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Tomita, N.; Kitakura, T.; Onmori, N.; Ikada, Y.; Aoyama, E. Prevention of fatigue cracks in ultrahigh molecular weight polyethylene joint components by the addition of vitamin E. J. Biomed. Mater. Res. 1999, 48, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Wood, W.J.; M, R.G.; Zhong, W.H. Improved wear and mechanical properties of UHMWPE-carbon nanofiber composites through an optimized paraffin-assisted melt-mixing process. Compos. Part B 2011, 42, 584–891. [Google Scholar] [CrossRef]
- Rachel, L.; Price, K.A.T.J.W. Improved osteoblast viability in the presence of smaller nanometre dimensioned carbon fibres. Nanotechnology 2004, 15, 892–900. [Google Scholar] [CrossRef]
- Galetz, M.C.; Blaβ, T.; Ruckdäschel, H.; Sandler, J.K.W.; Altstädt, V.; Glatzel, U. Carbon nanofibre-reinforced ultrahigh molecular weight polyethylene for tribological applications. J. Appl. Polymer Sci. 2007, 104, 4173–4181. [Google Scholar] [CrossRef]
- Wood, B.L.; Zhong, W. Influence of phase morphology on the sliding wear of polyethylene blends filled with carbon nanofibers. Polymer Eng. Sci. 2010, 50, 613–623. [Google Scholar] [CrossRef]
- Xu, S.; Aydar, A.; Liu, T.; Weston, W.; Tangpong, X.W.; Akhatov, I.S.; Zhong, W.-H. Wear of carbon nanofiber reinforced HDPE nanocomposites under dry sliding condition. J. Nanotechnol. Eng. Med. 2012, 3, 041003. [Google Scholar] [CrossRef]
- Price, R.L.; Haberstroh, K.M.; Webster, T.J. Improved osteoblast viability in the presence of smaller nanometre dimensioned carbon fibres. Nanotechnology 2004, 15, 892–900. [Google Scholar] [CrossRef]
- Bakshi, S.R.; Tercero, J.E.; Agarwal, A. Synthesis and characterization of multiwalled carbon nanotube reinforced ultra high molecular weight polyethylene composite by electrostatic spraying technique. Compos. Part A 2007, 38, 2493–2499. [Google Scholar] [CrossRef]
- Campo, N.; Visco, A.M. Incorporation of carbon nanotubes into ultra high molecular weight polyethylene by high energy ball milling. Int. J. Polymer Anal. Charact. 2010, 15, 438–449. [Google Scholar] [CrossRef]
- Ruan, S.L.; Gao, P.; Yang, X.G.; Yu, T.X. Toughening high performance ultrahigh molecular weight polyethylene using multiwalled carbon nanotubes. Polymer 2003, 44, 5643–5654. [Google Scholar] [CrossRef]
- Xue, Y.; Wu, W.; Jacobs, O.; Schadel, B. Tribological behaviour of UHMWPE/HDPE blends reinforced with multi-wall carbon nanotubes. Polymer Test. 2006, 25, 221–229. [Google Scholar] [CrossRef]
- Kuilla, T.; Bhadra, S.; Yao, D.; Kim, N.H.; Bose, S.; Lee, J.H. Recent advances in graphene based polymer composites. Prog. Polymer Sci. 2010, 35, 1350–1375. [Google Scholar] [CrossRef]
- Min, C.; Nie, P.; Song, H.-J.; Zhang, Z.; Zhao, K. Study of tribological properties of polyimide/graphene oxide nanocomposite films under seawater-lubricated condition. Tribol. Int. 2014, 80, 131–140. [Google Scholar] [CrossRef]
- Puértolas, J.A.; Kurtz, S.M. Evaluation of carbon nanotubes and graphene as reinforcements for UHMWPE-based composites in arthroplastic applications: A review. J. Mech. Behav. Biomed. Mater. 2014, 39, 129–145. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wang, J.; Jiang, B.; Yang, Y. Hindered phenol grafted carbon nanotubes for enhanced thermal oxidative stability of polyethylene. Polymer 2013, 54, 1167–1176. [Google Scholar] [CrossRef]
- Soldano, C.; Mahmood, A.; Dujardin, E. Production, properties and potential of graphene. Carbon 2010, 48, 2127–2150. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Ge, S.; Zhang, D.; Wang, Q. Biotribological behaviour of ultra-high molecular weight polyethylene composites containing Ti in a hip joint simulator. Proc. Inst. Mech. Eng. Part J. 2007, 221, 307–313. [Google Scholar]
- Lahiri, D.; Hec, F.; Thiesse, M.; Durygin, A.; Zhang, C.; Agarwal, A. Nanotribological behavior of graphene nanoplatelet reinforced ultra high molecular weight polyethylene composites. Tribol. Int. 2014, 70, 165–169. [Google Scholar] [CrossRef]
- Plumlee, K.; Schwartz, C.J. Improved wear resistance of orthopaedic UHMWPE by reinforcement with zirconium particles. Wear 2009, 267, 710–717. [Google Scholar] [CrossRef]
- Schwartz, C.J.; Bahadur, S.; Mallapragada, S.K. Effect of crosslinking and Pt-Zr quasicrystal fillers on the mechanical properties and wear resistance of uhmwpe for use in artificial joints. Wear 2007, 263, 1072–1080. [Google Scholar] [CrossRef]
- Zoo, Y.-S.; An, J.-W.; Lim, D.-P.; Lim, D.-S. Effect of carbon nanotube addition on tribological behavior of UHMWPE. Tribol. Lett. 2004, 16, 305–309. [Google Scholar] [CrossRef]
- Kanagaraj, S.; Mathew, M.T.; Fonseca, A.; Oliveira, M.S.A.; Simões, J.A.O.; Rocha, L.A. Tribological characterisation of carbon nanotubes/ultrahigh molecular weight polyethylene composites: The effect of sliding distance. Int. J. Surf. Sci. Eng. 2010, 4, 305–321. [Google Scholar] [CrossRef]
- Meschi Amoli, B.; Ahmad Ramazani, S.A.; Izadi, H. Preparation of ultrahigh-molecular-weight polyethylene/carbon nanotube nanocomposites with a ziegler-Natta catalytic system and investigation of their thermal and mechanical properties. J. Appl. Polym. Sci. 2012, 125, E453–E461. [Google Scholar]
- Maksimkin, A.V.; Kaloshkin, S.D.; Kaloshkina, M.S.; Gorshenkov, M.V.; Tcherdyntsev, V.V.; Ergin, K.S.; Shchetinin, I.V. Ultra-high molecular weight polyethylene reinforced with multi-walled carbon nanotubes: Fabrication method and properties. J. Alloys Compd. 2012, 536, 538–540. [Google Scholar] [CrossRef]
- Fonseca, M.A.; Subramani, K.; Oliveira, M.S.A.; Simões, J.A. Enhanced UHMWPE reinforced with mwcnt through mechanical ball-milling. Defect Diffus. Forum 2011, 312–315, 1238–1243. [Google Scholar] [CrossRef]
- Lee, J.K.; Rhee, K.Y.; Lee, J.H. Wear properties of 3-aminopropyltriethoxysilanefunctionalized carbon nanotubes reinforced ultra high molecular weight polyethylene nanocomposites. Polymer Eng. Sci. 2010, 50, 1433–1439. [Google Scholar] [CrossRef]
- Liu, Y.; Sinha, S.K. Wear performances and wear mechanism study of bulk UHMWPE composites with nacre and cnt fillers and pfpe overcoat. Wear 2013, 300, 44–54. [Google Scholar] [CrossRef]
- Martínez-Morlanes, M.J.; Castell, P.; Alonso, P.J.; Martinez, M.T.; Puértolas, J.A. Multi-walled carbon nanotubes acting as free radical scavengers in gamma-irradiated ultrahigh molecular weight polyethylene composites. Carbon 2012, 50, 2442–2452. [Google Scholar] [CrossRef]
- Balani, K.; Anderson, R.; Laha, T.; Andara, M.; Tercero, J.; Crumpler, E.; Agarwal, A. Plasma-sprayed carbon nanotube reinforced hydroxyapatite coatings and their interaction with human osteoblasts in vitro. Biomaterials 2007, 28, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, D.; Ge, S. Biotribological behavior of ultra-high molecular weight polyethylene composites containing ti ina hip joint simulator. Proc. Inst. Mech. Eng. Part J. 2007, 221, 307–313. [Google Scholar] [CrossRef]
- Ge, S.; Wang, S.; Huang, X. Increasing the wear resistance of UHMWPE acetabular cups by adding natural biocompatible particles. Wear 2009, 267, 770–776. [Google Scholar] [CrossRef]
- Xie, X.L.; Tang, C.Y.; Chan, K.Y.Y.; Wu, X.C.; Tsui, C.P.; Cheung, C.Y. Wear performance of ultrahigh molecular weight polyethylene/quartz composites. Biomaterials 2003, 24, 1889–1896. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sinha, S.K. Wear performances of uhmwpe composites with nacre and cnts, and pfpe coatings for bio-medical applications. Wear 2013, 300, 44–54. [Google Scholar] [CrossRef]
- Habibovic, P.; van der Valk, C.M.; van Blitterswijk, C.A.; de Groot, K.; Meijer, G. Influence of octacalcium phosphate coating on osteoinductive properties of biomaterials. J. Mater. Sci. Mater. Med. 2004, 15, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Calderon, V.S.; Sánchez-López, J.C.; Cavaleiro, A.; Carvalho, S. Biotribological behavior of Ag-Zrcxn1-x coatings against uhmwpe for joint prostheses devices. J. Mech. Behav. Biomed. Mater. 2015, 41, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Firouzi, D.; Youssef, A.; Amer, M.; Srouji, R.; Amleh, A.; Foucher, D.A.; Bougherara, H. A new technique to improve the mechanical and biological performance of ultra high molecular weight polyethylene using a nylon coating. J. Mech. Behav. Biomed. Mater. 2014, 32, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Ruan, F.; Bao, L. Mechanical enhancement of uhmwpe fibers by coating with carbon nanoparticles. Fibers Polymer 2014, 15, 723–728. [Google Scholar] [CrossRef]
- Zhang, Y.-C.; He, J.-X.; Wu, H.-Y.; Qiu, Y.-P. Surface characterization of helium plasma treated nano-sio2 sol-gel coated uhmwpe filaments by contact angle experiments and atr-ftir. J. Fiber Bioeng. Inf. 2010, 3, 50–54. [Google Scholar] [CrossRef]
- Chu, P.K.; Chen, J.Y.; Wang, L.P.; Huang, N. Plasma-surface modification of biomaterials. Mater. Sci. Eng. 2002, 36, 143–206. [Google Scholar] [CrossRef]
- Puértolas, J.A.; Martínez-Nogués, V.; Martínez-Morlanes, M.J.; Mariscal, M.D.; Medel, F.J.; López-Santos, C.; Yubero, F. Improved wear performance of ultra high molecular weight polyethylene coated with hydrogenated diamond like carbon. Wear 2010, 269, 458–465. [Google Scholar] [CrossRef] [Green Version]
- He, W.; Gonsalves, K.E.; Halberstadt, C.R. Micro/nanomachining and fabrication of materials for biomedical applications. In Biomedical Nanostructures; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; pp. 25–47. [Google Scholar]
- Dong, H.; Bell, T. State-of-the-art overview: Ion beam surface modification of polymers towards improving tribological properties. Surf. Coat. Technol. 1999, 111, 29–40. [Google Scholar] [CrossRef]
- Boampong, D.K.; Green, S.M.; Unsworth, A. N+ ion implantation of ti6al4v alloy and UHMWPE for total joint replacement application. J. Appl. Biomater. Biomech. 2003, 1, 164–171. [Google Scholar] [PubMed]
- Qiu, Z.-Y.; Chen, C.; Wang, X.-M.; Lee, I.-S. Advances in the surface modification techniques of bone-related implants for last 10 years. Regen. Biomater. 2014, 1, 67–79. [Google Scholar] [CrossRef]
- Ensinger, W.; Höchbauer, T.; Rauschenbach, B. Treatment uniformity of plasma immersion ion implantation studied with three-dimensional model systems. Surf. Coat. Technol. 1998, 103–104, 218–221. [Google Scholar] [CrossRef]
- Powles, R.C.; McKenzie, D.R.; Fujisawa, N.; McCulloch, D.G. Production of amorphous carbon by plasma immersion ion implantation of polymers. Diam. Relat. Mater. 2005, 14, 1577–1582. [Google Scholar] [CrossRef]
- Shi, W.; Li, X.Y.; Dong, H. Improved wear resistance of ultra-high molecular weight polyethylene by plasma immersion ion implantation. Wear 2001, 250, 544–552. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, W.; Wang, J.; Wang, X. Comparison of the effects of surface texture on the surfaces of steel and UHMWPE. Tribol. Int. 2013, 65, 138–145. [Google Scholar] [CrossRef]
- Kustandi, T.S.; Choo, J.H.; Low, H.Y.; Sinha, S.K. Texturing of UHMWPE surface via nil for low friction and wear properties. J. Phys. D 2010, 43, 015301. [Google Scholar] [CrossRef]
- Tan, J.; Saltzman, W.M. Biomaterials with hierarchically defined micro- and nanoscale structure. Biomaterials 2004, 25, 3593–3601. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Kaneda, K.; Yuhta, T.; Nishimura, I.; Yasuda, K.; Matsuno, T. Reduction of polyethylene wear by concave dimples on the frictional surface in artificial hip joints. J. Arthropl. 2000, 15, 332–338. [Google Scholar] [CrossRef]
- Kurella, A.; Dahotre, N.B. Review paper: Surface modification for bioimplants: The role of laser surface engineering. J. Biomater. Appl. 2005, 20, 5–50. [Google Scholar] [CrossRef] [PubMed]
- Chyr, A.; Qiu, M.; Speltz, J.; Jacobsen, R.L.; Sanders, A.P.; Raeymaekers, B. A patterned microtexture to reduce friction and increase longevity of prosthetic hip joints. Wear 2014, 315, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Cervantes, A.; Dominguez-Lopez, I.; Barceinas-Sanchez, J.D.; Garcia-Garcia, A.L. Effects of surface texturing on the performance of biocompatible UHMWPE as a bearing material during in vitro lubricated sliding/rolling motion. J. Mech. Behav. Biomed. Mater. 2013, 20, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Sagbas, B.; Durakbasa, M.N. Effect of surface patterning on frictional heating of vitamin E blended UHMWPE. Wear 2013, 303, 313–320. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Zhang, B.; Huang, W. Design principles for the area density of dimple patterns. Proc. Inst. Mech. Eng. Part J. 2014. [Google Scholar] [CrossRef]
- Kelly, N.H.; Fu, R.H.; Wright, T.M.; Padgett, D.E. Wear damage in mobile-bearing TKA is as severe as that in fixed-bearing TKA. Clin. Orthop. Relat. Res. 2011, 469, 123–130. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baena, J.C.; Wu, J.; Peng, Z. Wear Performance of UHMWPE and Reinforced UHMWPE Composites in Arthroplasty Applications: A Review. Lubricants 2015, 3, 413-436. https://doi.org/10.3390/lubricants3020413
Baena JC, Wu J, Peng Z. Wear Performance of UHMWPE and Reinforced UHMWPE Composites in Arthroplasty Applications: A Review. Lubricants. 2015; 3(2):413-436. https://doi.org/10.3390/lubricants3020413
Chicago/Turabian StyleBaena, Juan C., Jingping Wu, and Zhongxiao Peng. 2015. "Wear Performance of UHMWPE and Reinforced UHMWPE Composites in Arthroplasty Applications: A Review" Lubricants 3, no. 2: 413-436. https://doi.org/10.3390/lubricants3020413





